Selecting a Bedside Cognitive Vital Sign to Monitor Cognition in Hospital: Feasibility, Reliability, and Responsiveness of Logical Memory
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcomes
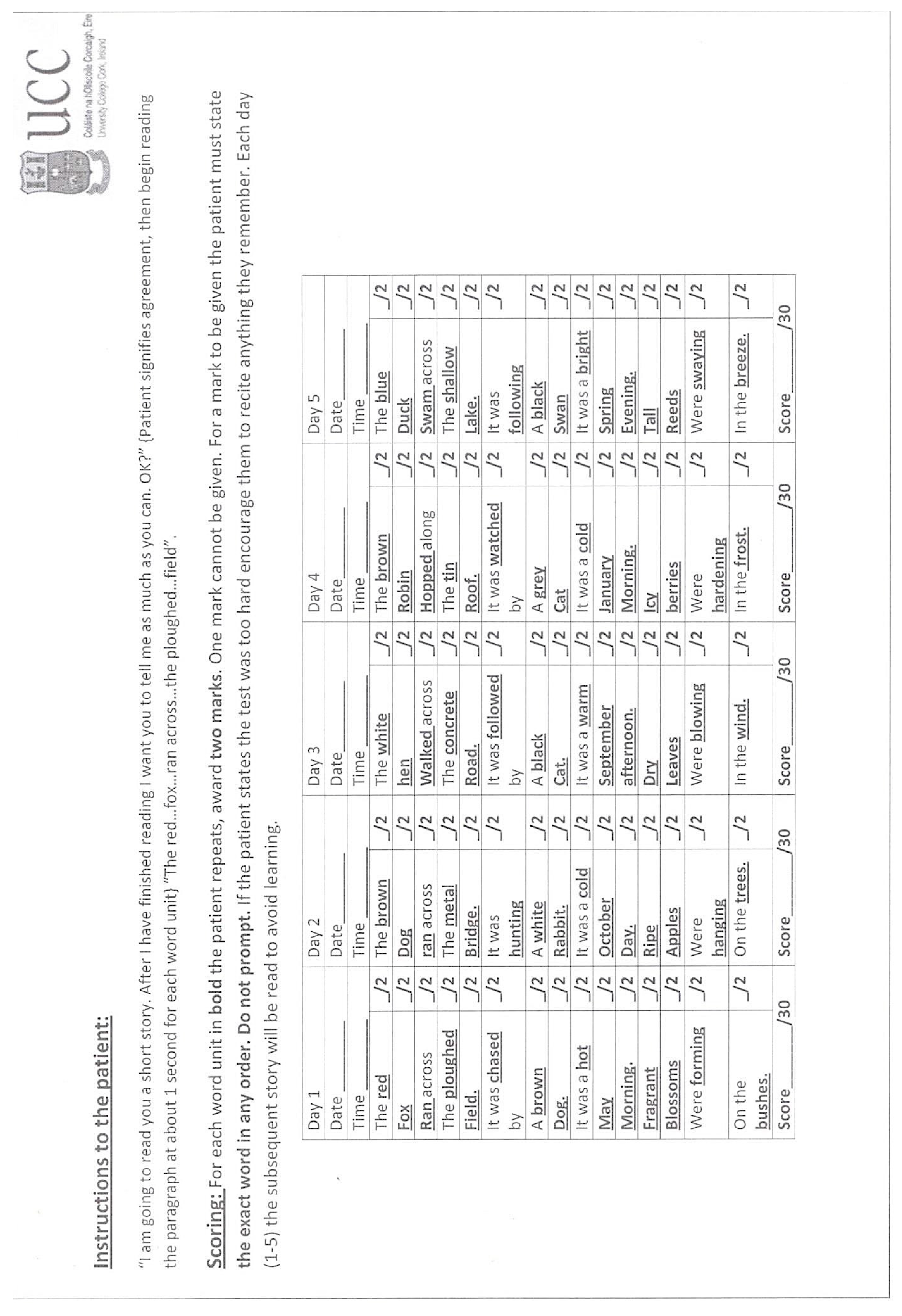
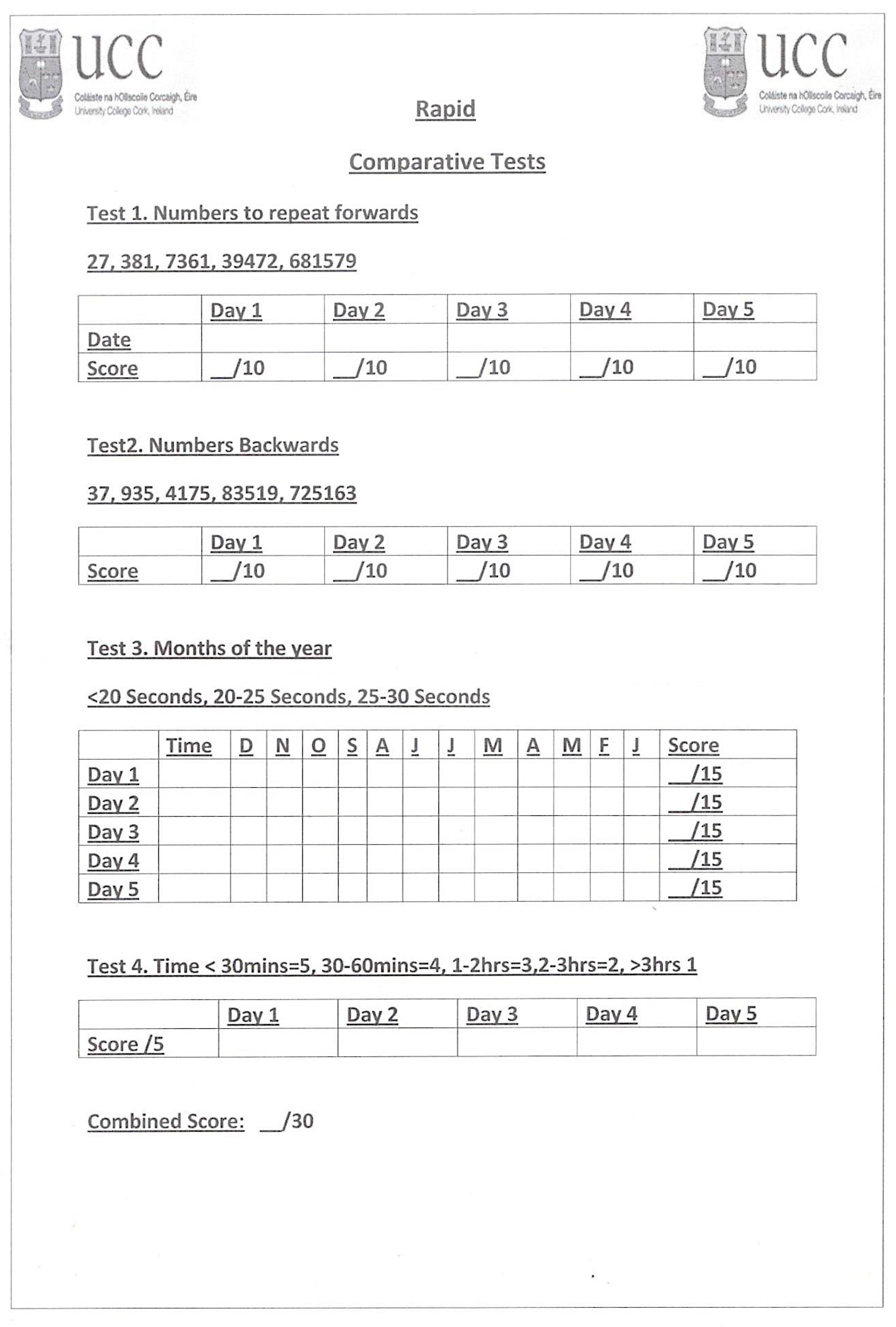
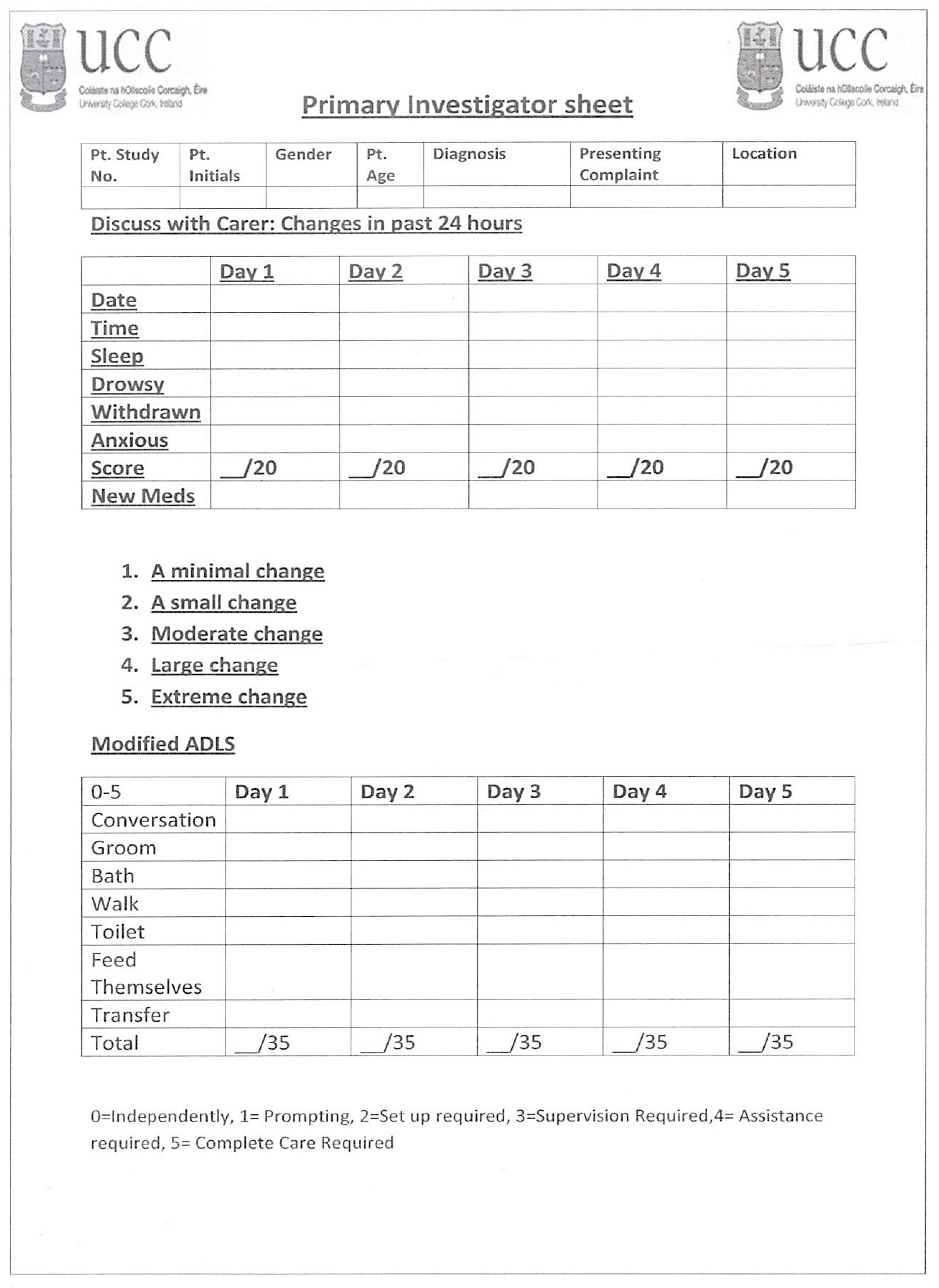
2.3. Study Measures and Procedures
2.4. The Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
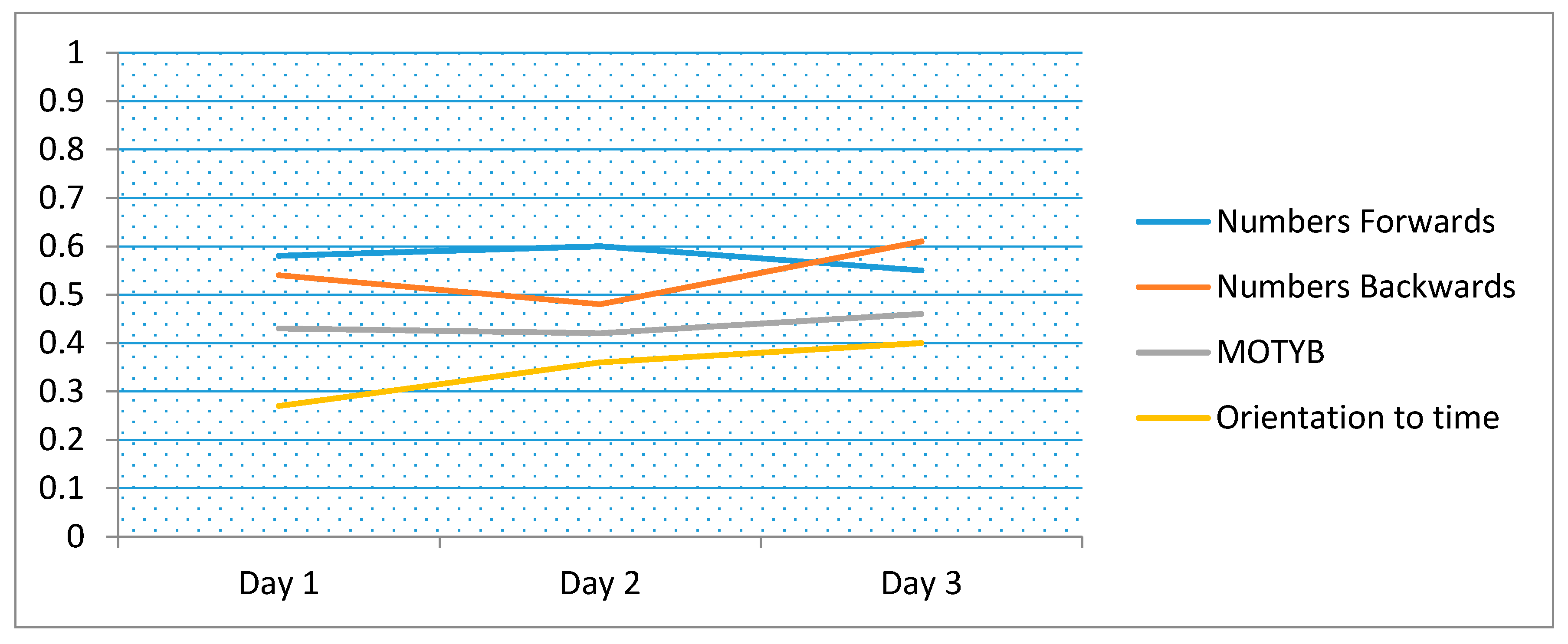
3.2. Reliability, Variance Day-to-Day (Diurnal), and Learning Effects
3.3. Comparison of Logical Memory with Short Tests of Attention and Orientation
3.4. Clinical Correlation
3.5. Feasibility of Using Logical Memory as a Cognitive Vital Sign
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Mathews, S.B.; Arnold, S.E.; Epperson, C.N. Hospitalization and cognitive decline: Can the nature of the relationship be deciphered? Am. J. Geriatr. Psychiatry 2014, 22, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.A.; Gladman, J.R.; Harwood, R.H.; MacLullich, A.M.; Sampson, E.L.; Sheehan, B.; Davis, D.H. Challenges and opportunities in understanding dementia and delirium in the acute hospital. PLoS Med. 2017, 14, e1002247. [Google Scholar] [CrossRef] [PubMed]
- Fick, D.M. The Critical Vital Sign of Cognitive Health and Delirium: Whose Responsibility Is It? J. Gerontol. Nurs. 2018, 44, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Voyer, P.; Champoux, N.; Desrosiers, J.; Landreville, P.; McCusker, J.; Monette, J.; Savoie, M.; Carmichael, P.H.; Richard, H.; Richard, S. RADAR: A measure of the sixth vital sign? Clin. Nurs. Res. 2016, 25, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.J.; O’Regan, N.A.; O’Caoimh, R.; Clare, J.; O’Connor, M.; Leonard, M.; McFarland, J.; Tighe, S.; O’Sullivan, K.; Trzepacz, P.T.; et al. Delirium in an adult acute hospital population: Predictors, prevalence and detection. BMJ Open 2013, 3, e001772. [Google Scholar] [CrossRef]
