Evaluating the Integrated Methadone and Anti-Retroviral Therapy Strategy in Tanzania Using the RE-AIM Framework
Abstract
:1. Introduction
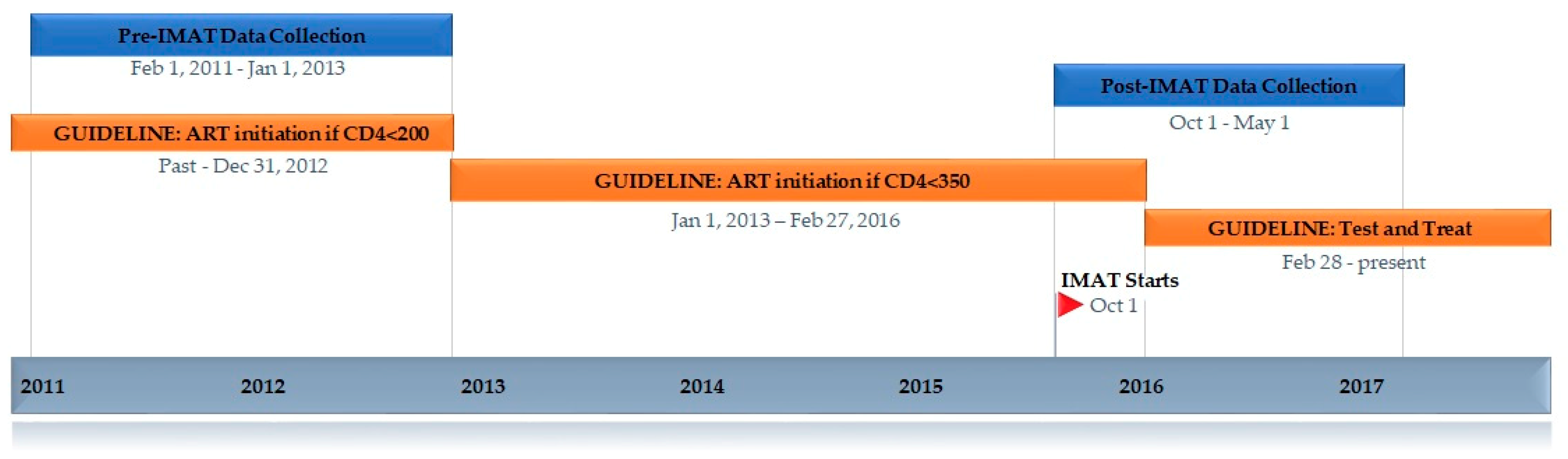
2. Methods
2.1. Setting
2.2. Eligibility Criteria for Starting ART
2.3. Developing IMAT
2.4. The IMAT Program
2.5. Laboratory Data Collection
2.6. Evaluation Framework
2.7. Quantitative Data for Evaluation
2.8. Qualitative Data for Evaluation
3. Results
3.1. Reach
“The current system is better than the past. In the past, we were given the medications to take at home, but many people were not taking them, and we had a calamity. Many people died. Now you have to go to take your methadone and then go to the back to take your ARVs [anti-retrovirals]. They will know that you are not taking them if you don’t show up. This system is better.”
