“The Dop System of Alcohol Distribution is Dead, but It’s Legacy Lives On….”
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Patterns of Alcohol, Drug, and Tobacco Use
3.2. Dop Prevalence over Time by Sample and Residence
3.3. Rates of Total FASD and by Specific Diagnoses
3.4. Association Analysis: The Direct Relationship between Heavy Drinking, FASD Diagnoses, and Dop Experience
4. Discussion
4.1. The Dop System in Contemporary Western Cape Communities
4.2. Has the Dop System Been Influential in Today’s Alcohol Use Patterns?
5. Limitations and Strengths of the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- London, L.; Sanders, D.; te Water Naude, J. Farm workers in South Africa-the challenge of eradicating alcohol abuse and the legacy of the “dop” system. S. Afr. Med. J. 1998, 88, 1092–1095. [Google Scholar] [PubMed]
- London, L. The “dop” system, alcohol abuse and social control amongst farm workers in South Africa: a public health challenge. Soc. Sci. Med. 1999, 48, 1407–1414. [Google Scholar] [CrossRef]
- London, L. Alcohol consumption amongst South African farm workers: A challenge for post-apartheid health sector transformation. Drug Alcohol Depend. 2000, 59, 199–206. [Google Scholar] [CrossRef]
- Falletisch, L.A. Understanding the legacy of dependency and powerlessness experienced by farm workers on wine farms in the Western Cape. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, March 2008. [Google Scholar]
- Phillip Gossage, J.; Snell, C.L.; Parry, C.D.H.; Marais, A.S.; Barnard, R.; De Vries, M.; Blankenship, J.; Seedat, S.; Hasken, J.M.; May, P.A. Alcohol use, working conditions, job benefits, and the legacy of the “dop” system among farm workers in the Western Cape Province, South Africa: Hope despite high levels of risky drinking. Int. J. Environ. Res. Public Health 2014, 11, 7406–7424. [Google Scholar] [CrossRef]
- te Water Naude, J.; London, L.; Pitt, B.; Mahomed, C. The “dop” system around Stellenbosch—Reults of a farm survey. S. Afr. Med. J. 1998, 88, 1102–1105. [Google Scholar]