- Sampson, E.L.; Blanchard, M.R.; Jones, L.; Tookman, A.; King, M. Dementia in the acute hospital: Prospective cohort study of prevalence and mortality. Br. J. Psychiatry 2009, 195, 61–66. [Google Scholar] [CrossRef]
- Timmons, S.; Manning, E.; Barrett, A.; Brady, N.M.; Browne, V.; O’shea, E.; Molloy, D.W.; O’regan, N.A.; Trawley, S.; Cahill, S.; et al. Dementia in older people admitted to hospital: A regional multi-hospital observational study of prevalence, associations and case recognition. Age Ageing 2015, 44, 993–999. [Google Scholar] [CrossRef]
- Clegg, A.; Westby, M.; Young, J.B. Under-reporting of delirium in the NHS. Age Ageing 2011, 40, 283–286. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia 2011, 7, 263–269. [Google Scholar] [CrossRef]
- O’Regan, N.A.; Ryan, D.J.; Boland, E.; Connolly, W.; McGlade, C.; Leonard, M.; Clare, J.; Eustace, J.A.; Meagher, D.; Timmons, S. Attention! A good bedside test for delirium? J. Neurol. Neurosurg. Psychiatry 2014, 85, 1122–1131. [Google Scholar] [CrossRef]
- Adamis, D.; Meagher, D.; Murray, O.; O’neill, D.; O’mahony, E.; Mulligan, O.; McCarthy, G. Evaluating attention in delirium: A comparison of bedside tests of attention. Geriatr. Gerontol. Int. 2016, 16, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.; Brady, N.; Manning, E.; O’shea, E.; O’grady, S.; O‘Regan, N.; Timmons, S. Validation of the 6-Item Cognitive Impairment Test and the 4AT test for combined delirium and dementia screening in older Emergency Department attendees. Age Ageing 2018, 47, 61–68. [Google Scholar]