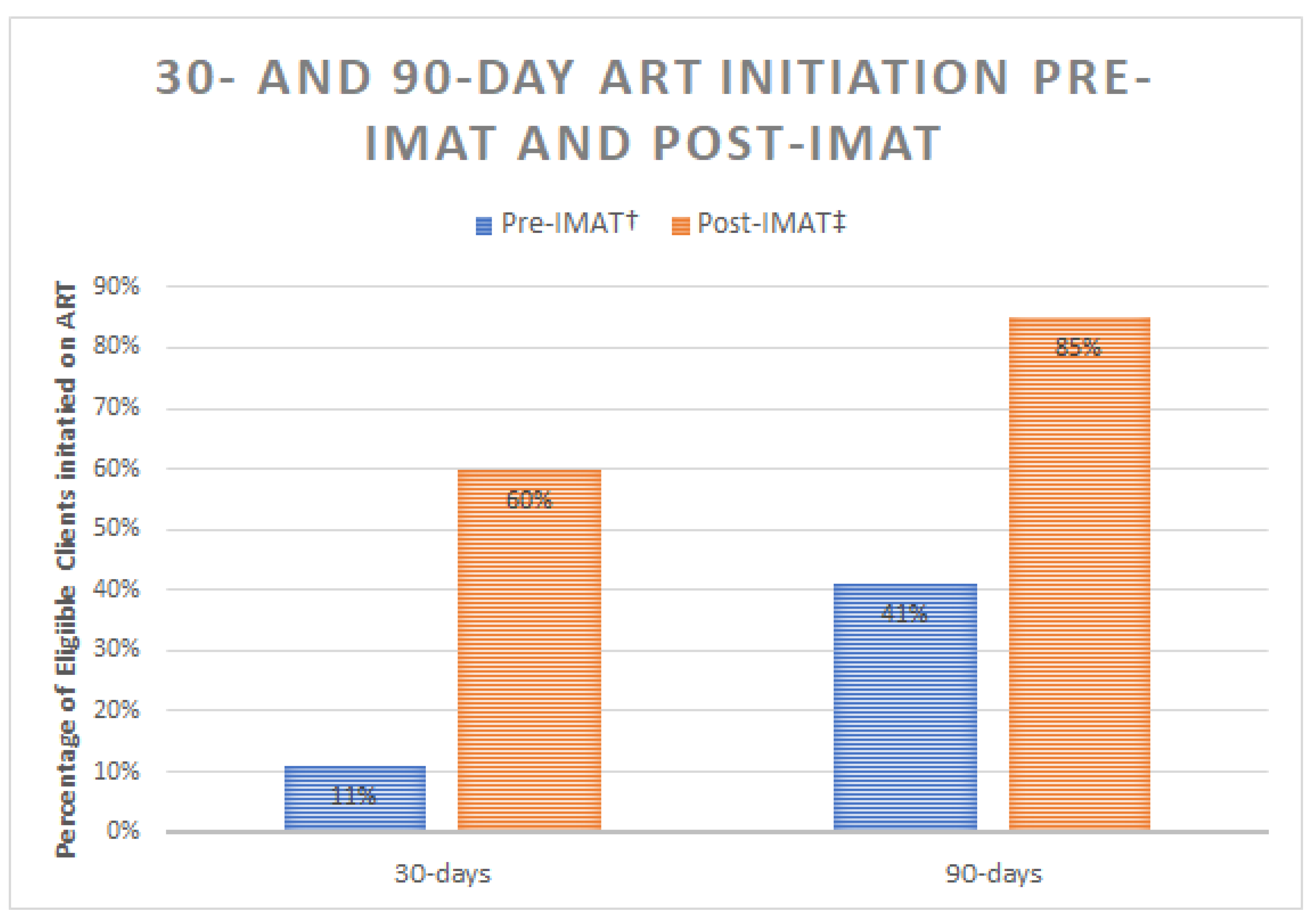
3.2. Effectiveness
ART Initiation
3.3. Adoption
“Drug users are at an increased risk of getting HIV, so it is [appropriate] for [the OTP clinic] to have HIV services integrated. It is not okay to separate HIV services from this program.”
“This program has added the work load on us.…. We now have a lot of works to do in such a way that you cannot do an assessment on them. We have to leave other work and do this because of the shortage.”
“Yes, it is very helpful because we are working as a team though we don’t get to run it directly. It reminds and guides us on who is supposed to do what…. It reminds us of who needs certain services and who is due for follow up. I am imagining it if we were doing it in the file system, it would have been such a challenge.”
