Fatigue-Related Factors for Community-Dwelling Older Adults with Diabetes: A Theory-Guided Multi-Dimensional Approach Using the Dynamic Biopsychosocial Model
Abstract
1. Introduction
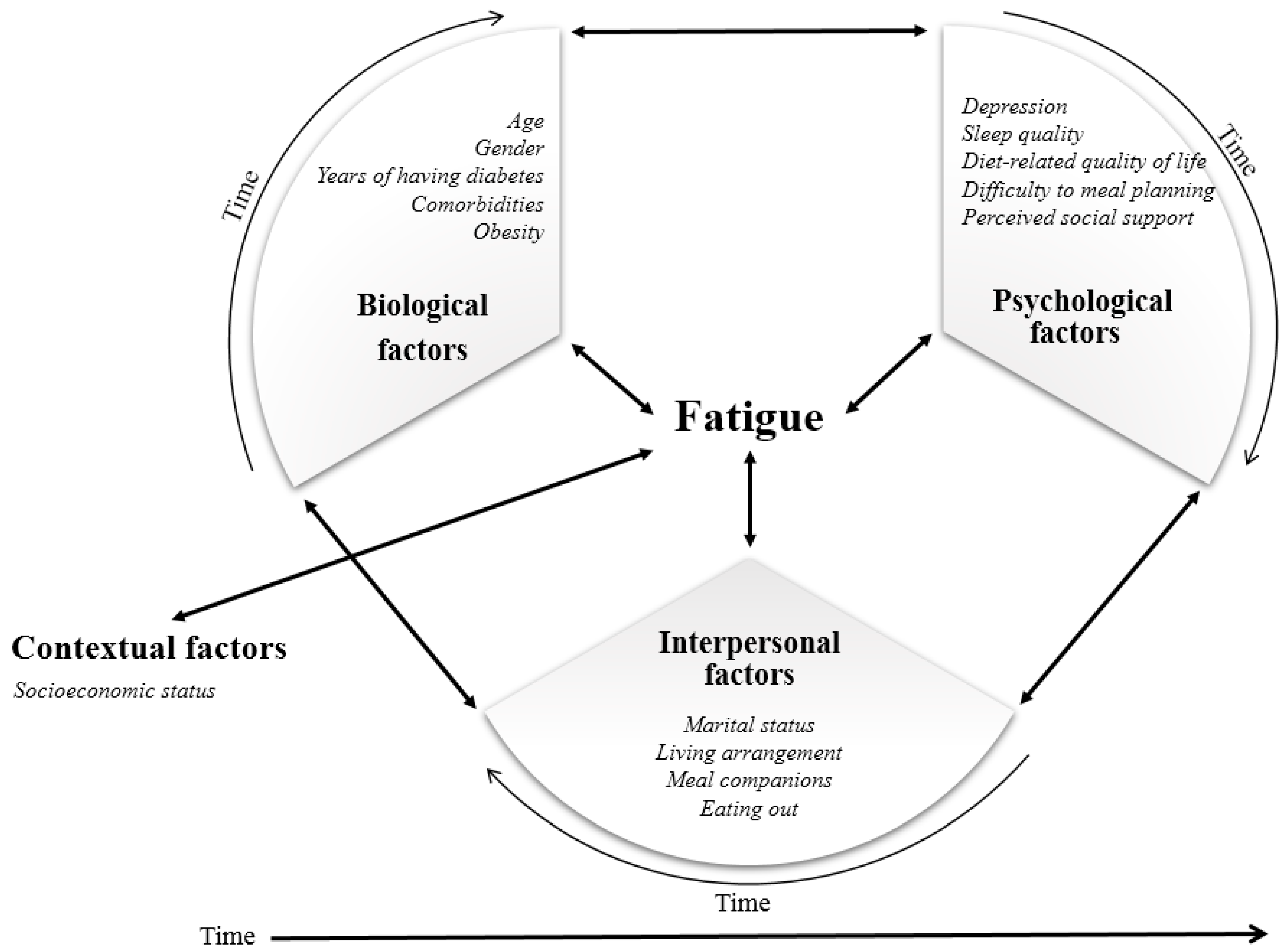
Theoretical Framework
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Instruments
2.3.1. Fatigue
2.3.2. Depression
2.3.3. Sleep Quality
2.3.4. Diabetes Diet-Related Quality of Life
2.3.5. Perceived Social Support
2.4. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| DBPS Domain | Potential Predictor | Fatigue | |||
|---|---|---|---|---|---|
| n (%) | M (SD) | F(p) | |||
| Biological | Age | <75-years-old | 45 (35.4) | 3.73 (1.79) | 0.634 (0.428) |
| ≥75-years-old | 82 (64.6) | 4.00 (1.81) | |||
| Gender | Men | 50 (39.4) | 3.88 (1.57) | 0.006 (0.937) | |
| Women | 77 (60.6) | 3.91 (1.94) | |||
| Years with diabetes | <10 years | 58 (45.7) | 3.82 (1.77) | 0.192 (0.662) | |
| ≥10 years | 69 (54.3) | 3.97 (1.84) | |||
| Comorbidities | <2 | 44 (34.6) | 3.98 (1.83) | 0.421 (0.518) | |
| ≥2 | 83 (65.4) | 3.76 (1.75) | |||
| Body mass index (kg/m2) | Normal (<23.0) | 46 (36.2) | 3.83 (1.88) | 0.012 (0.988) | |
| Overweight (23.0–24.9) | 34 (26.8) | 3.89 (1.83) | |||
| Obesity (≥25.0) | 44 (34.6) | 3.88 (1.73) | |||
| Psychological | Depression | Non-Depressed | 63 (49.6) | 3.30 (1.68) | 12.475 (<0.001) |
| Moderate Depression | 45 (35.4) | 4.11 (1.76) | |||
| Severe Depression | 19 (15.0) | 5.41 (1.24) | |||
| Sleep quality | Good | 37 (29.1) | 3.09 (1.53) | 11.547 (0.001) | |
| Poor | 90 (70.0) | 4.24 (1.80) | |||
| Difficulty with meal planning | Not at all difficult | 48 (37.8) | 3.45 (1.83) | 4.548 (0.012) | |
| Not so difficult/ Somewhat | 39 (30.7) | 3.78 (1.59) | |||