- Trangenstein, P.J.; Morojele, N.K.; Lombard, C.; Jernigan, D.H.; Parry, C.D.H. Heavy drinking and contextual risk factors among adults in South Africa: Findings from the International Alcohol Control study. Subst. Abus. Treat. Prev. Policy 2018, 13, 43. [Google Scholar] [CrossRef]
- National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), ICF. South Africa Demographic and Health Survey 2016; National Department of Health: Pretoria, South Africa; Rockville, MD, USA, 2019.
- Crome, I.; Glass, Y. The DOP system: A manifestation of social exclusion. A personal commentary on “Alcohol consumption amongst South African farm workers: A post-apartheid challenge, by L. London 1999”. Drug Alcohol Depend. 2000, 59, 207–208. [Google Scholar]
- Roozen, S.; Peters, G.J.Y.; Kok, G.; Townend, D.; Nijhuis, J.; Curfs, L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol. Clin. Exp. Res. 2016, 40, 18–32. [Google Scholar] [CrossRef]
- May, P.A.; Brooke, L.; Gossage, J.P.; Croxford, J.; Adnams, C.; Jones, K.L.; Robinson, L.; Viljoen, D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am. J. Public Health 2000, 90, 1905–1912. [Google Scholar]
- Viljoen, D.L.; Gossage, J.P.; Brooke, L.; Adnams, C.M.; Jones, K.L.; Robinson, L.K.; Hoyme, H.E.; Snell, C.; Khaole, N.C.O.; Kodituwakku, P.; et al. Fetal alcohol syndrome epidemiology in a South African community: A second study of a very high prevalence area. J. Stud. Alcohol 2005, 66, 593–604. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Marais, A.S.; Adnams, C.M.; Hoyme, H.E.; Jones, K.L.; Robinson, L.K.; Khaole, N.C.O.; Snell, C.; Kalberg, W.O.; et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007, 88, 259–271. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Barnard, R.; De Vries, M.; Robinson, L.K.; Adnams, C.M.; Buckley, D.; et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2013, 37, 818–830. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Marais, A.S.; de Vries, M.M.; Kalberg, W.O.; Buckley, D.; Hasken, J.M.; Adnams, C.M.; Barnard, R.; Joubert, B.; Cloete, M.; et al. The continuum of fetal alcohol spectrum disorders in a community in South Africa: Prevalence and characteristics in a fifth sample. Drug Alcohol Depend. 2016, 168, 274–286. [Google Scholar] [CrossRef] [PubMed]