3.4. Implementation
3.4.1. Referral to the CTC
3.4.2. CD4 Testing
3.4.3. Viral Load Testing
3.4.4. Protocol Adaptation: Changes to Treatment Guidelines
3.4.5. Protocol Adaptation: Client Preference
3.4.6. Protocol Adaptation: System-Level Challenges
3.4.7. Barriers and Facilitators to Implementation
“The problem is with the baseline investigations results […] The clients cannot be started on ARVs if they have kidney problems […] Clients may be lost […]”(Provider)
“I am just requesting for more confidentiality […] Many people are scared of taking their [ART] medications here because they don’t want to be mocked […]”
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Anti-retroviral therapy |
| CTC | Care and Treatment Center |
| IMAT | Integrated Methadone and Anti-Retroviral Therapy |
| LIMS | Laboratory Information Management System |
| OTP | Opioid Treatment Program |
| PWID | People Who Inject Drugs |
| RE-AIM | Reach, Effectiveness, Adoption, Implementation, Maintenance |
| SOP | Standard Operating Procedures |
References
- UNAIDS. Ending AIDS: Progress towards the 90-90-90 Targets; Joint United Nations Program on HIV/AIDS (UNAIDS): Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ogbuagu, O.; Bruce, R.D. Reaching the unreached: Treatment as prevention as a workable strategy to mitigate HIV and its consequences in high-risk groups. Curr. HIV/AIDS Rep. 2014, 11, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.D. Is it time for treatment as prevention among people who inject drugs? J. Acquir. Immune Defic. Syndr. 2013, 63, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Stockman, J.K. Epidemiology of HIV Among Injecting and Non-injecting Drug Users: Current Trends and Implications for Interventions. Curr. HIV/AIDS Rep. 2010, 7, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, B.M.; Degenhardt, L.; Phillips, B.; Wiessing, L.; Hickman, M.; Strathdee, S.A.; Wodak, A.; Panda, S.; Tyndall, M.; Toufik, A.; et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet 2008, 372, 1733–1745. [Google Scholar] [CrossRef]
- Mathers, B.M.; Degenhardt, L.; Ali, H.; Wiessing, L.; Hickman, M.; Mattick, R.P.; Myers, B.; Ambekar, A.; Strathdee, S.A. HIV prevention, treatment, and care services for people who inject drugs: A systematic review of global, regional, and national coverage. Lancet 2010, 375, 1014–1028. [Google Scholar] [CrossRef]
- Forsyth, A.D.; Valdiserri, R.O. Reaping the prevention benefits of highly active antiretroviral treatment: Policy implications of HIV Prevention Trials Network 052. Curr. Opin. HIV AIDS 2012, 7, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.J.; Garnett, G.P.; Hallett, T.B. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. Aids 2010, 24, 729–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, O.C.; Bruce, R.D.; Masao, F.; Ubuguyu, O.; Sabuni, N.; Mbwambo, J.; Lambdin, B.H. Implementation and Operational Research: Linkage to Care Among Methadone Clients Living With HIV in Dar es Salaam, Tanzania. J. Acquir. Immune Defic. Syndr. 2015, 69, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.L.; McCurdy, S.A.; Bowen, A.M.; Kilonzo, G.P.; Atkinson, J.S.; Ross, M.W.; Leshabari, M.T. HIV seroprevalence in a sample of Tanzanian intravenous drug users. AIDS Educ. Prev. 2009, 21, 474–483. [Google Scholar] [CrossRef] [PubMed]
- National Aids Control Program, Dar-Es-Salaam, Tanzania. Consensus estimates on key population size and HIV prevalence in Tanzania. 2014. Available online: http://www.healthpolicyproject.com/pubs/ (accessed on 20 December 2018).
- Davies, C.F.; French, C.; Christensen, H.; May, M.T.; Hickman, M.; Welton, N.J.; Vickerman, P.; Low, A.J.; McLean, S.; Mburu, G.; et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2016, 63, 1094–1104. [Google Scholar]
- Bruce, R.D.; Altice, F.L. Clinical care of the HIV-infected drug user. Infect. Dis Clin. N. Am. 2007, 21, 149–179, ix. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Delamere, S.; McCullough, L.; Hopkins, S.; Bergin, C.; Mulcahy, F. Assessing limiting factors to the acceptance of antiretroviral therapy in a large cohort of injecting drug users. HIV Med. 2003, 4, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, A.H.; Scheyett, A.; Golin, C.E. HIV and stigma: Analysis and research program. Curr. HIV/AIDS Rep. 2005, 2, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Keenan, E.; Ryan, M.; Barry, M.; Mulcahy, F. Directly observed antiretroviral therapy for injection drug users with HIV infection. AIDS Read. 2002, 12, 305–307. [Google Scholar] [PubMed]