- Weinrebe, W.; Johannsdottir, E.; Karaman, M.; Füsgen, I. What does delirium cost? Z. Gerontol. Geriatr. 2016, 49, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Mulsant, B.H.; Herrmann, N.; Rapoport, M.; Nilsson, M.; Shulman, K. Canadian academy of geriatric psychiatry survey of brief cognitive screening instruments. Can. Geriatr. J. 2013, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Kelly, S.; Khan, A.; Cullum, S.; Dening, T.; Rait, G.; Fox, C.; Katona, C.; Cosco, T.; Brayne, C.; et al. Attitudes and preferences towards screening for dementia: A systematic review of the literature. BMC Geriatr. 2015, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.E.; Harvey, P.D.; Wesnes, K.A.; Snyder, P.J.; Schneider, L.S. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s Dementia Diagn. Assess Dis. Monit. 2015, 1, 103–111. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Svendovski, A.; Gallagher, P.; Eustace, J.; Molloy, D.W. Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. J. Alzheimer’s Dis. 2017, 57, 123–133. [Google Scholar] [CrossRef]
- Blatter, K.; Cajochen, C. Circadian rhythms in cognitive performance: Methodological constraints, protocols, theoretical underpinnings. Physiol. Behav. 2007, 90, 196–208. [Google Scholar] [CrossRef]
- Gildner, T.E.; Liebert, M.A.; Kowal, P.; Chatterji, S.; Snodgrass, J.J. Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: Results from the Study on Global Ageing and Adult Health (SAGE). J. Clin. Sleep Med. 2014, 10, 613–621. [Google Scholar] [CrossRef]
- Pye, A.; Charalambous, A.P.; Leroi, I.; Thodi, C.; Dawes, P. Screening tools for the identification of dementia for adults with age-related acquired hearing or vision impairment: A scoping review. Int. Psychoger. 2017, 29, 1771–1784. [Google Scholar] [CrossRef]