| Very difficult/ Extremely difficult | 40 (31.5) | 4.56 (1.80) | |||
| Satisfaction with diet | <median | 68 (53.5) | 4.32 (1.74) | 8.509 (0.004) | |
| ≥median | 59 (46.5) | 3.41 (1.75) | |||
| Burden of diet therapy | <median | 65 (51.2) | 4.00 (1.74) | 0.407 (0.525) | |
| ≥median | 62 (48.8) | 3.80 (1.87) | |||
| Perceived merits of diet therapy | <median | 70 (55.1) | 4.03 (1.81) | 0.752 (0.387) | |
| ≥median | 57 (44.9) | 3.75 (1.79) | |||
| Perceived social support | <median | 60 (47.2) | 4.12 (1.80) | 1.739 (0.190) | |
| ≥median | 67 (52.8) | 3.70 (1.79) | |||
| Interpersonal | Marital status | Widowed/divorced/ separated | 58 (45.7) | 3.97 (1.84) | 0.165 (0.685) |
| Married/partnered | 69 (54.3) | 3.84 (1.78) | |||
| Living arrangement | Living alone | 41 (32.3) | 4.14 (1.88) | 1.108 (0.295) | |
| Living with others | 86 (67.7) | 3.78 (1.76) | |||
| Exclusive eating alone * | No | 92 (72.4) | 3.70 (1.73) | 4.431 (0.037) | |
| Yes | 35 (27.6) | 4.44 (1.89) | |||
| Eating out | Less than 2 times a week | 93 (73.2) | 4.11 (1.81) | 4.972 (0.028) | |
| 3 times or more a week | 34 (26.8) | 3.32 (1.66) | |||
| Contextual | Education | <High School | 64 (50.4) | 4.01 (2.15) | 0.512 (0.476) |
| ≥High School | 63 (49.6) | 3.78 (1.52) | |||
| Household income | ≤1,000,000 Korean Won | 73 (57.5) | 4.21 (1.81) | 5.365 (0.022) | |
| >1,000,000 Korean Won | 54 (42.5) | 3.48 (1.72) |
References
- Statistics Korea. Population Projections for Korea. Available online: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1BPA003&vw_cd=MT_ETITLE&list_id=&scrId=&seqNo=&language=en&obj_var_id=&itm_id=&conn_path=A6&path=%252Feng%252F (accessed on 21 May 2019).