- Sorensen, J.L.; Haug, N.A.; Larios, S.; Gruber, V.A.; Tulsky, J.; Powelson, E.; Logan, D.P.; Shapiro, B. Directly administered antiretroviral therapy: Pilot study of a structural intervention in methadone maintenance. J. Subst. Abuse Treat. 2012, 43, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachireddy, C.; Soule, M.C.; Izenberg, J.M.; Dvoryak, S.; Dumchev, K.; Altice, F.L. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014, 134, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achmad, Y.M.; Istiqomah, A.N.; Iskandar, S.; Wisaksana, R.; van Crevel, R.; Hidayat, T. Integration of methadone maintenance treatment and HIV care for injecting drug users: A cohort study in Bandung, Indonesia. Acta Med. Indones 2009, 41 (Suppl. 1), 23–27. [Google Scholar] [PubMed]
- Tran, B.X.; Ohinmaa, A.; Duong, A.T.; Nguyen, L.T.; Vu, P.X.; Mills, S.; Houston, S.; Jacobs, P. Cost-effectiveness of integrating methadone maintenance and antiretroviral treatment for HIV-positive drug users in Vietnam’s injection-driven HIV epidemics. Drug Alcohol Depend. 2012, 125, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Nosyk, B.; Min, J.E.; Colley, G.; Lima, V.D.; Yip, B.; Milloy, M.J.; Wood, E.; Montaner, J.S. The causal effect of opioid substitution treatment on HAART medication refill adherence. Aids 2015, 29, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, K.M.; Bia, J.; Altice, F.L.; Feinberg, J. Integrated Models of Care for Individuals with Opioid Use Disorder: How Do We Prevent HIV and HCV? Curr. HIV/AIDS Rep. 2018, 15, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, B.J.; Munoz, N.; McGovern, M.P.; Funaro, M.; Villanueva, M.; Tetrault, J.M.; Edelman, E.J. Integration of care for HIV and opioid use disorder: A systematic review of interventions in clinical and community-based settings. Aids 2018. [Google Scholar] [CrossRef] [PubMed]
- Onken, L. PRECEDE-PROCEED and the NIDA stage model: The value of a conceptual framework for intervention research. J. Public Health Dent. 2011, 71 (Suppl. 1), S18–S19. [Google Scholar] [CrossRef] [PubMed]
- Crosby, R.; Noar, S.M. What is a planning model? An introduction to PRECEDE-PROCEED. J. Public Health Dent. 2011, 71, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.D.; Hersey, J.C.; Iverson, D.C. Health behavior models compared. Soc. Sci. Med. 1987, 24, 973–981. [Google Scholar] [CrossRef]
- Cooke, A.; Saleem, H.; Mushi, D.; Mbwambo, J.; Hassan, S.; Lambdin, B.H. Convenience without disclosure: A formative research study of a proposed integrated methadone and antiretroviral therapy service delivery model in Dar es Salaam, Tanzania. Addict. Sci Clin. Pract. 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.T.; Mushi, D.; Hassan, S.; Bruce, R.D.; Cooke, A.; Mbwambo, J.; Lambdin, B.H. “Can’t you initiate me here?”: Challenges to timely initiation on antiretroviral therapy among methadone clients in Dar es Salaam, Tanzania. Int. J. Drug Policy 2016, 30, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National AIDS Control Programme, Ministry of Health and Social Welfare. Tanzania National Guidelines for the Management of HIV and AIDS, 4th ed.; Ministry of Health and Social Welfare (Tanzania): Dar Es Salaam, Tanzania, 2012.
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, R.E.; Lichtenstein, E.; Marcus, A.C. Why Don’t We See More Translation of Health Promotion Research to Practice? Rethinking the Efficacy-to-Effectiveness Transition. Am. J. Public Health 2003, 93, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Finocchario-Kessler, S.; Odera, I.; Okoth, V.; Bawcom, C.; Gautney, B.; Khamadi, S.; Clark, K.; Goggin, K. Lessons learned from implementing the HIV Infant Tracking System (HITSystem): A web-based intervention to improve early infant diagnosis in Kenya. Healthcare 2015, 3, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Noar, S.M. Computer technology-based interventions in HIV prevention: State of the evidence and future directions for research. AIDS Care 2011, 23, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Graffy, J.; Goodhart, C.; Sennett, K.; Kamusiime, G.; Tukamushaba, H. Young people’s perspectives on the adoption of preventive measures for HIV/AIDS, malaria and family planning in South-West Uganda: Focus group study. BMC Public Health 2012, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Hassan, S.; Saleem, H.; Mushi, D.; Mbwambo, J.; Lambdin, B. Integration of Care: Patient and Provider Perspectives on Integration Effectiveness. Addict. Sci Clin. Pract. 2019. under review. [Google Scholar]
- National AIDS Control Programme, Ministry of Health and Social Welfare. Tanzanian National Guidelines for the Management of HIV and AIDS, 5th ed.; Ministry of Health and Social Welfare (Tanzania): Dar Es Salaam, Tanzania, 2015.
