Association between Maternal Smoking during Pregnancy and Missing Teeth in Adolescents
Abstract
1. Introduction
2. Materials and Methods
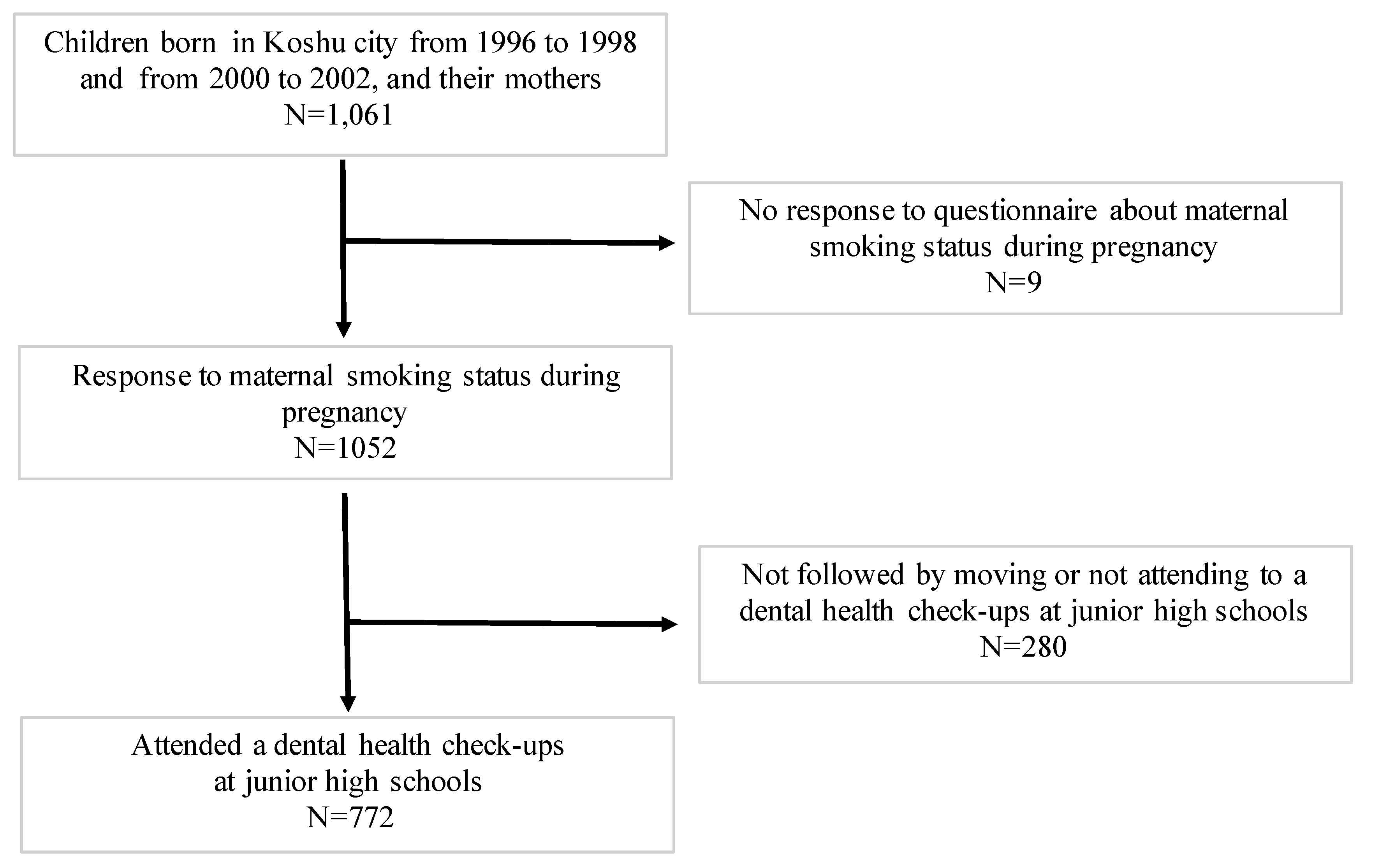
2.1. Participants and Study Design
2.2. Smoking during Pregnancy
2.3. Assessment of Missing Teeth
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Paula Junior, D.F.; Santos, N.C.; da Silva, E.T.; Nunes, M.F.; Leles, C.R. Psychosocial impact of dental esthetics on quality of life in adolescents. Angle Orthod. 2009, 79, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Higashihori, N.; Takada, J.I.; Katayanagi, M.; Takahashi, Y.; Moriyama, K. Frequency of missing teeth and reduction of mesiodistal tooth width in Japanese patients with tooth agenesis. Prog. Orthod. 2018, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Hobkirk, J.A.; Goodman, J.R.; Jones, S.P. Presenting complaints and findings in a group of patients attending a hypodontia clinic. Br. Dent. J. 1994, 177, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Silva Rodrigues, A.; Santos Freire, J.; Inacio Melandes da Silva, G.; Santos Antunes, L.; Azeredo Alves Antunes, L. Does dental agenesis have an impact on OHRQoL of children, adolescents and young adults? A systematic review. Acta Odontol. Scand. 2018, 76, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Gungor, A.Y.; Turkkahraman, H. Effects of severity and location of nonsyndromic hypodontia on craniofacial morphology. Angle Orthod. 2013, 83, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, A.; Lucchese, A.; Darnahal, A.; Kamali, Z.; Perillo, L. Cleft sidedness and congenitally missing teeth in patients with cleft lip and palate patients. Prog. Orthod. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Komazaki, Y.; Fujiwara, T.; Ogawa, T.; Sato, M.; Suzuki, K.; Yamagata, Z.; Moriyama, K. Association between malocclusion and headache among 12-to 15-year-old adolescents: A population-based study. Community Dent. Oral Epidemiol. 2014, 42, 572–580. [Google Scholar] [CrossRef]
- Basha, S.; Mohamed, R.N.; Swamy, H.S.; Parameshwarappa, P. Untreated gross dental malocclusion in adolescents: psychological impact and effect on academic performance in school. Oral Health Prev. Dent. 2016, 14, 63–69. [Google Scholar] [CrossRef]
- Bonczek, O.; Balcar, V.J.; Sery, O. PAX9 gene mutations and tooth agenesis: A review. Clin. Genet. 2017, 92, 467–476. [Google Scholar] [CrossRef]
- Kaste, S.C.; Hopkins, K.P.; Jenkins, J.J., 3rd. Abnormal odontogenesis in children treated with radiation and chemotherapy: Imaging findings. AJR Am. J. Roentgenol. 1994, 162, 1407–1411. [Google Scholar] [CrossRef]