- May, P.; De Vries, M.; Marais, A.S.; Kalberg, W.; Buckley, D.; Adnams, C.; Hasken, J.; Tabachnick, B.; Robinson, L.; Manning, M.; et al. Replication of High Fetal Alcohol Spectrum Disorders Prevalence Rates, Child Characteristics, and Maternal Risk Factors in a Second Sample of Rural Communities in South Africa. Int. J. Environ. Res. Public Health 2017, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Olivier, L.; Urban, M.; Chersich, M.; Temmerman, M.; Viljoen, D. Burden of fetal alcohol syndrome in a rural West Coast area of South Africa. S. Afr. Med. J. 2013, 103, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Chersich, M.F.; Fourie, L.A.; Chetty, C.; Olivier, L.; Viljoen, D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S. Afr. Med. J. 2008, 98, 877–882. [Google Scholar]
- Urban, M.F.; Olivier, L.; Viljoen, D.; Lombard, C.; Louw, J.G.; Drotsky, L.M.; Temmerman, M.; Chersich, M.F. Prevalence of fetal alcohol syndrome in a South African city with a predominantly Black African population. Alcohol Clin. Exp. Res. 2015, 39, 1016–1026. [Google Scholar] [CrossRef]
- Viljoen, D.; Hymbaugh, K. Fetal alcohol syndrome—South Arica, 2001. MMWR 2003, 52, 660–662. [Google Scholar]
- May, P.A.; Gossage, J.P.; Brooke, L.E.; Snell, C.L.; Marais, A.S.; Hendricks, L.S.; Croxford, J.A.; Viljoen, D.L. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: A population-based study. Am. J. Public Health 2005, 95, 1190–1199. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Marais, A.S.; Hendricks, L.S.; Snell, C.L.; Tabachnick, B.G.; Stellavato, C.; Buckley, D.G.; Brooke, L.E.; Viljoen, D.L. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: A third study. Alcohol. Clin. Exp. Res. 2008, 32, 738–753. [Google Scholar] [CrossRef]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Joubert, B.; Cloete, M.; Barnard, R.; De Vries, M.; Hasken, J.; et al. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): Quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013, 133, 502–512. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Hasken, J.M.; Marais, A.S.; Brooke, L.E.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod. Toxicol. 2014, 46, 31–39. