Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from The Aegean Sea, Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Heavy Metals Analyses and Quality Control
2.3. Risk Assessment Analyses
2.4. Statistics
3. Results
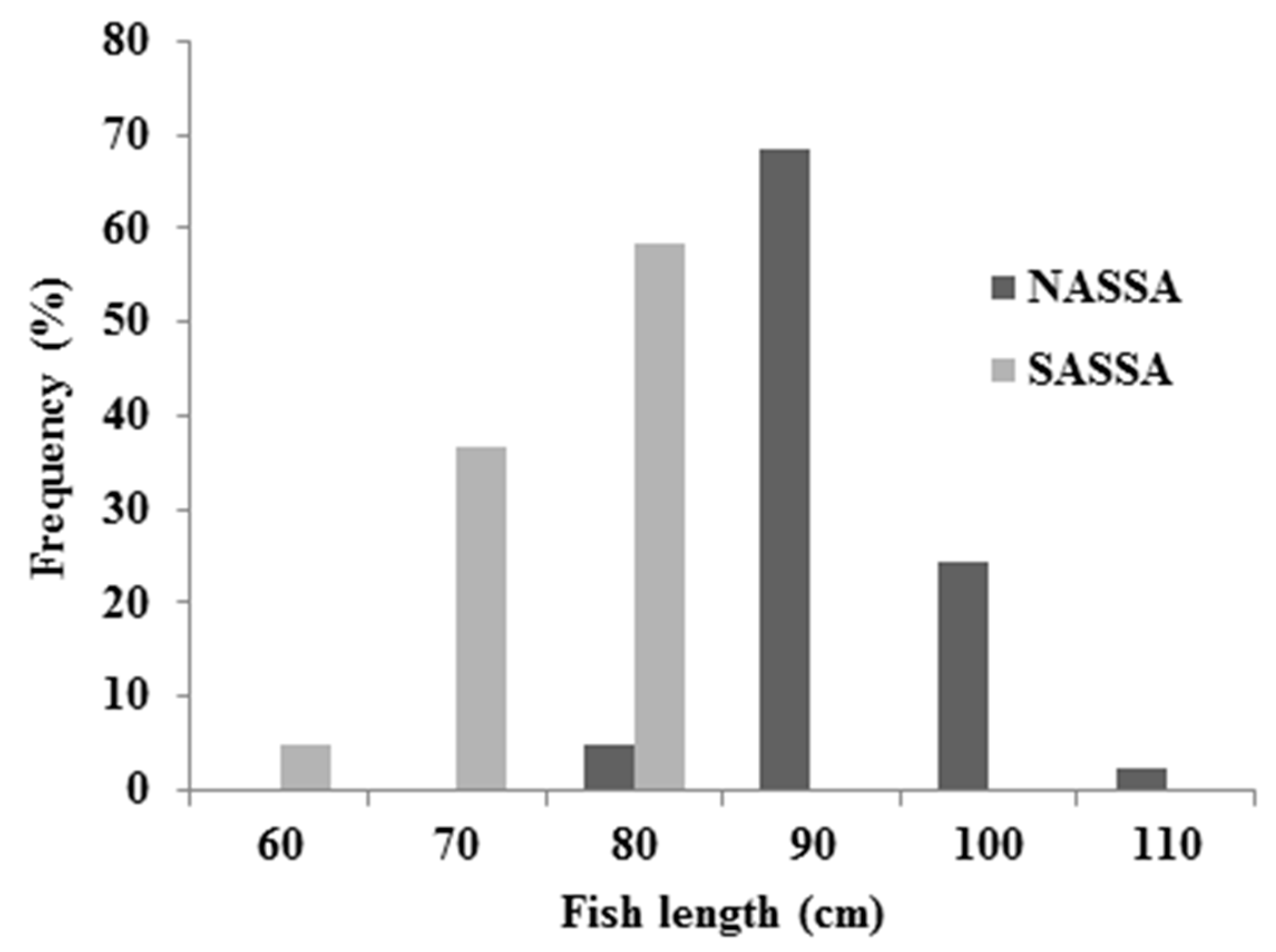
3.1. Descriptive Statistics and Biological Data