- Kaste, S.C.; Hopkins, K.P.; Jones, D.; Crom, D.; Greenwald, C.A.; Santana, V.M. Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia 1997, 11, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, M.; Qin, D.; Li, Y.; Cen, X.; Sun, X.; Lian, W.; Liao, B. Establishment of a congenital tooth agenesis related gene MSX1 knockout human embryonic stem cell lines by CRISPR-Cas9 technology. Stem Cell Res. 2017, 24, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Chaushu, S. Etiology of maxillary canine impaction: A review. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, H.M.; Bencharit, S.; Seaman, W.; Frazier-Bowers, S.A. In silico and functional evaluation of PTH1R mutations found in patients with primary failure of eruption (PFE). Orthod. Craniofac. Res. 2017, 20, 57–62. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.; Zadurska, M.; Czochrowska, E. Impacted Teeth: An Interdisciplinary Perspective. Adv. Clin. Exp. Med. 2016, 25, 575–585. [Google Scholar] [CrossRef]
- Peck, S.; Peck, L.; Kataja, M. The palatally displaced canine as a dental anomaly of genetic origin. Angle Orthod. 1994, 64, 249–256. [Google Scholar] [CrossRef]
- Juuri, E.; Balic, A. The Biology Underlying Abnormalities of Tooth Number in Humans. J. Dent. Res. 2017, 96, 1248–1256. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P., Jr.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar]
- Zhang, Y.D.; Chen, Z.; Song, Y.Q.; Liu, C.; Chen, Y.P. Making a tooth: Growth factors, transcription factors, and stem cells. Cell Res. 2005, 15, 301–316. [Google Scholar] [CrossRef]
- Holtta, P.; Hovi, L.; Saarinen-Pihkala, U.M.; Peltola, J.; Alaluusua, S. Disturbed root development of permanent teeth after pediatric stem cell transplantation—Dental root development after SCT. Cancer 2005, 103, 1484–1493. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Goyal, A.; Dubey, M.; Kapur, A.; Ritwik, P. Congenital Rubella Syndrome: Dental Manifestations and Management in a 5 year Old Child. J. Clin. Pediatric Dent. 2012, 37, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Merriman, T.R.; Farella, M. Maternal Smoking during Pregnancy Is Associated with Offspring Hypodontia. J. Dent. Res. 2017, 96, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Zhongpeng, Y.; Yanjun, G.; Jiaqi, D.; Yuchi, Z.; Bing, S.; Chenghao, L. Maternal active smoking and risk of oral clefts: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Watt, R.G. Emerging theories into the social determinants of health: Implications for oral health promotion. Community Dent. Oral Epidemiol. 2002, 30, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zuckerman, B.; Pearson, C.; Kaufman, G.; Chen, C.Z.; Wang, G.Y.; Niu, T.H.; Wise, P.H.; Bauchner, H.; Xu, X.P. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA-J. Am. Med Assoc. 2002, 287, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Buka, S.L.; Shenassa, E.D.; Niaura, R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. Am. J. Psychiatry 2003, 160, 1978–1984. [Google Scholar] [CrossRef]