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Hamrick, K.J.; Corbin, K.D.; Has ken, J.M.; Marais, A.S.; Blankenship, J.; Hoyme, H.E.; Gossage, J.P. Maternal nutritional status as a contributing factor for the risk of fetal alcohol spectrum disorders. Reprod. Toxicol. 2016, 59, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, D.; Croxford, J.; Gossage, J.P.; Kodituwakku, P.W.; May, P.A. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: A case control study. J. Stud. Alcohol 2002, 63, 6–17. [Google Scholar]
- Idrus, N.M.; Breit, K.R.; Thomas, J.D. Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol. Teratol. 2017, 59, 43–52. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; de Vries, M.M.; Marais, A.S.; Kalberg, W.O.; Adnams, C.M.; Hasken, J.M.; Tabachnick, B.; Robinson, L.K.; Manning, M.A.; Jones, K.L.; et al. The continuum of fetal alcohol spectrum disorders in four rural communities in south africa: Prevalence and characteristics. Drug Alcohol Depend. 2016, 159, 207–218. [Google Scholar] [CrossRef]
- Hoyme, H.; May, P.; Kalberg, W.; Kodituwakku, P.; Gossage, J.; Trujillo, P.; Buckley, D.; Miller, J.; Aragon, A.; Khaole, N.; et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 2005, 115, 39–47. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Stratton, K.; Howe, C.; Battaglia, F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment; Institute Of Medicine National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows; IBM: Armonk, NY, USA, 2018. [Google Scholar]
- Williams, P.P.; Morojele, N.; Londani, M.; Burnhams, N.H.; Parry, C.D.H. Alcohol Advertising, Affordability and Availability, and the Effect on Adult Heavy Drinking and Symptoms of Alcohol Problems: International Alcohol Control Study (South Africa). Subst. Use Misuse 2019, 54, 1751–1762. [Google Scholar] [CrossRef]
- Green, P.P.; McKnight-Eilky, L.R.; Tan, C.H.; Mejia, R.; Denny, C.H. Vital S.: Alcohol-Expo. Pregnancies—U.S., 2011–2013. MMWR Morb. Mortal Wkly. Rep. 2016, 65, 91–97. [Google Scholar] [CrossRef]
- de Vries, M.M.; Joubert, B.; Cloete, M.; Roux, S.; Baca, B.A.; Hasken, J.M.; Barnard, R.; Buckley, D.; Kalberg, W.O.; Snell, C.L.; et al. Indicated prevention of fetal alcohol spectrum disorders in South Africa: Effectiveness of case management. Int. J. Environ. Res. Public Health 2015, 13, 76. [Google Scholar] [CrossRef]