3.2. Heavy Metals Content in the Marine Organisms
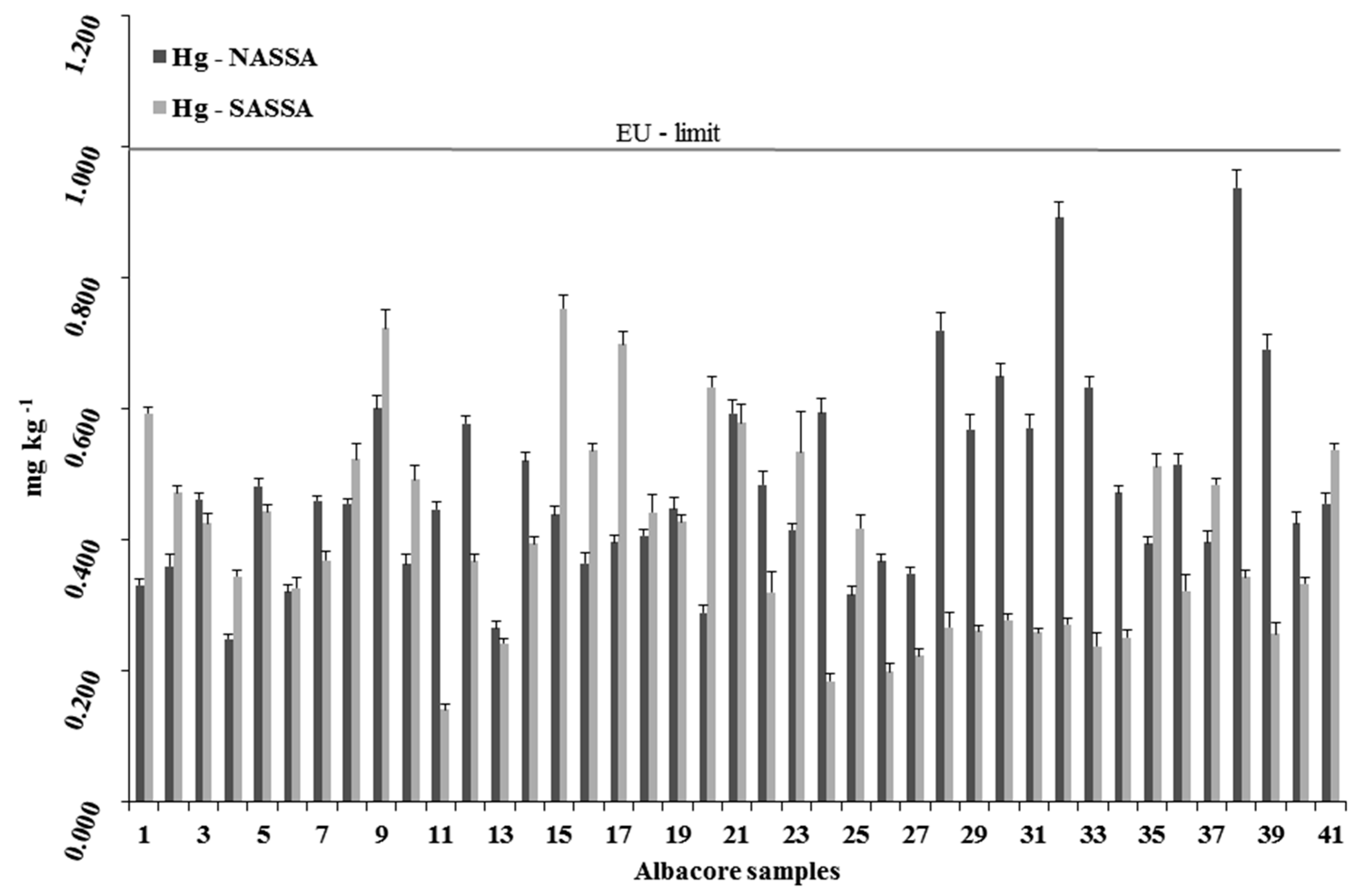
3.2.1. Mercury
3.2.2. Cadmium
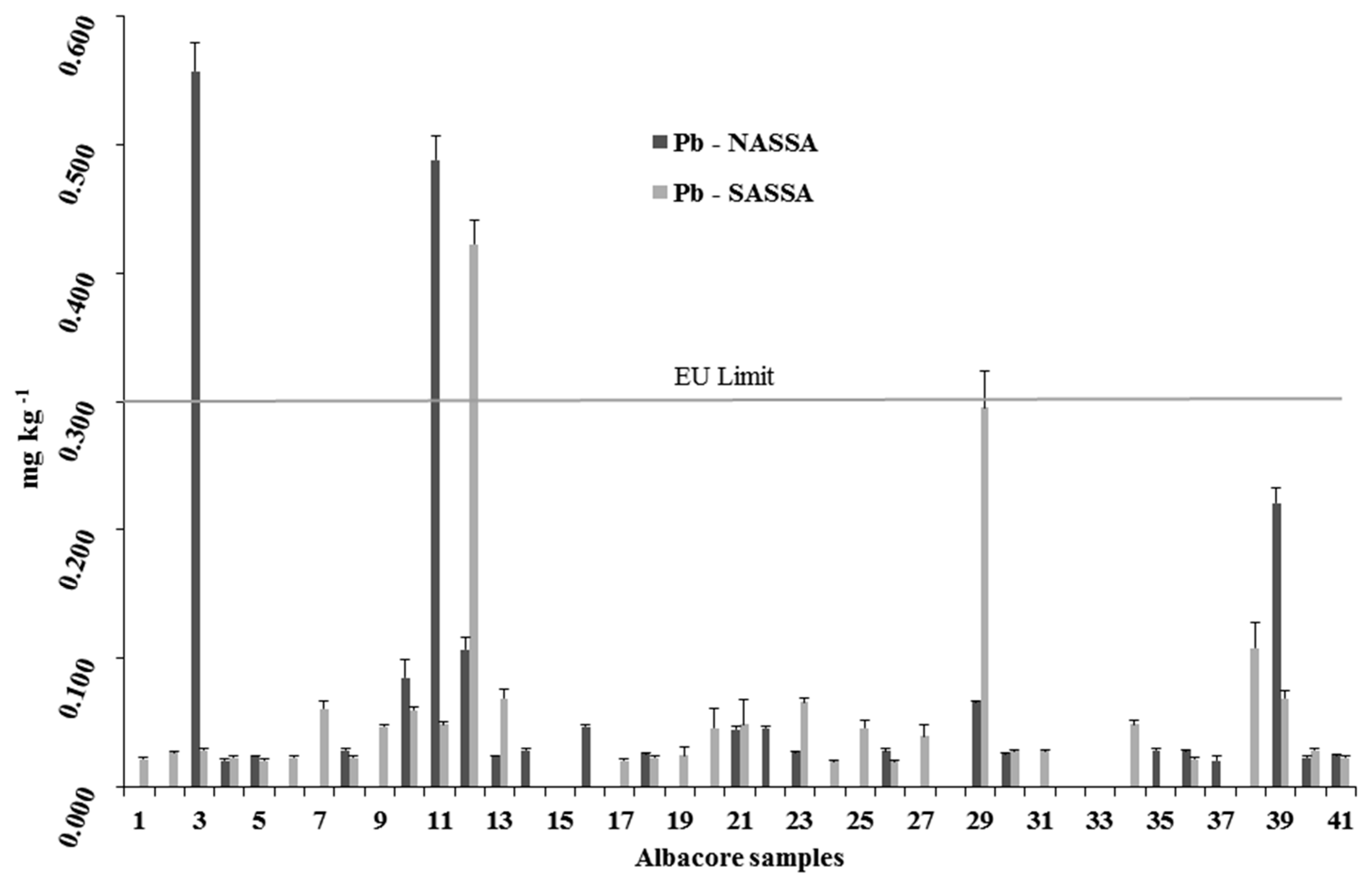
3.2.3. Lead
3.3. THQs of Mercury, Cadmium and Lead and TTHQs
4. Discussion
4.1. Metals Bioaccumulation
4.2. Possible Human Health Risks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). An overview of the global tuna market. In A quarterly update on World Seafood Markets; (Issue 4); GLOBEFISH Highlights: Rome, Italy, 2016; pp. 63–65. Available online: http://www.fao.org/3/a-i6592e.pdf (accessed on 31 October 2018).