- Miyake, K.; Kawaguchi, A.; Miura, R.; Kobayashi, S.; Tran, N.Q.V.; Kobayashi, S.; Miyashita, C.; Araki, A.; Kubota, T.; Yamagata, Z.; et al. Association between DNA methylation in cord blood and maternal smoking: The Hokkaido Study on Environment and Children’s Health. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Romitti, P.A.; Lidral, A.C.; Munger, R.G.; Daack-Hirsch, S.; Burns, T.L.; Murray, J.C. Candidate genes for nonsyndromic cleft lip and palate and maternal cigarette smoking and alcohol consumption: Evaluation of genotype-environment interactions from a population-based case-control study of orofacial clefts. Teratology 1999, 59, 39–50. [Google Scholar] [CrossRef]
- Shaw, G.M.; Wasserman, C.R.; Lammer, E.J.; O’Malley, C.D.; Murray, J.C.; Basart, A.M.; Tolarova, M.M. Orofacial clefts, parental cigarette smoking, and transforming growth factor-alpha gene variants. Am. J. Hum. Genet. 1996, 58, 551–561. [Google Scholar]
- Little, J.; Cardy, A.; Munger, R.G. Tobacco smoking and oral clefts: A meta-analysis. Bull. World Health Organ. 2004, 82, 213–218. [Google Scholar]
- Sakai, D.; Dixon, J.; Achilleos, A.; Dixon, M.; Trainor, P.A. Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat. Commun. 2016, 7, 10328. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Haruna, M.; Ota, E.; Murayama, R.; Yamaguchi, T.; Shioji, I.; Sasaki, S.; Yamaguchi, T.; Murashima, S. Effects of lifestyle factors on urinary oxidative stress and serum antioxidant markers in pregnant Japanese women: A cohort study. Biosci. Trends 2014, 8, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Reece, E.A. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Res. B Dev. Reprod. Toxicol. 2005, 74, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Sheldon, E.; Sharma, J.; Canturk, K.M.; Otu, H.H.; Nawshad, A. Nicotine Exposure During Pregnancy Results in Persistent Midline Epithelial Seam With Improper Palatal Fusion. Nicotine Tob. Res. 2016, 18, 604–612. [Google Scholar] [CrossRef]
- Larsen, L.G.; Clausen, H.V.; Jonsson, L. Stereologic examination of placentas from mothers who smoke during pregnancy. Am. J. Obstet. Gynecol. 2002, 186, 531–537. [Google Scholar] [CrossRef]
- Jauniaux, E.; Burton, G.J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 2007, 83, 699–706. [Google Scholar] [CrossRef]