| Community A | Community/Region B | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 [1997] (n = 86) | Sample 2 [1999] (n = 211) | Sample 3 [2002] (n = 222) | Sample 4 [2008] (n = 303) | Sample 5 [2010] (n = 466) | Test-Score | p | Sample 1 [2009] (n = 670) | Sample 2 [2011] (n = 450) | Test-Score | p | ||||||||
| Current Alcohol Consumption | ||||||||||||||||||
| Drank in past 12 months (% Yes) | 58.1 | 53.0 | 41.6 | 48.2 | 56.0 | χ2 = 15.532 | 0.004 | 54.3 | 66.6 | χ2 = 14.902 | <0.001 | |||||||
| Drank in past 30 days (% Yes) | 58.1 | 50.9 | 28.4 | 38.5 | 41.6 | χ2 = 32.505 | <0.001 | 42.6 | 50.2 | χ2 = 6.348 | 0.012 | |||||||
| Drank in the past week (% Yes) | 58.1 | 34.5 | 22.3 | 32.5 | 30.0 | χ2 = 38.301 | <0.0001 | 34.1 | 38.7 | χ2 = 2.466 | 0.116 | |||||||
| Total # of drinks per week 1–Mean (SD) | 11.7 | (10.5) | 12.9 | (13.7) | 8.1 | (8.8) | 8.1 | (8.8) | 9.6 | (9.6) | F = 3.110 | 0.015 | 10.2 | (9.4) | 10.1 | (7.5) | t = 0.057 | 0.955 |
| # of drinking days per week 1 | 1.9 | (0.9) | 1.9 | (1.5) | 1.9 | (1.0) | 1.8 | (1.0) | 1.8 | (1.2) | F = 0.303 | 0.876 | 1.8 | (1.1) | 1.8 | (1.0) | t = 0.534 | 0.811 |
| Drinks per drinking Day1 (DDD) | 5.5 | (3.6) | 5.4 | (3.9) | 3.9 | (2.8) | 4.2 | (3.0) | 4.3 | (2.8) | F = 3.106 | 0.016 | 5.7 | (5.2) | 5.4 | (3.2) | t = 0.798 | 0.425 |
| # drinks consumed on (Friday–Sunday)1 | 11.3 | (10.4) | 11.9 | (11.5) | 8.1 | (8.7) | 8.5 | (9.1) | 9.1 | (8.7) | F = 0.801 | 0.525 | 11.3 | (18.4) | 10.1 | (7.3) | t = 1.643 | 0.101 |
| Beverage of choice (% Yes) 1,2 | ||||||||||||||||||
| Beer | 54.0 | 78.4 | 81.6 | 71.6 | 79.1 | χ2 = 15.009 | 0.005 | 81.5 | 82.8 | χ2 = 0.106 | 0.744 | |||||||
| Fortified Wine | 0.0 | 0.0 | 4.1 | 2.1 | 0.0 | χ2 = 8.955 | 0.062 | 2.2 | 4.6 | χ2 = 1.801 | 0.180 | |||||||
| Spirits | 0.0 | 4.1 | 6.1 | 16.8 | 8.8 | χ2 = 15.537 | 0.004 | 9.3 | 15.5 | χ2 = 3.670 | 0.055 | |||||||
| Wine | 8.0 | 36.5 | 53.1 | 29.5 | 32.4 | χ2 = 24.211 | <0.001 | 46.3 | 48.9 | χ2 = 0.266 | 0.606 | |||||||