- Shin, E.C. Educational Strategies for Insulin Injection Therapy in Elderly Diabetic Patients. J. Korean Diabetes 2018, 19, 101–105. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; ISBN 978-930229-87-4. [Google Scholar]
- Korean Diabetes Association. Diabetes Fact Sheet in Korea 2018. Available online: http://www.diabetes.or.kr/pro/news/admin.php?category=A&code=admin&number=1546&mode=view (accessed on 2 Jun 2019).
- Abdelhafiz, A.H.; Sinclair, A.J. Management of type 2 diabetes in older people. Diabetes Ther. 2013, 4, 13–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregg, E.W.; Li, Y.; Wang, J.; Rios Burrows, N.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.S. Management of diabetes mellitus in older people with comorbidities. BMJ 2016, 353, i2200. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Cattan, M.; White, M.; Bond, J.; Learmouth, A. Preventing social isolation and loneliness among older people: A systematic review of health promotion interventions. Ageing Soc. 2005, 25, 41–67. [Google Scholar] [CrossRef]
- Song, Y.; Song, H.-J.; Han, H.-R.; Park, S.-Y.; Nam, S.; Kim, M.T. Unmet needs for social support and effects on diabetes self-care activities in Korean Americans with type 2 diabetes. Diabetes Educ. 2012, 38, 77–85. [Google Scholar] [CrossRef]
- Eldadah, B.A. Fatigue and fatigability in older adults. PM&R 2010, 2, 406–413. [Google Scholar] [CrossRef]
- Richardson, C.A.; Glynn, N.W.; Ferrucci, L.G.; Mackey, D.C. Walking Energetics, Fatigability, and Fatigue in Older Adults: The Study of Energy and Aging Pilot. J. Gerontol. A-Biol. 2015, 70, 487–494. [Google Scholar] [CrossRef]
- Moreh, E.; Jacobs, J.M.; Stessman, J. Fatigue, function, and mortality in older adults. J. Gerontol. A-Biol. 2010, 65, 887–895. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, R.; Choudhary, P.K.; Yadav, N.; Jain, G.; Maanju, M. Study of fatigue, depression, and associated factors in type 2 diabetes mellitus in industrial workers. Ind. Psychiatry J. 2015, 24, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jensen, Ø.; Bernklev, T.; Jelsness-Jørgensen, L.P. Fatigue in type 1 diabetes: A systematic review of observational studies. Diabetes Res. Clin. Pract. 2017, 123, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Sahay, R. Diabetes Fatigue Syndrome. Diabetes Ther. 2018, 9, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Griggs, S.; Morris, N.S. Fatigue among adults with type 1 diabetes mellitus and implications for self-management: An integrative review. Diabetes Educ. 2018, 44, 325–339. [Google Scholar] [CrossRef]
- Singh, R.; Teel, C.; Sabus, C.; McGinnis, P.; Kluding, P. Fatigue in Type 2 Diabetes: Impact on Quality of Life and Predictors. PLoS ONE 2016, 11, e0165652.1–e0165652.13. [Google Scholar] [CrossRef]
- Tomas-Carus, P.; Ortega-Alonso, A.; Pietilainen, K.H.; Santos, V.; Goncalves, H.; Ramos, J.; Raimundo, A. A randomized controlled trial on the effects of combined aerobic-resistance exercise on muscle strength and fatigue, glycemic control and health-related quality of life of type 2 diabetes patients. J. Sports Med. Phys. Fit. 2016, 56, 572–578. [Google Scholar]
- Lehman, B.J.; David, D.M.; Gruber, J.A. Rethinking the biopsychosocial model of health: Understanding health as a dynamic system. Soc. Personal. Psychol. Compass 2017, 11, e12328.1–e12328.17. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Ecology of the family as a context for human development: Research perspectives. Dev. Psychol. 1986, 22, 723–742. [Google Scholar] [CrossRef]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef]