- Wechsler, D. A standardized memory scale for clinical use. J. Psychol. 1945, 19, 87–95. [Google Scholar] [CrossRef]
- Gavett, B.E.; Gurnani, A.S.; Saurman, J.L.; Chapman, K.R.; Steinberg, E.G.; Martin, B.; Chaisson, C.E.; Mez, J.; Tripodis, Y.; Stern, R.A. Practice effects on story memory and list learning tests in the neuropsychological assessment of older adults. PLoS ONE 2016, 11, e0164492. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Gao, Y.; McGlade, C.; Healy, L.; Gallagher, P.; Timmons, S.; Molloy, D.W. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 2012, 41, 624–629. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Svendrovski, A.; Johnston, B.C.; Gao, Y.; McGlade, C.; Eustace, J.; Timmons, S.; Guyatt, G.; Molloy, D.W. The Quick Mild Cognitive Impairment screen correlated with the Standardized Alzheimer’s Disease Assessment Scale–cognitive section in clinical trials. J. Clin. Epidemiol. 2014, 67, 87–92. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sato, S.; Wall, J.; Igras, E.; Foley, M.J.; Timmons, S.; Molloy, W. Potential for a “Memory Gym” intervention to delay conversion of mild cognitive impairment to dementia. J. Am. Med. Directors Assoc. 2015, 16, 998–999. [Google Scholar]
- Bunt, S.; O’Caoimh, R.; Krijnen, W.P.; Molloy, D.W.; Goodijk, G.P.; van der Schans, C.P.; Hobbelen, H.J. Validation of the Dutch version of the quick mild cognitive impairment screen (Qmci-D). BMC Geriatr. 2015, 15, 115. [Google Scholar] [CrossRef]
- Clarnette, R.; O’Caoimh, R.; Antony, D.N.; Svendrovski, A.; Molloy, D.W. Comparison of the quick mild cognitive impairment (Qmci) screen to the Montreal cognitive assessment (MoCA) in an Australian geriatrics clinic. Int. J. Geriatr. Psychiatry 2017, 32, 643–649. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Gallagher, P.F.; Eustace, J.; McGlade, C.; Molloy, D.W. Which part of the Quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 2013, 42, 324–330. [Google Scholar] [CrossRef]