- International Seafood Sustainability Foundation (ISSF). Status of the World Fisheries for Tuna, (Technical Report 2018-21), Washington, DC, USA. Available online: https://iss-foundation.org/download-monitor-demo/download-info/distribution-of-stocks-of-major-comercial-tunas-2011-oct-2018 (accessed on 1 November 2018).
- Khan, A.M.A.; Gray, T.S.; Mill, A.C.; Polunin, N.V.C. Impact of a fishing moratorium on a tuna pole-and-line fishery in eastern Indonesia. Mar. Policy 2018, 94, 143–149. [Google Scholar] [CrossRef]
- International Commission for the Conservation of Atlantic Tunas (ICCAT), ICCAT: Madrid, Spain. Available online: https://www.iccat.int/en/t1.asp (accessed on 31 October 2018).
- Alvarez-Berastegui, D.; Sámar Saber, S.; Ingram, G.W.; Díaz-Barroso, L.; Reglero, P.; Macías, D.; García-Barcelona, S.; de Urbina, J.O.; Tintoré, J.; Alemany, F. Integrating reproductive ecology, early life dynamics and mesoscale oceanography to improve albacore tuna assessment in the Western Mediterranean. Fish. Res. 2018, 208, 329–338. [Google Scholar] [CrossRef]
- Megalofonou, P. Age and growth of Mediterranean albacore. J. Fish Biol. 2000, 57, 700–715. [Google Scholar] [CrossRef]
- Andayesh, S.; Hadiani, M.R.; Mousavi, Z.; Shoeibi, S. Lead, cadmium, arsenic and mercury in canned tuna fish marketed in Tehran, Iran. Food Addit. Contam. Part B 2015, 8, 93–98. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Núñez, R.; Alonso, J.; Melgar, M.J. Total mercury in fresh and processed tuna marketed in Galicia (NW Spain) in relation to dietary exposure. Environ. Sci. Pollut. Res. 2016, 23, 24960–24969. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Pla, A.; Hernández, A.F.; Barbier, F.; Ayouni, L.; Gil, F. Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ. Int. 2013, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Barone, G.; Cuttone, G.; Giungato, D.; Garofalo, R. Occurrence of toxic metals (Hg, Cd and Pb) in fresh and canned tuna: Public health implications. Food Chem. Toxicol. 2010, 48, 3167–3170. [Google Scholar] [CrossRef] [PubMed]
- Ruelas-Inzunza, J.; Soto-Jiménez, M.; Ruiz-Fernández, A.; Bojórquez-Leyva, H.; Pérez-Bernal, H.; Páez-Osuna, F. 210Po activity and concentrations of selected trace elements (As, Cd, Cu, Hg, Pb, Zn) in the muscle tissue of tunas Thunnus albacares and Katsuwonus pelamis from the Eastern Pacific Ocean. Biol. Trace Elem. Res. 2010, 149, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Do fattening process and biological parameters affect the accumulation of metals in Atlantic bluefin tuna? Food Addit. Contam. A 2015, 32, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.; Caetano, M.; Vale, C.; Coelho, R.; Mil-Homens, M.; Dos Santos, M.N. Searching relationships between tissue elemental concentrations and geographical distribution of bigeye tuna (Thunnus obesus) from the South Atlantic Ocean. J. Fishscicom 2017, 11, 64–70. [Google Scholar] [CrossRef]
- Ugarte, A.; Abrego, Z.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Evaluation of the bioaccumulation of trace elements in tuna species by correlation analysis between their concentrations in muscle and first dorsal spine using microwave-assisted digestion and ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 1761–1775. [Google Scholar] [CrossRef]
- Voegborlo, R.B.; El-Methnani, A.M.; Abedin, M.Z. Mercury, cadmium and lead content of canned tuna fish. Food Chem. 1999, 67, 341–345. [Google Scholar] [CrossRef]
- Renieri, E.A.; Alegakis, A.K.; Kiriakakis, M.; Vinceti, M.; Ozcagli, E.; Wilks, M.F.; Tsatsakis, A.M. Cd, Pb and Hg Biomonitoring in Fish of the Mediterranean Region and Risk Estimations on Fish Consumption. Toxics 2014, 2, 417–442. [Google Scholar] [CrossRef]
- Mason, R.P. Trace Metals in Aquatic Systems; Blackwell Publishing Ltd.: Oxford, UK, 2013; p. 431. ISBN 978-1-40-516048-3. [Google Scholar]
