Valuation of Estimation Toxic Chemical Release Inventory Method-Focusing on Paint Manufacturing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Chemical Substances and the Discharge Route
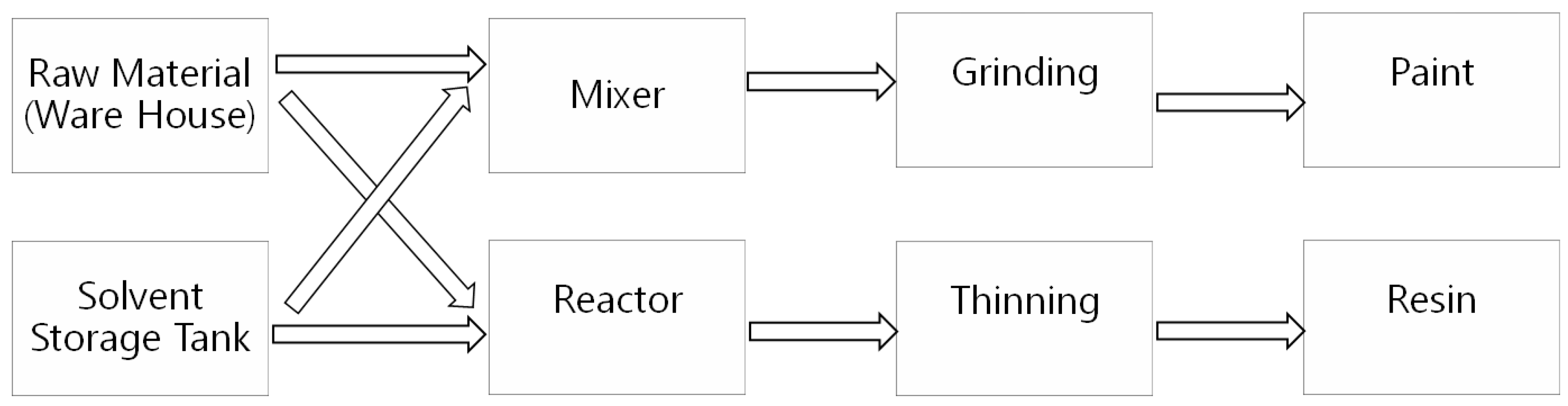
2.2. Selection of Industries and Process
2.3. TRI Emission Method
2.3.1. Source Testing Method
2.3.2. Material Balance Method
2.3.3. Emission Factor Method
2.3.4. Emission Model Method
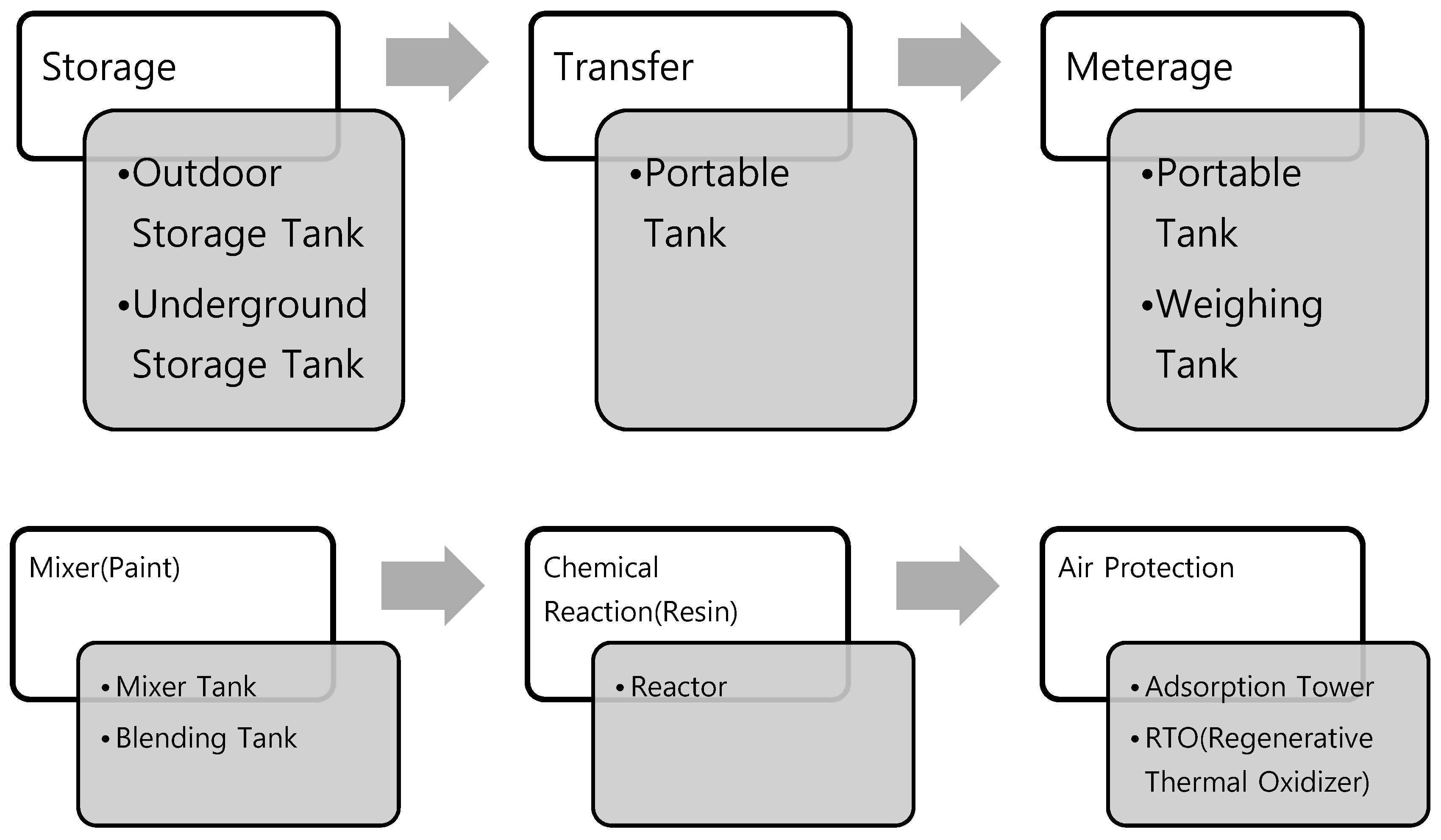
2.4. Selection of TRI Emission Estimation Method by Process
2.5. Theories and Factors of TRI Emission Estimation by Process
2.5.1. Storage Process, Metering Process, Mixing Process
2.5.2. Transfer Process
2.5.3. Reaction Process
2.5.4. Air Pollution Prevention Process
3. Results
3.1. TRI Estimation Result by Process
3.2. Application Method by Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kleindorfer, R.P.; Belke, C.J. Accident Epidemiology and the U.S. Chemical Industry: Accident History and Worst-Case Data from RMP*Info. Risk Anal. 2003, 23, 5. [Google Scholar] [CrossRef]
- Falkenberry, E.M. The Emergency Planning and Community Right-to-Know Act: A Toolfor Toxic Release Reduction in the 90’s. Buffalo Environ. Law J. 1995, 3, 1. [Google Scholar]
- Hong, J.S.; Kim, K.Y. A Study on Integrated Approaching Factors of Environmentally-Friendly Companies Certification Scheme and Environmental Impact Assessment of Korea. J. Environ. Impact Assess. 2008, 17, 113–124. [Google Scholar]
- National Law Information Center. Chemicals Control Act Article 11 (Pollutant Release and Transfer Registers). Available online: http://www.law.go.kr/ (accessed on 20 July 2019).