- Chapman, K.R.; Bing-Canar, H.; Alosco, M.L.; Steinberg, E.G.; Martin, B.; Chaisson, C.; Kowall, N.; Tripodis, Y.; Stern, R.A. Mini Mental State Examination and Logical Memory scores for entry into Alzheimer’s disease trials. Alzheimer’s Res. Ther. 2016, 8, 9. [Google Scholar] [CrossRef]
- Weintraub, S.; Salmon, D.; Mercaldo, N.; Ferris, S.; Graff-Radford, N.R.; Chui, H.; Cummings, J.; DeCarli, C.; Foster, N.L.; Galasko, D.; et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 2009, 23, 91–101. [Google Scholar] [CrossRef]
- Dikmen, S.S.; Heaton, R.K.; Grant, I.; Temkin, N.R. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J. Int. Neuropsychol. Soc. 1999, 5, 346–356. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Molloy, D.W. The Quick Mild Cognitive Impairment Screen (Qmci). In Cognitive Screening Instruments; A Practical Approach, 2nd ed.; Larner, A., Ed.; Springer-Verlag: London, UK, 2017; Volume 1, pp. 255–272. [Google Scholar]
- Cunje, A.; Molloy, D.W.; Standish, T.I.; Lewis, D.L. Alternate forms of logical memory and verbal fluency tasks for repeated testing in early cognitive changes. Int. Psychogeriatr. 2007, 19, 65–75. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Screening for mild cognitive impairment: Comparison of “MCI specific” screening instruments. J. Alzheimer’s Dis. 2016, 51, 619–629. [Google Scholar] [CrossRef]
- Leung, J.L.; Lee, G.T.; Lam, Y.H.; Chan, R.C.; Wu, J.Y. The use of the Digit Span Test in screening for cognitive impairment in acute medical inpatients. Int. Psychogeriatr. 2011, 23, 1569–1574. [Google Scholar] [CrossRef]