- Copat, C.; Conti, G.O.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy Metals in Fish from the Mediterranean Sea: Potential Impact on Diet. In The Mediterranean Diet; Preedy, V.R., Watson, R.R., Eds.; Academic Press: New York, NY, USA, 2015; pp. 547–562. [Google Scholar] [CrossRef]
- Christoforidis, A.; Stamatis, N.; Schmieder, K.; Tsachalidis, A. Organochlorine and mercury contamination in fish tissues from Nestos River, Greece. Chemosphere 2008, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Manta, D.S.; Mirto, S.; Quinci, E.M.; Ape, F.; Valeria Montalto, V.; Michele Gristina, M.; Traina, A.; Sprovieri, M. Bioaccumulation of heavy metals in fish, crustaceans, molluscs and echinoderms from the Tuscany coast. Ecotoxicol. Environ. Saf. 2018, 162, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, K.A. Toxic metal species and food regulations-Making a healthy choice. Analyst 2007, 132, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration. Toxic Metals, Washington, DC: U.S. Department of Labor. Available online: https://www.osha.gov/SLTC/metalsheavy/index.html (accessed on 15 November 2018).
- Munoz-Olivas, R.; Camara, C. Speciation related to human health. In Trace Element Speciation for Environment, Food and Health; Ebdon, L., Pitts, L., Cornelis, R., Crews, H., Donard, O.F.X., Quevauviller, P., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2001; pp. 331–353. [Google Scholar]
- European Food Safety Authority (EFSA). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Torres, P.; Rodrigues, A.; Soares, L.; Garcia, P. Metal concentrations in two commercial tuna species from an active volcanic region in the mid-Atlantic Ocean. Arch. Environ. Contam. Toxicol. 2016, 70, 341–347. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A. Metal concentrations in selected brands of canned fish in Nigeria: Estimation of dietary intakes and target hazard quotients. Environ. Monit. Assess. 2015, 187, 85. [Google Scholar] [CrossRef] [PubMed]
- Pappa, F.K.; Tsabaris, C.; Ioannidou, A.; Patiris, D.L.; Kaberi, H.; Pashalidis, I.; Eleftheriou, G.; Androulakaki, E.G.; Vlastou, R. Radioactivity and metal concentrations in marine sediments associated with mining activities in Ierissos Gulf, North Aegean Sea, Greece. Appl. Radiat. Isotopes 2016, 116, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zabetoglou, Κ.; Voutsa, D.; Samara, C. Toxicity and heavy metal contamination of surficial sediments from the Bay of Thessaloniki (Northwestern Aegean Sea) Greece. Chemosphere 2002, 49, 17–26. [Google Scholar] [CrossRef]
- Stamatis, N.; Ioannidou, D.; Christoforidis, A.; Koutrakis, E. Sediment pollution by heavy metals in the Strymonikos and Ierissos Gulfs, North Aegean Sea, Greece. Environ. Monit. Assess. 2002, 80, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, N.; Kamidis, N.; Sylaios, G. Sediment and Suspended Matter Lead Contamination in the Gulf of Kavala-Greece. Environ. Monit. Assess. 2006, 115, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, A.; Stamatis, N. Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 2009, 151, 257–263. [Google Scholar] [CrossRef]
- Stamatis, N.; Ioannidou, D.; Koutrakis, E. Monitoring of key eutrophication parameters at three inshore stations of Strymonikos Gulf, North Aegean Sea. Fresen. Environ. Bull. 2001, 10, 706–710. [Google Scholar]
- Mol, S.; Ozden, O.; Karakulak, S. Levels of Selected Metals in Albacore (Thunnus alalunga, Bonnaterre, 1788) from the Eastern Mediterranean. J. Aquat. Food Prod. Technol. 2012, 21, 111–117. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission regulation (EU) no. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJEU 2006, 364, 5–24. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants; Fifty-Seventh Report of the Joint FAO/WHO Expert Committee on Foods Additives; WHO Technical Report Series; WHO: Geneva, Switzerland, 2002; Volume 909, pp. 1–186. [Google Scholar]
- Adams, D.H.; McMicheal, R.H., Jr.; Henderson, G.E. Mercury Levels in Marine and Estuarine Fishes of Florida: 1989–2001, 2nd ed.; Technical Report TR-9; Florida Marine Research Institute: Petersburg, FL, USA, 2003; p. 57.