- Kinahan, J.C.; Surah, S.; Keating, S.; Bergin, C.; Mulcahy, F.; Lyons, F.; Keenan, E. Effect of integrating HIV and addiction care for non-engaging HIV-infected opiate-dependent patients. Irish J. Med. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nosyk, B.; Krebs, E.; Min, J.E.; Ahamad, K.; Buxton, J.; Goldsmith, C.; Hull, M.; Joe, R.; Krajden, M.; Lima, V.D.; et al. The ‘Expanded HIV care in opioid substitution treatment’ (EHOST) cluster-randomized, stepped-wedge trial: A study protocol. Contemp. Clin. Trials 2015, 45, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sylla, L.; Bruce, R.D.; Kamarulzaman, A.; Altice, F.L. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int. J. Drug Policy 2007, 18, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, R.D.; Lambdin, B.; Chang, O.; Masao, F.; Mbwambo, J.; Mteza, I.; Nyandindi, C.; Zamudio-Haas, S.; Buma, D.; Dunbar, M.S.; et al. Lessons from Tanzania on the integration of HIV and tuberculosis treatments into methadone assisted treatment. Int. J. Drug Policy 2014, 25, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Suthar, A.B.; Rutherford, G.W.; Horvath, T.; Doherty, M.C.; Negussie, E.K. Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS 2014, 28 (Suppl. 2), S175–185. [Google Scholar] [CrossRef] [PubMed]
- Guise, A.; Seguin, M.; Mburu, G.; McLean, S.; Grenfell, P.; Islam, Z.; Filippovych, S.; Assan, H.; Low, A.; Vickerman, P.; et al. Integrated opioid substitution therapy and HIV care: A qualitative systematic review and synthesis of client and provider experiences. AIDS Care 2017, 29, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
| IMAT Strategy Components | Description | |
|---|---|---|
| PREDISPOSING FACTORS | Opioid treatment program (OTP) provider education |
|
| OTP client sensitization |
| |
| ENABLING FACTORS | OTP provider familiarization with IMAT Standard Operating Procedures (SOP) |
|
| OTP clinic space and facilities physical adjustments |
| |
| Alere® point-of-care CD4 machine |
| |
| Scheduling flexibility |
| |
| ART dispensing modalities |
| |
| REINFORCING FACTORS | Laboratory information management system (LIMS) |
|
| Reach | |
| |
| Effectiveness | |
| |
| Adoption | |
| |
| Implementation | |
| |
| Maintenance | Not assessed; future work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.; Cooke, A.; Saleem, H.; Mushi, D.; Mbwambo, J.; Lambdin, B.H. Evaluating the Integrated Methadone and Anti-Retroviral Therapy Strategy in Tanzania Using the RE-AIM Framework. Int. J. Environ. Res. Public Health 2019, 16, 728. https://doi.org/10.3390/ijerph16050728
Hassan S, Cooke A, Saleem H, Mushi D, Mbwambo J, Lambdin BH. Evaluating the Integrated Methadone and Anti-Retroviral Therapy Strategy in Tanzania Using the RE-AIM Framework. International Journal of Environmental Research and Public Health. 2019; 16(5):728. https://doi.org/10.3390/ijerph16050728
Chicago/Turabian StyleHassan, Saria, Alexis Cooke, Haneefa Saleem, Dorothy Mushi, Jessie Mbwambo, and Barrot H. Lambdin. 2019. "Evaluating the Integrated Methadone and Anti-Retroviral Therapy Strategy in Tanzania Using the RE-AIM Framework" International Journal of Environmental Research and Public Health 16, no. 5: 728. https://doi.org/10.3390/ijerph16050728
APA StyleHassan, S., Cooke, A., Saleem, H., Mushi, D., Mbwambo, J., & Lambdin, B. H. (2019). Evaluating the Integrated Methadone and Anti-Retroviral Therapy Strategy in Tanzania Using the RE-AIM Framework. International Journal of Environmental Research and Public Health, 16(5), 728. https://doi.org/10.3390/ijerph16050728