- St. Clair-Thompson, H.L.; Allen, R.J. Are forwards and backwards digit recall the same? A dual task study of digit recall. Mem. Cognit. 2013, 41, 519–532. [Google Scholar] [CrossRef]
- O’Keeffe, E.; Mukhtar, O.; O’Keeffe, S.T. Orientation to time as a guide to the presence and severity of cognitive impairment in older hospital patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 500–504. [Google Scholar] [CrossRef]
- Pirozzo, S.; Papinczak, T.; Glasziou, P. Whispered voice test for screening for hearing impairment in adults and children: Systematic review. BMJ 2003, 327. [Google Scholar] [CrossRef]
- Sullivan, K. Estimates of interrater reliability for the Logical Memory subtest of the Wechsler Memory Scale-Revised. J. Clin. Exp. Neuropsychol. 1996, 18, 707–712. [Google Scholar] [CrossRef]
- Gavett, B.E.; Ashendorf, L.; Gurnani, A.S. Reliable change on neuropsychological tests in the Uniform Data Set. J. Int. Neuropsychol. Soc. 2015, 21, 558–567. [Google Scholar] [CrossRef]
- Larner, A.J. Speed versus accuracy in cognitive assessment when using CSIs. Prog. Neurol. Psychiatry 2015, 19, 21–24. [Google Scholar] [CrossRef]
- Teng, E.L.; Hasegawa, K.; Homma, A.; Imai, Y.; Larson, E.; Graves, A.; Sugimoto, K.; Yamaguchi, T.; Sasaki, H.; Chiu, D.; et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 1994, 6, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.; O’Neill, B.; Evans, J.J.; Coen, R.F.; Lawlor, B.A. A review of screening tests for cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2007, 78, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified early warning score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef]
| Patients (%) | 84 | 5 (6%) | 10 (11.9%) | 31 (36.9%) | 33 (39.2%) | 5 (6%) |
|---|---|---|---|---|---|---|
| Age | Total | 18–49 | 50–65 | 66–79 | 80–89 | ≥90 |