- United States Environmental Protection Agency (US EPA). Risk Assessment Guidance for Superfund: Human Health Evaluation Manual. (Part A), (Interim Final); 1989. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 8 November 2018).
- Food and Agriculture Organization of the United Nations (FAO). Statistical Databases. 2005. Available online: http://faostat.fao.org (accessed on 10 December 2012).
- United States Environmental Protection Agency (US EPA). Risk Assessment Guidance for Superfund Volume I. Human Health Evaluation Manual (Part A) I. 2010. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls (accessed on 8 November 2018).
- Hallenbeck, W.H. Quantitative Risk Assessment for Environmental and Occupational Health; Lewis: Boca Raton, FL, USA, 1993; p. 240. ISBN 0-87371-801-1. [Google Scholar]
- Chien, L.C.; Hung, T.C.; Choang, K.Y.; Yeh, C.Y.; Meng, P.J.; Shieh, M.J.; Han, B.C. Daily intake of TBT, Cu, Zn, Cd and as for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef]
- Çulha, S.T.; Yabanli, M.; Baki, B.; Yozukmaz, A. Heavy metals in tissues of scorpionfish (Scorpaena porcus) caught from Black Sea (Turkey) and potential risks to human health. Environ. Sci. Pollut. Res. 2016, 23, 20882–20892. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Compilation of Legal limits for Hazardous Substances in Fish and Fishery Products; FAO Fishery Circular No. 464; FAO: Rome, Italy, 1983; pp. 5–100. [Google Scholar]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Addit. Contam. 2002, 19, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Marcotrigiano, G.O. Content of mercury and cadmium in fish (Thunnus alalunga) and cephalopods (Eledone moschata) from the south-eastern Mediterranean Sea. Food Add. Contam. 2004, 21, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Besada, V.; Gonzalez, J.J.; Schultze, F. Mercury, cadmium, lead, arsenic, copper, and zinc concentrations in albacore, yellowfin tuna and bigeye tuna from the Atlantic Ocean. Cienc. Mar. 2006, 32, 439–445. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, G.; Iwata, H.; Subramanian, A.; Ismail, A.; Tanabe, S. Concentrations of trace elements in marine fish and its risk assessment in Malaysia. Mar. Pollut. Bull. 2005, 51, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Love, J.L.; Rush, G.M.; McGrath, H. Total mercury and methylmercury levels in some New Zealand commercial marine fish species. Food Addit. Contam. 2003, 20, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.T.; Geise, L.A. Mercury Content in Pacific Albacore Tuna (Thunnus alalunga) during 2006 Season; Oregon State University Seafood Laboratory: Astoria, OR, USA, 2006; pp. 1–8. Available online: http://www.seafarepacific.com/wp-content/uploads/2015/12/2006-Mercury-Content-Study.pdf (accessed on 8 November 2018).