- Xi, G.; Yan, L. Associations between maternal residential proximity to air emissions from industrial facilities and low birth weight in Texas, USA. Environ. Int. 2018, 120, 181–198. [Google Scholar]
- Ryan, J.; Kim, R.W. Socio-demographic Differences in Toxic Release Inventory Siting and Emissions in Metro Atlanta. Int. J. Environ. Res. Public Health 2016, 13, 747. [Google Scholar] [CrossRef]
- Lee, S.M. Measures to Improve Accuracy and Reliability in Estimating Chemical TRI Emissions. In Proceedings of the Chemical Emission Reduction Information Exchange Meeting, Seoul, Korea, 20 November 2009. [Google Scholar]
- Sweet, C.W.; Vermette, S.J. Toxic volatile organic compounds in urban air in Illinois. Environ. Sci. Technol. 1992, 26, 165–173. [Google Scholar] [CrossRef]
- Pollutant Release and Transfer Register. Available online: https://icis.me.go.kr/prtr/main.do (accessed on 20 July 2019).
- Ahn, S.C.; Hong, S.I. Comprehensive Assessment of Chemical TRI Emissions System and Development of Future Development Plan, 1st ed.; Ministry of Environment: Seoul, Korea, 2009; pp. 22–57.
- Lim, S.R.; Lam, C.W. Quantity-based and toxicity-based evaluation of the U.S. Toxics Release Inventory. J. Hazard. Mater. 2010, 178, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S. Prepare a Plan to Improve the Chemical TRI Emission Survey System, 1st ed.; Ministry of Environment: Seoul, Korea, 2007; pp. 6–45.
- Dursun, D.; Sengul, F. Waste minimization study in a solvent-based paint manufacturing plant. Resour. Conserv. Recycl. 2006, 47, 316–331. [Google Scholar] [CrossRef]
- Ministry of Environment. Guidelines for Estimating TRI Emissions of Volatile Substances; Ministry of Environment: Seoul, Korea, 2013; pp. 4–75.
- Gong, S.Y.; Lee, S.M. Environmental Forum: Achievements and Challenges of Chemical TRI Emissions Inspection System. Environ. Forum 2010, 164, 1–8. [Google Scholar]
- Barnes, L.; Betterton, B. Preferred and Alternative Methods for Estimating Air Emissions from the Printing, Packaging, and Graphic Arts Industry; Environmental Protection Agency: Washington, DC, USA, 2002; Volume 2, pp. 11–30.
- National Institute of Chemical Safety. Guidelines for the Investigation of Chemical TRI Emissions; Ministry of Environment: Daejeon, Korea, 2019; pp. 13–41.
- Chemical TRI Emission Reporting Service. Available online: http://icis.me.go.kr/prtr/tri (accessed on 20 July 2019).
- EPA (Environmental Protection Agency) Emissions Estimation Tools. Available online: https://www3.epa.gov/ttn/chief/software/ (accessed on 20 July 2019).
- Jeon, M.J.; Pak, S.H. Practical Study of Low-temperature Vacuum Swing Adsorption Process for VOCs Removal. Appl. Chem. Eng. 2017, 28, 332–338. [Google Scholar]
- Velagapudi, S.; Kumar, A. Application of the US EPA’s Risk-Screening Indicators Model and Toxic Release Inventory Database to Study Pollution Prevention Trends in Paper Manufacturing Industry. Environ. Prog. Sustain. 2017, 36, 3. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Toxic Chemical Release Inventory Reporting Forms and Instructions; 2018 Version; Environmental Protection Agency: Washington, DC, USA, 2018; pp. 52–80.