- Van Seben, R.; Reichardt, L.A.; Aarden, J.J.; van der Schaaf, M.; van der Esch, M.; Engelbert, R.H.H.; Twisk, J.W.R.; Bosch, J.A.; Buurman, B.M. Hospital-ADL study Group. The Course of Geriatric Syndromes in Acutely Hospitalized Older Adults: The Hospital-ADL Study. J. Am. Med. Dir. Assoc. 2019, 20, 152–158. [Google Scholar] [CrossRef]
- Soyuer, T. Fatigue and physical performance in the elderly aged 65 and over living in a nursing home. Int. J. Fam. Commun. Med. 2018, 2, 371–373. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.I.; Song, C.H. Clinical usefulness of fatigue severity scale for patients with fatigue, and anxiety or depression. Korean J. Psychosom. Med. 2001, 9, 164–173. [Google Scholar]
- Singh, R.; Kluding, P.M. Fatigue and related factors in people with type 2 diabetes. Diabetes Educ. 2013, 39, 320–326. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Jang, Y.; Small, B.J.; Haley, W.E. Cross-cultural comparability of the Geriatric Depression Scale: Comparison between older Koreans and older Americans. Aging Ment. Health 2001, 5, 31–37. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Bird, V.; Rizzo, M.; Meader, N. Diagnostic validity and added value of the Geriatric Depression Scale for depression in primary care: A meta-analysis of GDS30 and GDS15. J. Affect. Disord. 2010, 125, 10–17. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Sato, E.; Ochiai, R.; Shibayama, T.; Nishigaki, M.; Abe, Y.; Sawa, T.; Suzukamo, Y.; Kazuma, K. Reliability and validity of revised and short form versions of diabetes diet-related quality of life scale. Diabetol. Int. 2017, 8, 181–192. [Google Scholar] [CrossRef]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, G.K. The multidimensional scale of perceived social support. J. Pers. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Alavi, M.; Molavi, R.; Eslami, P. A structural equation model of self-care activities in diabetic elderly patients. Iran. J. Nurs. Midwifery Res. 2018, 23, 61–65. [Google Scholar] [CrossRef] [PubMed]
- De Maria, M.; Vellone, E.; Durante, A.; Biagioli, V.; Matarese, M. Psychometric evaluation of the Multidimensional Scale of Perceived Social Support (MSPSS) in people with chronic diseases. Ann. Ist. Super. Sanita 2018, 54, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, Q.; Huang, Z.; Huang, L.; Kong, F. Psychometric Properties of multi-dimensional scale of perceived social support in Chinese parents of children with cerebral palsy. Front. Psychol. 2017, 8, 2020.1–2020.6. [Google Scholar] [CrossRef]
- Lin, W.-Q.; Jing, M.-J.; Tang, J.; Wang, J.-J.; Zhang, H.-S.; Yuan, L.-X.; Wang, P.-X. Factors Associated with Fatigue among Men Aged 45 and Older: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2015, 12, 10897–10909. [Google Scholar] [CrossRef]
- Seo, Y.-M.; Hahm, J.-R.; Kim, T.-K.; Choi, W.-H. Factors Affecting Fatigue in Patients with Type II Diabetes Mellitus in Korea. Asian Nurs. Res. 2015, 9, 60–64. [Google Scholar] [CrossRef]
- Burke, S.E.; Samuel, I.B.H.; Zhao, Q.; Cagle, J.; Cohen, R.A.; Kluger, B.; Ding, M. Task-based cognitive fatigability for older adults and validation of mental fatigability subscore of Pittsburgh Fatigability Scale. Front. Aging Neurosci. 2018, 10, 327.1–327.7. [Google Scholar] [CrossRef]
- Aylaz, R.; Karadağ, E.; Işik, K.; Yildirim, M. Relationship between social support and fatigue in patients with type 2 diabetes mellitus in the east of turkey. Jpn. J. Nurs. Sci. 2015, 12, 367–376. [Google Scholar] [CrossRef]
- Galland-Decker, C.; Marques-Vidal, P.; Vollenweider, P. Prevalence and factors associated with fatigue in the Lausanne middle-aged population: A population-based, cross-sectional survey. BMJ Open 2015, 9, e027070.1–e027070.10. [Google Scholar] [CrossRef]