- Ghanem, A.; Abduljabbar, T.; Akram, Z.; Vohra, F.; Kellesarian, S.V.; Javed, F. A systematic review and meta-analysis of pre-clinical studies assessing the effect of nicotine on osseointegration. Int. J. Oral Maxillofac. Surg. 2017, 46, 496–502. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic. Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakao, M.; Shinozaki, Y.; Yano, E. Validity of the self-reported smoking status of schizophrenia patients, taking gender-related differences into consideration. Int. J. Psychiatry Clin. Pract. 2010, 14, 282–286. [Google Scholar] [CrossRef]
- Wisborg, K.; Henriksen, T.B.; Hedegaard, M.; Secher, N.J. Smoking habits among Danish pregnant women from 1989 to 1996 in relation to sociodemographic and lifestyle factors. Acta Obstet. Gynecol. Scand. 1998, 77, 836–840. [Google Scholar] [CrossRef]

| Characteristics | Missing Teeth (−) | Missing Teeth (+) | p for Chi-Square Test | ||
|---|---|---|---|---|---|
| (n = 734: 95.1%) | (n = 38: 4.9%) | ||||
| n | % | n | % | ||
| Sex | |||||
| Male | 400 | 54.5 | 19 | 50.0 | 0.59 |
| Female | 334 | 45.5 | 19 | 50.0 | |
| Grade | |||||
| 1 | 228 | 31.1 | 8 | 21.1 | 0.42 |
| 2 | 265 | 36.1 | 16 | 42.1 | |
| 3 | 241 | 32.8 | 14 | 36.8 | |
| Time of registration of pregnancy | |||||
| Early (<16 gestational weeks) | 623 | 94.3 | 30 | 88.2 | 0.15 |
| Late (16 gestational weeks+) | 38 | 5.7 | 4 | 11.8 | |
| Birth Weight | |||||
| Normal (2500 g+) | 673 | 93.1 | 33 | 91.7 | 0.75 |
| Low (<2500 g) | 50 | 6.9 | 3 | 8.3 | |
| Gestational age | |||||
| Full term (37 weeks+) | 689 | 95.4 | 33 | 91.7 | 0.30 |
| Preterm (<37 weeks) | 33 | 4.6 | 3 | 8.3 | |
| Delivery | |||||
| Normal | 539 | 80 | 25 | 73.5 | 0.19 |
| Caesarean | 111 | 16.5 | 9 | 26.5 | |
| Suction | 24 | 3.5 | 0 | 0.0 | |
| Maternal age at delivery | |||||
| <20 years | 6 | 0.8 | 1 | 2.6 | 0.57 |
| 21–30 years | 362 | 49.7 | 19 | 50.0 | |
| 31–40 years | 346 | 47.6 | 18 | 47.4 | |
| 40+ years | 14 | 1.9 | 0 | 0.0 | |
| Duration of Exclusive breastfeeding | |||||
| Never | 45 | 6.3 | 2 | 5.7 | 0.42 |
| <6.0 months | 547 | 76.8 | 30 | 85.7 | |
| 6.0 months+ | 120 | 16.9 | 3 | 8.6 | |
| Maternal Education | |||||
| JHS or HS | 288 | 44.7 | 16 | 47.1 | 0.42 |
| Some college | 268 | 41.6 | 16 | 47.1 | |
| College or more | 88 | 13.7 | 2 | 5.8 | |
| Paternal Education | |||||
| JHS or HS | 312 | 48.6 | 21 | 61.8 | 0.32 |
| Some college | 93 | 14.5 | 4 | 11.8 | |
| College or more | 237 | 36.9 | 9 | 26.4 | |
| Variables | Number of Non-Missing Teeth | Number of Missing Teeth | Crude | Adjusted | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Maternal smoking status | ||||||
| Never or stopped smokers before pregnancy | 694 | 32 | Ref | Ref | ||
| Sustained smoking during pregnancy (1–5 per day) | 15 | 2 | 2.89 | 0.63–13.19 | 2.80 | 0.52–15.06 |