| Mean LM score | 10.7 | 17.6 | 10.2 | 11.81 | 9.7 | 7.20 |
| Test | LM (Independent Rater) | LM (Trained Nurses) | Numbers Forwards | Numbers Backwards | MOTYB | Orientation (Time) |
|---|---|---|---|---|---|---|
| Overall scores | ||||||
| Mean ± SD | 10.9 ± 5.5 | 10.9 ± 5.9 | 9.1 ± 1.4 | 5.1 ± 2.4 | 11.1 ± 4.1 | 4.2 ± 1.0 |
| Scores by day of administration (Mean ± SD) | ||||||
| Day 1 | 10.9 ± 5.4 | 10.9 ± 5.4 | 8.8 ± 1.6 | 4.8 ± 2.6 | 11.1 ± 4.1 | 4.2 ± 1.2 |
| Day 2 | 10.7 ± 5.1 | 11.5 ± 5.7 | 9.3 ± 1.3 | 5.3 ± 2.4 | 11.1 ± 4.1 | 4.2 ± 0.9 |
| Day 3 | 10.8 ± 5.5 | 10.5 ± 5.9 | 9.3 ± 1.4 | 5.3 ± 2.3 | 11.3 ± 4.1 | 4.0 ± 0.9 |
| Day 4 | 11.5 ± 7.3 | 11.5 ± 8.0 | 9.7 ± 0.8 | 4.3 ± 2.2 | 10.3 ± 4.0 | 4.3 ± 0.7 |
| Day 5 | 12.3 ± 5.9 | 9.3 ± 5.9 | 10.0 ± 0.0 | 5.3 ± 1.2 | 11.7 ± 3.5 | 4.7 ± 0.6 |
| Variance components analysis (% of variance explained by various factors) | ||||||
| Time (day of follow-up) | 0.3% | 0.8% | 3.5% | 1.5% | 0.3% | 1.0% |
| Patient | 90.7% | 86.7% | 78.7% | 88.7% | 95.5% | 65.7% |
| Day-to-day variability | 9.0% | 12.5% | 17.8% | 9.8% | 4.2% | 33.3% |
| Variation from day to day based on max difference from patient-level mean | ||||||
| Mean/Median | 1.8/1.3 | 2.2/2.0 | 0.5/0.0 | 1.0/1.3 | 0.9/0.7 | 0.7/0.7 |
| % overall mean | 16.6% | 20.3% | 5.9% | 18.8% | 7.7% | 17.2% |
| % patients with zero fluctuation | 11.9% | 14.6% | 63.1% | 28.9% | 32.1% | 10.8% |
| Variation from day to day (based on standard deviation at the patient level) | ||||||
| Mean/Median | 1.5/1.2 | 1.9/2.0 | 0.5/0.0 | 0.8/1.2 | 0.8/0.6 | 0.6/0.6 |
| Learning effect (Linear Mixed Model, significance of study day) | ||||||
| Categorical | p = 0.70 | p = 0.54 | p < 0.001 a | p = 0.001 c | p = 0.23 | p = 0.28 |
| Continuous | p = 0.30 | p = 0.33 | p < 0.001 b | p = 0.002 d | p = 0.40 | p = 0.46 |