- Murphy, S.L.; Alexander, N.B.; Levoska, M.; Smith, D.M. Relationship between fatigue and subsequent physical activity among older adults with symptomatic osteoarthritis. Arthritis Care Res. 2013, 65, 1617–1624. [Google Scholar] [CrossRef]
- Egerton, T.; Chastin, S.F.; Stensvold, D.; Helbostad, J.L. Fatigue may contribute to reduced physical activity among older people: An observational study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, S.; Nayfield, S.G.; Patel, K.V.; Eldadah, B.; Cesari, M.; Ferrucci, L.; Ceresini, G.; Guralnik, J.M. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J. Gerontol. A-Biol. 2009, 64, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Choi-Kwon, S. Characteristics and factors related to fatigue in older adults at a senior welfare center. Korean J. Adult Nurs. 2018, 30, 516–526. [Google Scholar] [CrossRef]
- Amtmann, D.; Kim, J.; Chung, H.; Bamer, A.M.; Askew, R.L.; Wu, S.; Cook, K.F.; Johnson, K.L. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil. Psychol. 2014, 59, 220–229. [Google Scholar] [CrossRef]
- Huibers, M.J.; Leone, S.S.; van Amelsvoort, L.G.; Kant, I.; Knottnerus, J.A. Associations of fatigue and depression among fatigued employees over time: A 4-year follow-up study. J. Psychosom. Res. 2007, 63, 137–142. [Google Scholar] [CrossRef]
- Patrick, S.; Connick, P. Psychometric properties of the PHQ-9 depression scale in people with multiple sclerosis: A systematic review. PLoS ONE 2019, 14, e0197943.1–e0197943.12. [Google Scholar] [CrossRef]
- Park, M.; Reynolds, C.F., III. Depression among older adults with diabetes mellitus. Clin. Geriatr. Med. 2015, 31, 117–137. [Google Scholar] [CrossRef]
- Cuellar, N.G.; Ratcliffe, S.J. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J. Clin. Sleep Med. 2008, 4, 50–56. [Google Scholar]
- Dean, M.; Raats, M.M.; Grunert, K.G.; Lumbers, M. Factors influencing eating a varied diet in old age. Public Health Nutr. 2009, 12, 2421–2427. [Google Scholar] [CrossRef]
- Lane, K.; Poland, F.; Fleming, S.; Lambert, N.; MacDonald, H.; Potter, J.; Raats, M.; Skidmore, P.; Vince, C.; Wellings, A.; et al. Older women’s reduced contact with food in the Changes Around Food Experience (CAFE) study: Choices, adaptations and dynamism. Ageing Soc. 2014, 34, 645–669. [Google Scholar] [CrossRef]
- Cho, J.H.J.; Olmstead, R.; Choi, H.; Carrillo, C.; Seeman, T.E.; Irwin, M.R. Associations of objective versus subjective social isolation with sleep disturbance, depression, and fatigue in community-dwelling older adults. Aging Ment. Health 2019, 23, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, L.; Qian, J.; Peng, J. Social support moderates stress effects on depression. Int. J. Ment. Health Syst. 2014, 8, 41.1–41.5. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.D.; Seery, E.; Kent, J.A. Physical activity, sleep quality, and self-reported fatigue across the adult lifespan. Exp. Gerontol. 2016, 77, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Meléndez, M.Á.; Jimenez-Correa, U.; Gallegos-Cari, A.; Ayala-Guerrero, F.; Jiménez-Anguiano, A. Prevalence of sleep disorders, daytime sleepiness and clinical symptomatology in older adults. Rev. Med. Hosp. Gen. 2016, 79, 136–143. [Google Scholar] [CrossRef]
- Chattu, V.K.; Chattu, S.K.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. Mellitus. In Healthcare; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2019; Volume 7, p. 37. [Google Scholar]