| Combination | 38.0 | 1.4 | 4.1 | 2.1 | 1.4 | χ2 = 98.292 | <0.001 | 3.5 | 3.4 | χ2 = 0.002 | 0.967 | |||||||
| Past alcohol consumption | ||||||||||||||||||
| Drank during pregnancy (% Yes) | 74.4 | 41.4 | 48.4 | 49.9 | 58.1 | χ2 = 35.838 | <0.001 | 57.7 | 67.5 | χ2 = 11.376 | 0.001 | |||||||
| Tobacco and Other Drug Use | ||||||||||||||||||
| Used tobacco in lifetime | 75.6 | 46.9 | 63.0 | 70.3 | 63.4 | χ2 = 49.050 | <0.001 | 70.0 | 67.6 | χ2 = 0.782 | 0.377 | |||||||
| Use tobacco in past 30 days 3 | 83.1 | 83.2 | 77.0 | 91.1 | 79.7 | χ2 = 14.094 | 0.029 | 81.9 | 77.3 | χ2 = 2.347 | 0.126 | |||||||
| # of cigarettes per day–Mean (SD) | 9.8 | (7.7) | 5.1 | (5.2) | 4.7 | (3.7) | 5.8 | (5.0) | 6.4 | (4.9) | F = 8.536 | <0.001 | 4.9 | (4.2) | 8.8 | (12.5) | t = -4.279 | <0.001 |
| Used drugs–past year (% Yes) | -- | 0.5 | 0.4 | 0.7 | 1.9 | χ2 = 4.548 | 0.208 | 0.6 | 1.5 | χ2 = 2.623 | 0.105 | |||||||
| Used drugs–pregnancy (% Yes) | -- | 0.0 | 0.9 | 0.7 | 1.5 | χ2 = 4.787 | 0.310 | 1.0 | 2.8 | χ2 = 5.33 | 0.201 | |||||||
| Community A | Community B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 [1997] (n = 86) | Sample 2 [1999] (n = 211) | Sample 3 [2002] (n = 222) | Sample 4 [2008] (n = 303) | Sample 5 [2010] (n = 466) | χ2 | p | Sample 1 [2009] (n = 670) | Sample 2 [2011] (n = 450) | χ2 | p | |
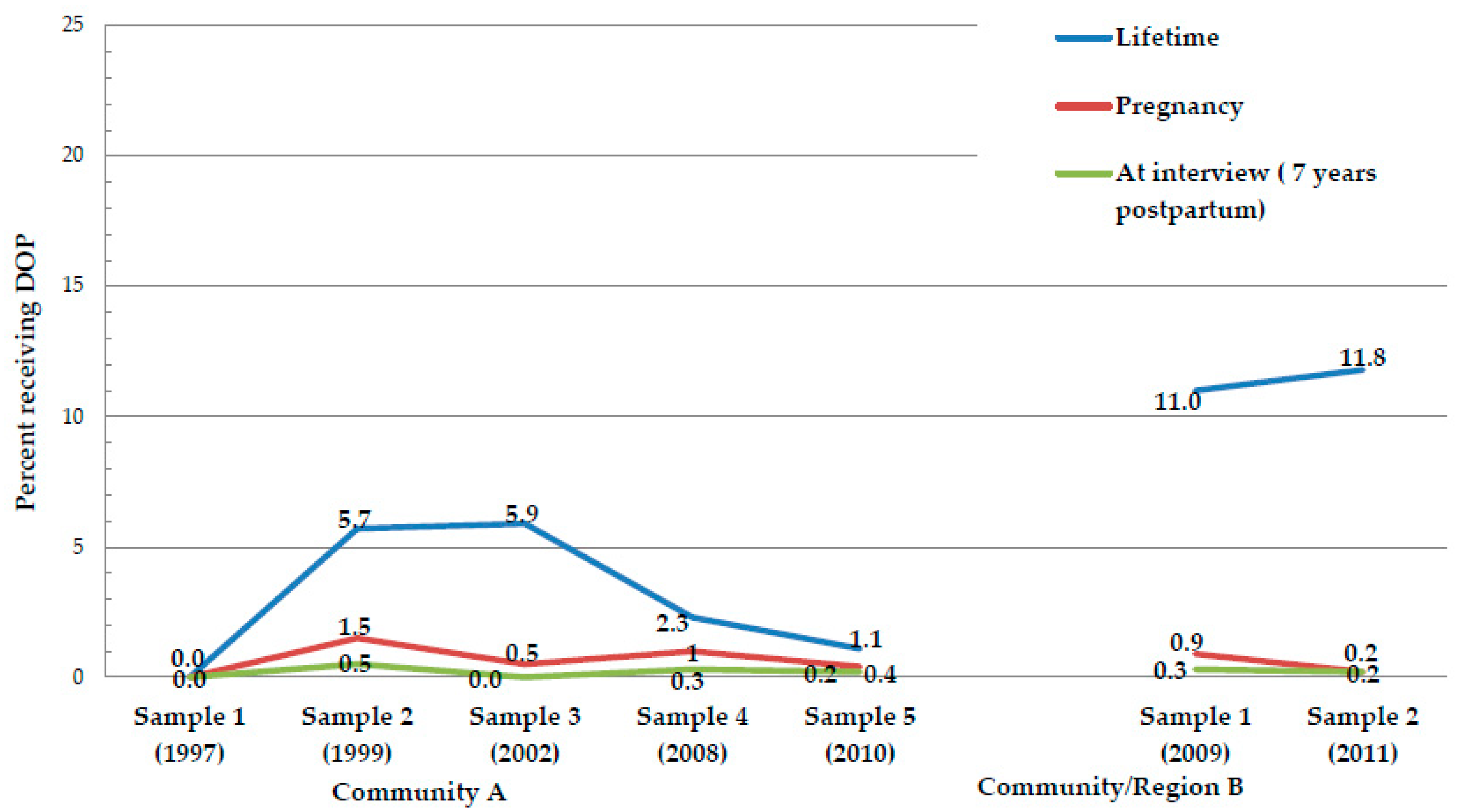
| Received Dop in Lifetime (%Yes) | -- | 5.7 | 5.9 | 2.3 | 1.1 | 17.435 | 0.001 | 11.0 | 11.8 | 0.144 | 0.704 |
| Received Dop in Pregnancy (%Yes) | -- | 1.5 | 0.5 | 1.0 | 0.4 | 2.585 | 0.460 | 0.9 | 0.2 | 1.950 | 0.163 |
| Received Dop at time of interview | 0.0 | 0.5 | 0.0 | 0.3 | 0.2 | 0.113 | 0.774 | 0.3 | 0.2 | 0.054 | 0.816 |
| Community A | Community/Region B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 [1997] | Sample 2 [1999] | Sample 3 [2002] | Sample 4 [2008] | Sample 5 [2010] | Sample 1 [2009] | Sample 2 [2011] | ||||||||
| FASD | Controls | FASD | Controls | FASD | Controls | FASD | Controls | FASD | Controls | FASD | Controls | FASD | Controls | |
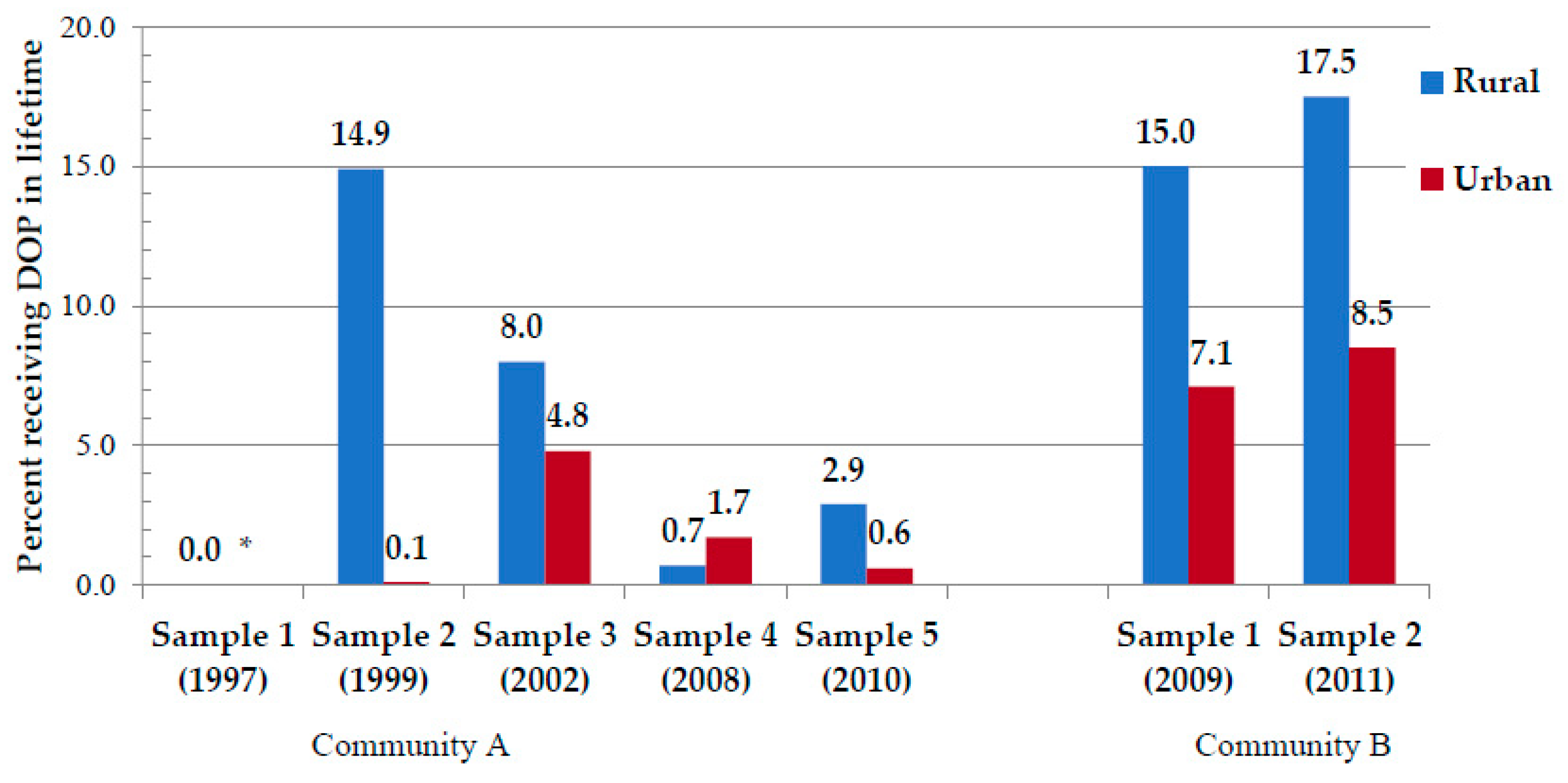
| Received Dop in Lifetime (% Yes) | -- | -- | 11.5 | 2.8 * | 9.5 | 7.3 | 5.9 | 0.0 *** | 1.6 | 0.7 | 16.4 | 6.4 *** | 15.8 | 6.5 ** |