- Chen, C.Y.; Lai, C.C.; Chen, K.S.; Hsu, C.C.; Hung, C.H.; Chen, M.H. Total and organic mercury concentrations in the muscles of Pacific albacore (Thunnus alalunga) and bigeye tuna (Thunnus obesus). Mar. Pollut. Bull. 2014, 85, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Bacci, E. Mercury in the Mediterranean. Mar. Pollut. Bull. 1989, 20, 59–63. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; Marcotrigiano, G.O. Accumulation of mercury, cadmium, lead and arsenic in swordfish and bluefin tuna from the Mediterranean Sea: A comparative study. Mar. Poll. Bull. 2005, 50, 993–1018. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.M.; Cedeño-Macías, L.A. Heavy metals in yellowfin tuna (Thunnus albacares) and common dolphinfish (Coryphaena hippurus) landed on the Ecuadorian coast. Sci. Total Environ. 2016, 541, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Agyekum Akwasi, A.; Frimpong Clifford, E.; Armah, D.; Agyei-Mensah, R.; Adongo Abdul-Malik, A.; Opoku-Danquah, J.; Derry, D. Occurrence of toxic heavy metals (Hg, Pb and Cd) in fish on Ghanaian markets. Elixir Food Sci. 2012, 48, 9669–9671. [Google Scholar]
- Mendil, D.; Demirci, Z.; Tuzen, M.; Soylak, M. Seasonal investigation of trace element contents in commercially valuable fish species from the Black sea, Turkey. Food Chem. Toxicol. 2010, 48, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005, 99, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Ganjavi, M.; Ezzatpanah, H.; Hadi Givianrad, M.; Shams, A. Effect of canned tuna fish processing steps on lead and cadmium contents of Iranian tuna fish. Food Chem. 2010, 118, 525–528. [Google Scholar] [CrossRef]
- Agah, H.; Leermakers, M.; Elskens, M.; Reza Fatemi, S.M.; Baeyens, W. Total mercury and methyl mercury concentrations in fish from the Persian Gulf and the Caspian Sea Homira. Water Air Soil Pollut. 2007, 181, 95–105. [Google Scholar] [CrossRef]




| Albacores | NASSA Area | SASSA Area | ||||||
|---|---|---|---|---|---|---|---|---|
| THQHg | THQCd | THQPb | TTHQ | THQHg | THQCd | THQPb | TTHQ | |
| 1 | 1.779 | 0.015 | 0.000 | 1.794 | 3.186 | 0.011 | 0.003 | 3.201 |
| 2 | 1.934 | 0.016 | 0.000 | 1.950 | 2.536 | 0.012 | 0.004 | 2.552 |
| 3 | 2.482 | 0.024 | 0.075 | 2.581 | 2.289 | 0.033 | 0.004 | 2.326 |
| 4 | 1.338 | 0.012 | 0.003 | 1.353 | 1.854 | 0.012 | 0.003 | 1.869 |
| 5 | 2.595 | 0.022 | 0.003 | 2.620 | 2.386 | 0.012 | 0.003 | 2.400 |
| 6 | 1.725 | 0.035 | 0.000 | 1.760 | 1.757 | 0.015 | 0.003 | 1.775 |
| 7 | 2.472 | 0.025 | 0.000 | 2.497 | 1.983 | 0.016 | 0.008 | 2.006 |
| 8 | 2.450 | 0.023 | 0.004 | 2.477 | 2.816 | 0.012 | 0.003 | 2.831 |
| 9 | 3.229 | 0.023 | 0.000 | 3.252 | 3.890 | 0.012 | 0.006 | 3.909 |
| 10 | 1.956 | 0.013 | 0.011 | 1.980 | 2.644 | 0.013 | 0.008 | 2.665 |
| 11 | 2.402 | 0.012 | 0.066 | 2.479 | 0.758 | 0.013 | 0.007 | 0.778 |
| 12 | 3.106 | 0.023 | 0.014 | 3.143 | 1.977 | 0.014 | 0.057 | 2.048 |
| 13 | 1.429 | 0.036 | 0.003 | 1.468 | 1.300 | 0.015 | 0.009 | 1.324 |
| 14 | 2.799 | 0.025 | 0.004 | 2.828 | 2.122 | 0.016 | 0.000 | 2.138 |
| 15 | 2.359 | 0.047 | 0.000 | 2.406 | 4.046 | 0.012 | 0.000 | 4.058 |
| 16 | 1.961 | 0.359 | 0.006 | 2.327 | 2.885 | 0.012 | 0.000 | 2.897 |