- Surani, S.; Brito, V.; Surani, A.; Ghamande, S. Effect of diabetes mellitus on sleep quality. World J. Diabetes 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Thichumpa, W.; Howteerakul, N.; Suwannapong, N.; Tantrakul, V. Sleep quality and associated factors among the elderly living in rural Chiang Rai, northern Thailand. Epidemiol. Health 2018, 40, 40. [Google Scholar] [CrossRef]
- Budhiraja, R.; Roth, T.; Hudgel, D.W.; Budhiraja, P.; Drake, C.L. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep (Basel) 2011, 34, 859–867. [Google Scholar] [CrossRef]
- Bani-issa, W.; Al-Shujairi, A.M.; Patrick, L. Association between quality of sleep and health-related quality of life in persons with diabetes mellitus type 2. J. Clin. Nurs. 2018, 27, 1653–1661. [Google Scholar] [CrossRef]
- Chasens, E.R.; Luyster, F.S. Effect of sleep disturbances on quality of life, diabetes self-care behavior, and patient-reported outcomes. Diabetes Spectr. 2016, 29, 20–23. [Google Scholar] [CrossRef]
- Archundia Herrera, M.C.A.; Subhan, F.B.; Chan, C.B. Dietary patterns and cardiovascular disease risk in people with type 2 diabetes. Curr. Obes. Rep. 2017, 6, 405–413. [Google Scholar] [CrossRef]
- García-Mayor, R.V.; García-Soidán, F.J. Eating disorders in type 2 diabetic people: Brief review. Diabetes Metab. Syndr. 2017, 11, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Ruggiero, L.; Prohaska, T.R.; Chavez, N.; Boughton, S.W.; Peacock, N.; Zhao, W.; Nouwen, A. A cross-sectional study of depressive symptoms and diabetes self-care in African Americans and Hispanics/Latinos with diabetes: The role of self-efficacy. Diabetes Educ. 2016, 42, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Martyn-Nemeth, P.; Ruggiero, L.; Park, C.G.; Zhang, Y.; Fritschi, C. Associations between fatigue, sleep disturbance and eating style in adults with type 2 diabetes: A correlational study. J. Clin. Nurs. 2019, 28, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Stanley, K. Nutrition Considerations for the Growing Population of Older Adults with Diabetes. Diabetes Spectr. 2014, 27, 29–36. [Google Scholar] [CrossRef]
- Lee, K.H.; Mo, J. The Factors Influencing Meal Satisfaction in Older Adults: A Systematic Review and Meta-analysis. Asian Nurs. Res. 2019, 13, 169–176. [Google Scholar] [CrossRef]
- Best, R.L.; Appleton, K.M. The consumption of protein-rich foods in older adults: An exploratory focus group study. J. Nutr. Educ. Behav. 2013, 45, 751–755. [Google Scholar] [CrossRef]
- Edfors, E.; Westergren, A. Home-living elderly people’s views on food and meals. J. Aging Res. 2012, 2012, 761291.1–761291.9. [Google Scholar] [CrossRef]
- Quandt, S.A.; Chen, H.; Bell, R.A.; Savoca, M.R.; Anderson, A.M.; Leng, X.; Kohrman, T.; Gilbert, G.H.; Arcury, T.A. Food avoidance and food modification practices of older rural adults: Association with oral health status and implications for service provision. Gerontologist 2010, 50, 100–111. [Google Scholar] [CrossRef]
- Araki, A.; Ito, H. Development of elderly diabetes burden scale for elderly patients with diabetes mellitus. Geriatr. Gerontol. Int. 2003, 3, 212–224. [Google Scholar] [CrossRef]
- Kaya, U.P.; Caydam, O.D. Association between social support and diabetes burden among elderly patients with diabetes: A cross-sectional study from Turkey. Saudi. J. Med. Med. Sci. 2019, 7, 86–92. [Google Scholar] [CrossRef]
- Bradley, C.; Speight, J. Patient perceptions of diabetes and diabetes therapy: Assessing quality of life. Diabetes Metab. Res. Rev. 2002, 18, S64–S69. [Google Scholar] [CrossRef] [PubMed]