| (6+ per day) | 25 | 4 | 3.47 | 1.14–10.56 | 4.59 | 1.07–19.67 |
| p-value | 0.014 | 0.024 | ||||
| Sex | ||||||
| Male | 400 | 19 | Ref | |||
| Female | 334 | 19 | 1.92 | 0.89–4.14 | ||
| Gestational age | ||||||
| Full term (37 weeks+) | 689 | 33 | Ref | |||
| Preterm (<37 weeks) | 33 | 3 | 3.82 | 0.96–15.15 | ||
| Maternal BMI before pregnancy | ||||||
| Normal weight (<25.0 kg/m2) | 624 | 29 | Ref | |||
| Overweight (25.0–30.0 kg/m2) | 52 | 4 | 1.84 | 0.58–5.80 | ||
| Obesity (30.0 kg/m2+) | 19 | 1 | 0.74 | 0.07–8.01 | ||
| Maternal age at delivery | ||||||
| <20 years | 6 | 1 | 1.87 | 0.16–21.45 | ||
| 21–30 years | 362 | 19 | Ref | |||
| 31–40 years | 346 | 18 | 1.10 | 0.52–2.33 | ||
| 40+ years | 14 | 0 | NA | |||
| Maternal Education | ||||||
| JHS or HS | 288 | 16 | Ref | |||
| Some college | 268 | 16 | 1.19 | 0.54–2.61 | ||
| College or more | 88 | 2 | 0.48 | 0.10–2.25 | ||
| Maternal alcohol consumption | ||||||
| (−) | 639 | 37 | Ref | |||
| (+) | 87 | 1 | NA | |||
| Paternal smoking status during maternal pregnancy | ||||||
| (−) | 187 | 6 | Ref | |||
| (+) | 46 | 2 | 1.57 | 0.64–3.84 | ||
| Maternal breakfast consumption habits | ||||||
| Every day | 569 | 29 | Ref | |||
| 3–5 times a week | 51 | 1 | 0.36 | 0.04–2.95 | ||
| 1–2 times a week | 49 | 5 | 1.54 | 0.49–4.79 | ||
| None | 63 | 3 | 0.61 | 0.13–2.85 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa Kang, J.; Unnai Yasuda, Y.; Ogawa, T.; Sato, M.; Yamagata, Z.; Fujiwara, T.; Moriyama, K. Association between Maternal Smoking during Pregnancy and Missing Teeth in Adolescents. Int. J. Environ. Res. Public Health 2019, 16, 4536. https://doi.org/10.3390/ijerph16224536
Nakagawa Kang J, Unnai Yasuda Y, Ogawa T, Sato M, Yamagata Z, Fujiwara T, Moriyama K. Association between Maternal Smoking during Pregnancy and Missing Teeth in Adolescents. International Journal of Environmental Research and Public Health. 2019; 16(22):4536. https://doi.org/10.3390/ijerph16224536
Chicago/Turabian StyleNakagawa Kang, Junka, Yuko Unnai Yasuda, Takuya Ogawa, Miri Sato, Zentaro Yamagata, Takeo Fujiwara, and Keiji Moriyama. 2019. "Association between Maternal Smoking during Pregnancy and Missing Teeth in Adolescents" International Journal of Environmental Research and Public Health 16, no. 22: 4536. https://doi.org/10.3390/ijerph16224536
APA StyleNakagawa Kang, J., Unnai Yasuda, Y., Ogawa, T., Sato, M., Yamagata, Z., Fujiwara, T., & Moriyama, K. (2019). Association between Maternal Smoking during Pregnancy and Missing Teeth in Adolescents. International Journal of Environmental Research and Public Health, 16(22), 4536. https://doi.org/10.3390/ijerph16224536