- Furry, D.W.; Harris, G. Evaluation of Instrument Leak Detection Capabilities for Smart LDAR Application: Chemical Plant Testing. Environ. Prog. 2007, 26, 2. [Google Scholar] [CrossRef]
- Mandal, P.C. Gas Leak Detection in Pipelines & Repairing System of Titas Gas. Int. J. Appl. Eng. Res. 2014, 2, 2. [Google Scholar]
- Baek, S.O.; Jeon, C.G. Current Status and Future Directions of Management of Hazardous Air Pollutatns in Korea-Focusing on Ambient Air Monitoring Issues. J. Korean Soc. Atmos. Environ. 2013, 29, 513–527. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, J.W. Design standard of activated carbon vessel for the intermittent emission sources of volatie organic compounds. J. Korean Soc. Atmos. Environ. 2007, 23, 250–260. [Google Scholar] [CrossRef]
- Ciolek, M. Emission Factor Documentation for AP-42 Section 7.1 Organic Liquid Storage Tanks; Environmental Protection Agency: Washington, DC, USA, 2006; pp. 12–146.
- Pulles, T. Quality of emission data: Community right to know and national reporting. Environ. Sci. 2008, 5, 151–160. [Google Scholar] [CrossRef][Green Version]
- Ellenbecker, M.; Geiser, K. At the source: The origins of the Massachusetts toxics use reduction program and an overview of this special issue. J. Clean. Prod. 2011, 19, 389–396. [Google Scholar] [CrossRef]
| Rank | The United States | Japan | Australia | Korea |
|---|---|---|---|---|
| 1 | Hydrochloric acid (23.4%) | Toluene (42.2%) | Ammonia water (22.1%) | Xylene (28.1%) |
| 2 | Sulfuric acid (6.4%) | Xylene (16.7%) | Hydrochloric acid (12.4%) | Toluene (15.6%) |
| 3 | Methanol (6.3%) | Methyl ethyl ketone (8.7%) | Methanol (8.7%) | Ethyl acetate (9.5%) |
| 4 | Toluene (1.8%) | Dichloromethane (3.1%) | Toluene (6.9%) | Methyl ethyl ketone (7.2%) |
| 5 | Xylene (1.6%) | Ethyl benzene (2.9%) | Xylene (5.5%) | Dichloromethane (5.5%) |
| Rank | The United States | Japan | Australia | Korea |
|---|---|---|---|---|
| 1 | Air (61.6%) | Air (95.1%) | Air (98.1%) | Air (99.6%) |
| 2 | Water (10.9%) | Water (3.9%) | Water (1.7%) | Water (0.03%) |
| 3 | Soil (27.5%) | Soil (1.0%) | Soil (0.11%) | Soil (0.01%) |
| Method | Ratio (%) |
|---|---|
| Emission Model Method | 10 |
| Emission Factor Method | 4 |
| Material Balance Method | 47 |
| Source Testing Method | 8 |
| Mixture of methods | 31 |
| Sum | 100 |
| Source | State | Emission Factor |
|---|---|---|
| (kg/h/source) | ||
| Valve | Gas | 0.00597 |
| Light oil | 0.00403 | |
| Heavy oil | 0.00023 | |
| Pump | Light oil | 0.0199 |
| Heavy oil | 0.00862 | |
| Compressor | Gas/steam | 0.228 |
| Safety valve | Gas/steam | 0.104 |
| Connector (flange, manhole) | All | 0.00183 |
| Open lines | All | 0.0017 |
| Sampling points | All | 0.0150 |
| Injection conditions | Coefficient (a) |
|---|---|
| Empty tank, infusion under the face | 0.5 |
| Empty tank, spraying on top of liquid | 1.45 |
| Normal state, infusion under the face | 0.6 |
| Normal state, spraying on top of liquid | 1.45 |
| Normal state, infusion under the face with pressure control | 1.0 |
| Normal state, spraying on top of liquid with pressure control | 1.0 |
| Storage Process | Transfer Process | Metering Process | Mixing Process | Reaction Process | Air Pollution Prevention Process |
|---|---|---|---|---|---|