- Vesnaver, E.; Keller, H.H.; Payette, H.; Shatenstein, B. Dietary resilience as described by older community-dwelling adults from the NuAge study “If there is a will–there is a way!”. Appetite 2012, 58, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; MacLeod, J.; Evert, A.; Brown, C.; Gradwell, E.; Handu, D.; Reppert, A.; Robinson, M. Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: Systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J. Acad. Nutr. Diet. 2017, 117, 1659–1679. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.K.; Kristeller, J.L.; Headings, A.; Nagaraja, H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A randomized controlled trial. Health Educ. Behav. 2014, 41, 145–154. [Google Scholar] [CrossRef]
- Weijman, I.; Kant, I.; Swaen, G.M.; Ros, W.J.; Rutten, G.E.; Schaufeli, W.B.; Schabracq, M.J.; Winnubst, J.A.M. Diabetes, employment and fatigue-related complaints: A comparison between diabetic employees, “healthy” employees, and employees with other chronic diseases. J. Occup. Environ. Med. 2004, 46, 828–836. [Google Scholar] [CrossRef]
- Delahanty, L.M.; Hayden, D.; Ammerman, A.; Nathan, D.M. Medical nutrition therapy for hypercholesterolemia positively affects patient satisfaction and quality of life outcomes. Ann. Behav. Med. 2002, 24, 269–278. [Google Scholar] [CrossRef]
- De Morais, C.; Oliveira, B.; Afonso, C.; Lumbers, M.; Raats, M.; de Almeida, M.D.V. Nutritional risk of European elderly. Eur. J. Clin. Nutr. 2013, 67, 1215–1219. [Google Scholar] [CrossRef]
- Holmes, B.A.; Roberts, C.L. Diet quality and the influence of social and physical factors on food consumption and nutrient intake in materially deprived older people. Eur. J. Clin. Nutr. 2011, 65, 538–545. [Google Scholar] [CrossRef]
- Seo, K.; Song, M. Relationships of motivational factors and diabetes self-management behavior in community dwelling older adults. J. Muscle Jt. Health 2012, 19, 308–318. [Google Scholar] [CrossRef][Green Version]
- Kim, C.O. Food choice patterns among frail older adults: The associations between social network, food choice values, and diet quality. Appetite 2016, 96, 116–121. [Google Scholar] [CrossRef]
- Pyatak, E.A.; Florindez, D.; Weigensberg, M.J. Adherence decision making in the everyday lives of emerging adults with type 1 diabetes. Patient Prefer. Adherence 2013, 7, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Heckman, B.W.; Mathew, A.R.; Carpenter, M.J. Treatment burden and treatment fatigue as barriers to health. Curr. Opin. Psychol. 2015, 5, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chen, R.C.Y.; Chang, Y.H.; Huang, Y.C.; Wahlqvist, M.L. Physical Function Mitigates the Adverse Effects of Being Thin on Mortality in a Free-Living Older Taiwanese Cohort. J. Nutr. Health Aging 2012, 16, 776–783. [Google Scholar] [CrossRef] [PubMed]
| Level of Fatigue | Mean (SD; Range) | Frequency (%) |
|---|---|---|
| Fatigue (total score of 7 points) | 3.94 (1.81; 1–7) | |
| Normal (<4) | 66 (51.2) | |
| Moderate fatigue (4–4.9) | 22 (17.0) | |
| Severe fatigue (≥5) | 41 (31.8) |
| Predictors | Unstandardized | Standardized | Correlations | VIF | |||
|---|---|---|---|---|---|---|---|
| B (95% CI) | SE | β | p | Partial | Part | ||
| Biological | |||||||
| Age | 0.029 (−0.028, 0.086) | 0.029 | 0.102 | 0.317 | 0.091 | 0.090 | 1.288 |
| Gender | 0.083 (−0.582, 0.748) | 0.336 | 0.023 | 0.805 | 0.022 | 0.022 | 1.042 |
| Years with diabetes | 0.007 (−0.025,0.038) | 0.016 | 0.042 | 0.672 | 0.038 | 0.038 | 1.231 |
| Comorbidities | −0.090 (−0.365, 0.185) | 0.139 | −0.060 | 0.519 | −0.058 | −0.058 | 1.066 |
| Body mass index | 0.033 (−0.075, 0.141) | 0.054 | 0.056 | 0.548 | 0.055 | 0.054 | 1.084 |