| Received Dop in Pregnancy (% Yes) | -- | -- | 3.2 | 0.8 | 1.6 | 0.0 | 2.5 | 0.0 | 1.1 | 0.0 | 2.0 | 0.0** | 0.4 | 0.0 |
| Received Dop at time of interview | 0.0 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 0.6 | 0.0 | 0.7 | 0.0 | 0.4 | 0.0 |
| Current Maternal Age 20–29 | Current Maternal Age 30–39 | Current Maternal Age 40+ | p | |
|---|---|---|---|---|
| Received Dop in Lifetime (% Yes) | 2.8 | 6.8 | 13.7 | <0.001 |
| Received Dop in Pregnancy (% Yes) | 0.5 | 0.6 | 1.4 | 0.134 |
| Received Dop at time of interview (% Yes) | 0.5 | 0.1 | 0.4 | 0.300 |
| Rural residence (% Yes) | 36.7 | 34.7 | 38.5 | 0.276 |
| Rural | Urban | p | |||
|---|---|---|---|---|---|
| Drank in Lifetime (% Yes) | 91.8 | 79.5 | <0.001 | ||
| Drank in past 12 months (% Yes) | 58.2 | 52.7 | |||
| Drank in past 30 days (% Yes) | 49.1 | 39.7 | <0.001 | ||
| Drank in the past week (% Yes) | 42.6 | 28.9 | <0.001 | ||
| Total # of drinks per week 1–Mean (SD) | 12.3 | (15.9) | 8.4 | (7.9) | <0.001 |
| # of drinking days per week 1–Mean (SD) | 1.9 | (1.0) | 1.8 | (1.2) | 0.108 |
| Drinks per drinking day1 (DDD)–Mean (SD) | 5.9 | (4.7) | 4.4 | (2.9) | <0.001 |
| # of drinks consumed on (Friday–Sunday) 1 | 11.6 | (12.4) | 7.6 | (7.0) | <0.001 |
| Drank during pregnancy (% Yes) | 70.6 | 49.2 | <0.001 | ||
| Community A | Community/Region B | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 1 | Sample 2 | |||||||||||||||
| Rural | Urban | p^ | Rural | Urban | p^ | Rural | Urban | p^ | Rural | Urban | p^ | Rural | Urban | P^ | Rural | Urban | p^ | Rural | Urban | p^ | |
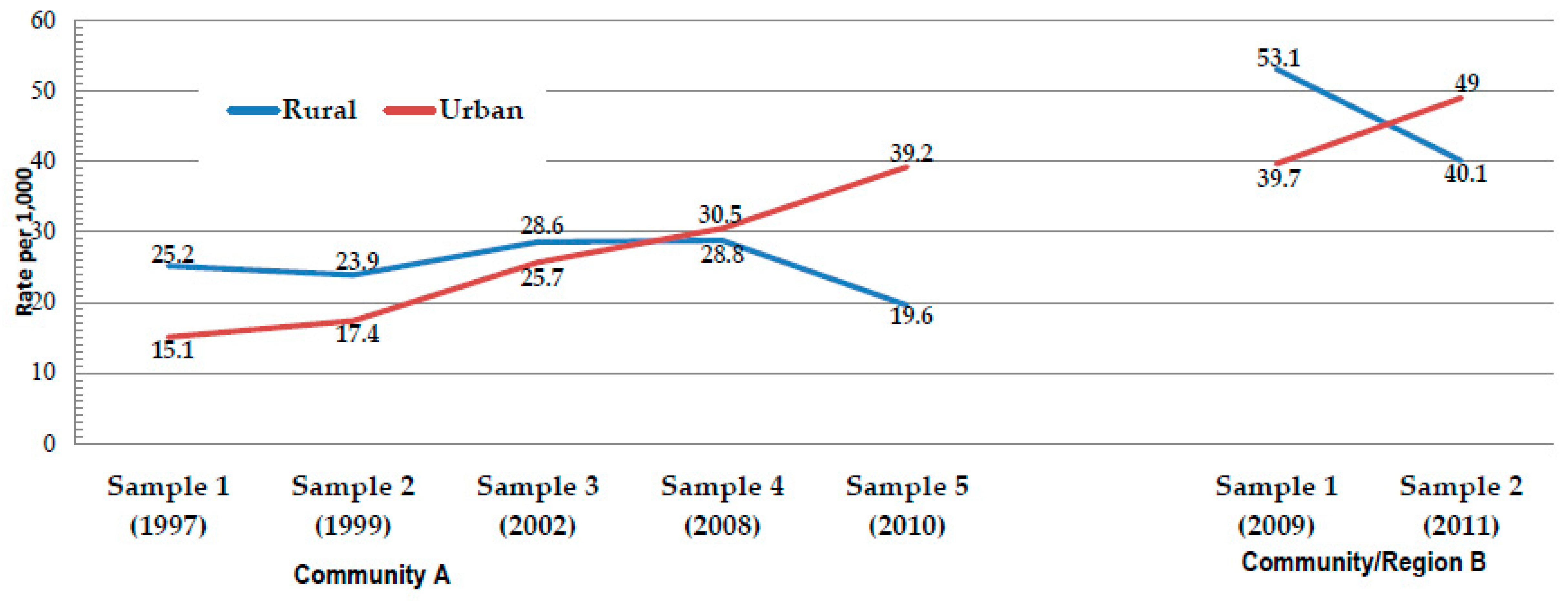
| FAS | 25.2 | 15.1 | <0.001 | 23.9 | 17.4 | <.0001 | 28.6 | 25.7 | 0.142 | 28.8 | 30.5 | 0.737 | 19.6 | 39.2 | <0.001 | 53.1 | 39.7 | <0.001 | 40.1 | 49.0 | <0.001 |
| PFAS * | 1.0 | 3.0 | 0.002 | 13.0 | 18.4 | 0.001 | 4.9 | 12.8 | <0.001 | 14.8 | 30.5 | <0.001 | 19.6 | 58.0 | <0.001 | 27.3 | 31.1 | <0.001 | 25.6 | 43.5 | <0.001 |
| ARND ** | 0.0 | 0.0 | -- | 3.3 | 7.6 | <0.001 | 0.0 | 0.0 | -- | 14.8 | 16.6 | 0.236 | 11.1 | 22.2 | <0.001 | 16.6 | 15.0 | <0.001 | 13.8 | 24.2 | <0.001 |
| Total FASD | 26.2 | 18.1 | <.001 | 40.1 | 43.4 | 0.151 | 41.5 | 38.5 | 0.168 | 58.4 | 77.6 | <.001 | 50.3 | 119.5 | <0.001 | 97.0 | 85.7 | <0.001 | 79.4 | 116.7 | <0.001 |