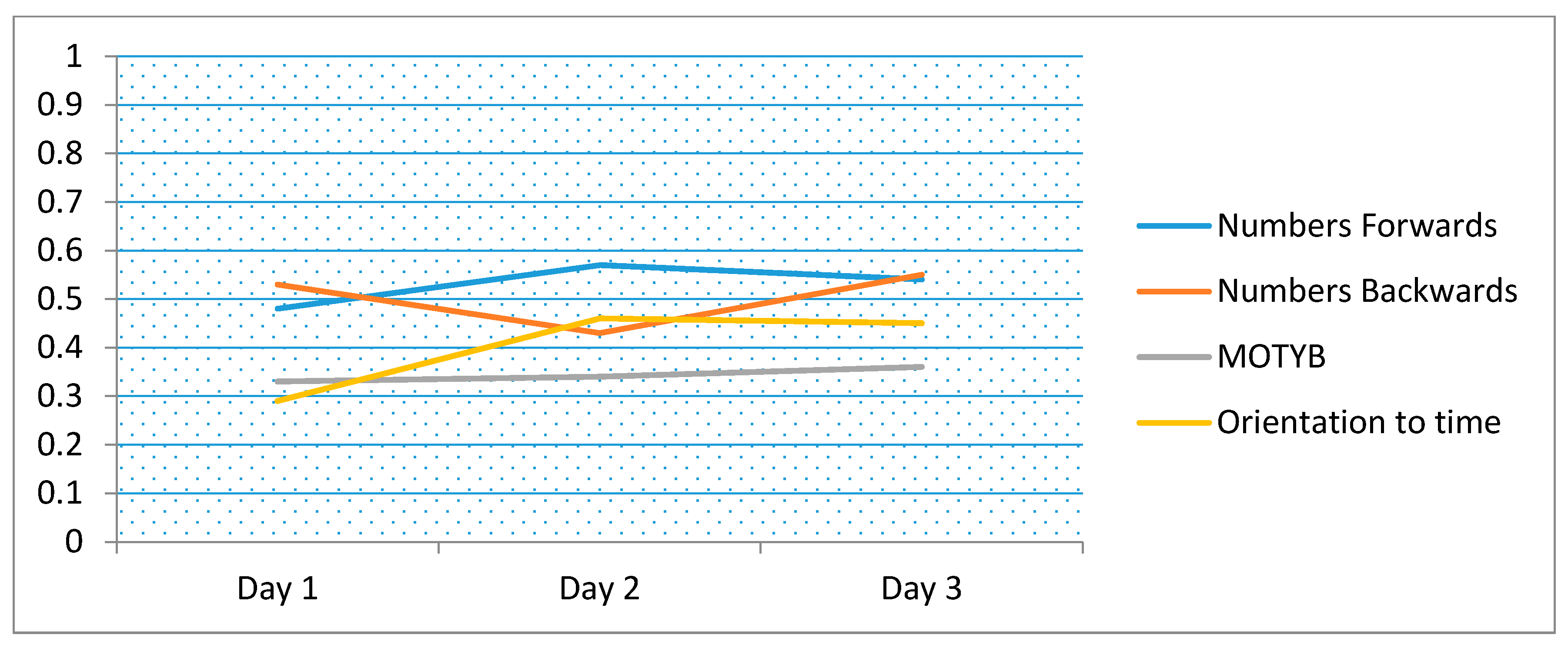
| Days | Digit-Span Forwards | Digit-Span Backwards | MOTYB | Orientation to Time |
|---|---|---|---|---|
| Day 1 | 0.58 | 0.54 | 0.43 | 0.27 |
| Day 2 | 0.60 | 0.48 | 0.42 | 0.36 |
| Day 3 | 0.55 | 0.61 | 0.46 | 0.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholas, P.; O’Caoimh, R.; Gao, Y.; Habib, A.; Mross, T.K.; Clarnette, R.; Molloy, D.W. Selecting a Bedside Cognitive Vital Sign to Monitor Cognition in Hospital: Feasibility, Reliability, and Responsiveness of Logical Memory. Int. J. Environ. Res. Public Health 2019, 16, 3545. https://doi.org/10.3390/ijerph16193545
Nicholas P, O’Caoimh R, Gao Y, Habib A, Mross TK, Clarnette R, Molloy DW. Selecting a Bedside Cognitive Vital Sign to Monitor Cognition in Hospital: Feasibility, Reliability, and Responsiveness of Logical Memory. International Journal of Environmental Research and Public Health. 2019; 16(19):3545. https://doi.org/10.3390/ijerph16193545
Chicago/Turabian StyleNicholas, Padraic, Rónán O’Caoimh, Yang Gao, Afsana Habib, Thomas Karol Mross, Roger Clarnette, and D. William Molloy. 2019. "Selecting a Bedside Cognitive Vital Sign to Monitor Cognition in Hospital: Feasibility, Reliability, and Responsiveness of Logical Memory" International Journal of Environmental Research and Public Health 16, no. 19: 3545. https://doi.org/10.3390/ijerph16193545
APA StyleNicholas, P., O’Caoimh, R., Gao, Y., Habib, A., Mross, T. K., Clarnette, R., & Molloy, D. W. (2019). Selecting a Bedside Cognitive Vital Sign to Monitor Cognition in Hospital: Feasibility, Reliability, and Responsiveness of Logical Memory. International Journal of Environmental Research and Public Health, 16(19), 3545. https://doi.org/10.3390/ijerph16193545