| Model fit | Adjusted R2 = −0.024, F = 0.415, p = 0.838; Durbin-Watson: 0.588 | ||||||
| Psychological | |||||||
| Depression | 0.187 (0.098, 0.276) | 0.045 | 0.368 | <0.001 | 0.356 | 0.312 | 1.387 |
| Poor sleep quality | 0.642 (0.029, 1.255) | 0.309 | 0.163 | 0.040 | 0.187 | 0.156 | 1.091 |
| Difficulty with meal planning | 0.233 (0.051, 0.415) | 0.092 | 0.204 | 0.013 | 0.226 | 0.190 | 1.146 |
| Satisfaction with diet | −0.090 (−0.172, −0.007) | 0.042 | −0.173 | 0.033 | −0.194 | −0.162 | 1.137 |
| Burden of diet therapy | 0.015 (−0.061, 0.092) | 0.039 | 0.031 | 0.695 | 0.036 | 0.029 | 1.119 |
| Perceived merits of diet therapy | 0.066 (−0.016, 0.149) | 0.042 | 0.128 | 0.115 | 0.144 | 0.119 | 1.156 |
| Perceived social support | −0.001 (−0.014, 0.013) | 0.007 | −0.008 | 0.923 | −0.007 | −0.007 | 1.156 |
| Model fit | Adjusted R2 = 0.290, F = 8.348, p < 0.001; Durbin-Watson: 1.026 | ||||||
| Interpersonal | |||||||
| Marital status | 0.174 (−0.779, 1.126) | 0.481 | 0.048 | 0.719 | 0.032 | 0.032 | 2.323 |
| Living arrangement | −0.075 (−1.132, 0.982) | 0.534 | −0.020 | 0.888 | −0.013 | −0.012 | 2.524 |
| Exclusive eating alone | 0.795 (−0.076, 1.666) | 0.440 | 0.199 | 0.073 | 0.160 | 0.158 | 1.596 |
| Eating out | 0.137 (0.415, 0.144) | 0.140 | 0.090 | 0.330 | 0.087 | 0.085 | 1.114 |
| Model fit | Adjusted R2 = 0.024, F = 1.771, p = 0.139; Durbin-Watson: 0.583 | ||||||
| Contextual | |||||||
| Education | −0.069 (−0.344, 0.205) | 0.139 | −0.050 | 0.617 | −0.046 | −0.046 | 1.158 |
| Household income | −0.001 (−0.004, 0.001) | 0.001 | −0.118 | 0.234 | −0.110 | −0.110 | 1.158 |
| Model fit | Adjusted R2 = 0.024, F = 1.771, p = 0.139; Durbin-Watson: 0.583 | ||||||
| Predictors | Unstandardized | Standardized | Correlations | VIF | |||
|---|---|---|---|---|---|---|---|
| B (95 % CI) | SE | β | p | Partial | Part | ||
| Comorbidity | 0.752 (0.096, 1.408) | 0.331 | 0.200 | 0.025 | 0.221 | 0.174 | 1.319 |
| Depression | 0.166 (0.066, 0.265) | 0.050 | 0.329 | 0.001 | 0.312 | 0.253 | 1.685 |
| Poor sleep quality | 0.762 (0.095, 1.428) | 0.336 | 0.191 | 0.026 | 0.220 | 0.174 | 1.211 |
| Difficulty with meal planning | 0.291 (0.091, 0.490) | 0.101 | 0.254 | 0.005 | 0.276 | 0.221 | 1.315 |
| Satisfaction with diet | −0.133 (−0.219, −0.047) | 0.043 | −0.261 | 0.003 | −0.293 | 0.236 | 1.226 |
| Model fit | Adjusted R2 = 0.319, F = 4.619, p < 0.001; Durbin-Watson: 1.142 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Son, H. Fatigue-Related Factors for Community-Dwelling Older Adults with Diabetes: A Theory-Guided Multi-Dimensional Approach Using the Dynamic Biopsychosocial Model. Int. J. Environ. Res. Public Health 2019, 16, 4502. https://doi.org/10.3390/ijerph16224502
Kim H, Son H. Fatigue-Related Factors for Community-Dwelling Older Adults with Diabetes: A Theory-Guided Multi-Dimensional Approach Using the Dynamic Biopsychosocial Model. International Journal of Environmental Research and Public Health. 2019; 16(22):4502. https://doi.org/10.3390/ijerph16224502
Chicago/Turabian StyleKim, Hyerang, and Heesook Son. 2019. "Fatigue-Related Factors for Community-Dwelling Older Adults with Diabetes: A Theory-Guided Multi-Dimensional Approach Using the Dynamic Biopsychosocial Model" International Journal of Environmental Research and Public Health 16, no. 22: 4502. https://doi.org/10.3390/ijerph16224502
APA StyleKim, H., & Son, H. (2019). Fatigue-Related Factors for Community-Dwelling Older Adults with Diabetes: A Theory-Guided Multi-Dimensional Approach Using the Dynamic Biopsychosocial Model. International Journal of Environmental Research and Public Health, 16(22), 4502. https://doi.org/10.3390/ijerph16224502