| 17 | 2.133 | 0.054 | 0.000 | 2.187 | 3.756 | 0.013 | 0.003 | 3.772 |
| 18 | 2.187 | 0.034 | 0.003 | 2.225 | 2.380 | 0.024 | 0.003 | 2.407 |
| 19 | 2.407 | 0.026 | 0.000 | 2.433 | 2.300 | 0.077 | 0.003 | 2.380 |
| 20 | 1.553 | 0.059 | 0.000 | 1.611 | 3.401 | 0.048 | 0.006 | 3.455 |
| 21 | 3.192 | 0.058 | 0.006 | 3.256 | 3.111 | 0.099 | 0.007 | 3.217 |
| 22 | 2.606 | 0.025 | 0.006 | 2.637 | 1.719 | 0.011 | 0.000 | 1.731 |
| 23 | 2.235 | 0.046 | 0.004 | 2.285 | 2.875 | 0.012 | 0.009 | 2.896 |
| 24 | 3.197 | 0.056 | 0.000 | 3.253 | 0.994 | 0.025 | 0.003 | 1.022 |
| 25 | 1.703 | 0.057 | 0.000 | 1.760 | 2.246 | 0.034 | 0.006 | 2.286 |
| 26 | 1.977 | 0.088 | 0.004 | 2.069 | 1.069 | 0.044 | 0.003 | 1.115 |
| 27 | 1.875 | 0.037 | 0.000 | 1.912 | 1.204 | 0.033 | 0.005 | 1.242 |
| 28 | 3.869 | 0.258 | 0.000 | 4.127 | 1.435 | 0.023 | 0.000 | 1.458 |
| 29 | 3.057 | 0.184 | 0.009 | 3.250 | 1.402 | 0.037 | 0.040 | 1.479 |
| 30 | 3.493 | 0.292 | 0.003 | 3.788 | 1.494 | 0.059 | 0.004 | 1.556 |
| 31 | 3.068 | 0.163 | 0.000 | 3.231 | 1.392 | 0.047 | 0.004 | 1.442 |
| 32 | 4.793 | 0.131 | 0.000 | 4.924 | 1.456 | 0.025 | 0.000 | 1.481 |
| 33 | 3.407 | 0.142 | 0.000 | 3.549 | 1.279 | 0.034 | 0.000 | 1.313 |
| 34 | 2.541 | 0.207 | 0.000 | 2.749 | 1.349 | 0.011 | 0.007 | 1.367 |
| 35 | 2.122 | 0.229 | 0.004 | 2.356 | 2.751 | 0.035 | 0.000 | 2.787 |
| 36 | 2.773 | 0.198 | 0.004 | 2.974 | 1.730 | 0.037 | 0.003 | 1.770 |
| 37 | 2.133 | 0.186 | 0.003 | 2.322 | 2.606 | 0.058 | 0.000 | 2.664 |
| 38 | 5.040 | 0.173 | 0.000 | 5.213 | 1.848 | 0.316 | 0.015 | 2.179 |
| 39 | 3.713 | 0.252 | 0.030 | 3.995 | 1.381 | 0.076 | 0.009 | 1.466 |
| 40 | 2.289 | 0.229 | 0.003 | 2.521 | 1.795 | 0.272 | 0.004 | 2.070 |
| 41 | 2.450 | 0.162 | 0.003 | 2.616 | 2.891 | 0.131 | 0.003 | 3.024 |
| MIN | 1.338 | 0.012 | 0.000 | 1.353 | 0.758 | 0.011 | 0.000 | 0.778 |
| MAX | 5.040 | 0.359 | 0.075 | 5.213 | 4.046 | 0.316 | 0.057 | 4.058 |
| MEAN | 2.581 | 0.099 | 0.007 | 2.687 | 2.153 | 0.044 | 0.006 | 2.204 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamatis, N.; Kamidis, N.; Pigada, P.; Stergiou, D.; Kallianiotis, A. Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from The Aegean Sea, Greece. Int. J. Environ. Res. Public Health 2019, 16, 821. https://doi.org/10.3390/ijerph16050821
Stamatis N, Kamidis N, Pigada P, Stergiou D, Kallianiotis A. Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from The Aegean Sea, Greece. International Journal of Environmental Research and Public Health. 2019; 16(5):821. https://doi.org/10.3390/ijerph16050821
Chicago/Turabian StyleStamatis, Nikolaos, Nikolaos Kamidis, Pelagia Pigada, Despoina Stergiou, and Argyris Kallianiotis. 2019. "Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from The Aegean Sea, Greece" International Journal of Environmental Research and Public Health 16, no. 5: 821. https://doi.org/10.3390/ijerph16050821
APA StyleStamatis, N., Kamidis, N., Pigada, P., Stergiou, D., & Kallianiotis, A. (2019). Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from The Aegean Sea, Greece. International Journal of Environmental Research and Public Health, 16(5), 821. https://doi.org/10.3390/ijerph16050821