| β | S.E. | Sig. | Odds Ratio | 95% C.I. for Odds Ratio | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Covariates | ||||||
| Estimated average # of drinks consumed per day in pregnancy | 0.302 | 0.022 | 0.000 | 1.352 | 1.296 | 1.410 |
| Age at time of pregnant with COI (in years) | 0.007 | 0.009 | 0.443 | 1.007 | 0.989 | 1.026 |
| Urban or rural residence during pregnancy | −0.280 | 0.114 | 0.014 | 0.756 | 0.605 | 0.945 |
| Maternal BMI | −0.048 | 0.008 | 0.000 | 0.953 | 0.938 | 0.969 |
| Gravidity | 0.124 | 0.043 | 0.004 | 1.132 | 1.040 | 1.232 |
| Predictor | ||||||
| Ever receive Dop–in lifetime | 0.304 | 0.228 | 0.183 | 1.355 | 0.866 | 2.121 |
| Constant | −0.119 | 0.313 | 0.702 | 0.887 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, P.A.; Marais, A.-S.; De Vries, M.; Hasken, J.M.; Stegall, J.M.; Hedrick, D.M.; Snell, C.L.; Seedat, S.; Parry, C.D.H. “The Dop System of Alcohol Distribution is Dead, but It’s Legacy Lives On….”. Int. J. Environ. Res. Public Health 2019, 16, 3701. https://doi.org/10.3390/ijerph16193701
May PA, Marais A-S, De Vries M, Hasken JM, Stegall JM, Hedrick DM, Snell CL, Seedat S, Parry CDH. “The Dop System of Alcohol Distribution is Dead, but It’s Legacy Lives On….”. International Journal of Environmental Research and Public Health. 2019; 16(19):3701. https://doi.org/10.3390/ijerph16193701
Chicago/Turabian StyleMay, Philip A., Anna-Susan Marais, Marlene De Vries, Julie M. Hasken, Julie M. Stegall, Dixie M. Hedrick, Cudore L. Snell, Soraya Seedat, and Charles D.H. Parry. 2019. "“The Dop System of Alcohol Distribution is Dead, but It’s Legacy Lives On….”" International Journal of Environmental Research and Public Health 16, no. 19: 3701. https://doi.org/10.3390/ijerph16193701
APA StyleMay, P. A., Marais, A.-S., De Vries, M., Hasken, J. M., Stegall, J. M., Hedrick, D. M., Snell, C. L., Seedat, S., & Parry, C. D. H. (2019). “The Dop System of Alcohol Distribution is Dead, but It’s Legacy Lives On….”. International Journal of Environmental Research and Public Health, 16(19), 3701. https://doi.org/10.3390/ijerph16193701