| Emission Model Method | Emission Factor Method | Emission Model Method | Emission Model Method | Material Balance Method | Emission Factor Method |
| Source Testing Method | Source Testing Method | Source Testing Method | Source Testing Method | Source Testing Method | Source Testing Method |
| Toluene | Xylene | Methyl Ethyl Ketone | |
|---|---|---|---|
| CAS No (Chemical abstracts service registered number) | 108-883 | 1330-20-7 | 78-93-3 |
| Molecular weight | 92.14 | 106.16 | 72.11 |
| Density (kg/m3) | 805 | 867 | 864 |
| Vapor pressure (mmHg at 25 °C) | 28.4 | 6.65 | 90.6 |
| Storage Tank | Weighing Tank | Mixing Tank | |
|---|---|---|---|
| Volume of tank m3 | 25 | 1 | 2.5 |
| Diameter, Height mm | (D 1: 3100, H 2: 4500) | (D 1: 500, H 2: 575) | (D 1: 1000, H 2: 1200) |
| Tank color coefficient | 1, 2 3 | 1, 2 3 | 1, 2 3 |
| Number of injections per year | 50 | 1250 | 1250 |
| Absolute temperature in tank (K) | 298 | 298 | 298 |
| Coefficient according to injection condition | Empty tank, infusion under face | Empty tank, spraying on top of liquid | Normal state, infusion under the face |
| 0.5 | 1.45 | 0.6 |
| TRI Emission (kg/year) | Toluene | Xylene | Methyl Ethyl Ketone |
|---|---|---|---|
| Storage process Emission Model Method | 210.7 | 62.1 | 503.0 |
| Storage process Source Testing Method | 106.3 | 12.25 | 83.2 |
| Metering process Emission Model Method | 101.6 | 27.5 | 214.8 |
| Metering process Source Testing Method | 54.3 | 6.3 | 42.5 |
| Mixing process Emission Model Method | 118.3 | 53.9 | 627.3 |
| Mixing process Source Testing Method | 27.1 | 31.3 | 212.5 |
| Transfer process Emission Factor method | 571.3 | 559.2 | 535.1 |
| Transfer process Source Testing Method | 93.8 | 81.4 | 123.7 |
| Reaction process Material Balance Method | 0 | 0 | 0 |
| Reaction process Source Testing Method | 488.4 | 382.2 | 562.7 |
| Air pollution prevention process Emission Factor method | 77.3 | 25.4 | 235.3 |
| Air pollution prevention process Source Testing Method | 86.8 | 67.9 | 100.1 |
| Storage Process | Transfer Process | Metering Process | Mixing Process | Reaction Process | Air Pollution Prevention Process | |
|---|---|---|---|---|---|---|
| Toluene | A | C | A | A | D | D |
| Xylene | A | C | A | A | D | D |
| Methyl ethyl ketone | A | C | A | A | D | C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.E.; Huh, E.-H.; Yoon, Y.; Yoon, S.J.; Huh, D.-A.; Moon, K.W. Valuation of Estimation Toxic Chemical Release Inventory Method-Focusing on Paint Manufacturing Process. Int. J. Environ. Res. Public Health 2019, 16, 3260. https://doi.org/10.3390/ijerph16183260
Lee HE, Huh E-H, Yoon Y, Yoon SJ, Huh D-A, Moon KW. Valuation of Estimation Toxic Chemical Release Inventory Method-Focusing on Paint Manufacturing Process. International Journal of Environmental Research and Public Health. 2019; 16(18):3260. https://doi.org/10.3390/ijerph16183260
Chicago/Turabian StyleLee, Hyo Eun, Eun-Hae Huh, Yi Yoon, Seok J. Yoon, Da-An Huh, and Kyong Whan Moon. 2019. "Valuation of Estimation Toxic Chemical Release Inventory Method-Focusing on Paint Manufacturing Process" International Journal of Environmental Research and Public Health 16, no. 18: 3260. https://doi.org/10.3390/ijerph16183260
APA StyleLee, H. E., Huh, E.-H., Yoon, Y., Yoon, S. J., Huh, D.-A., & Moon, K. W. (2019). Valuation of Estimation Toxic Chemical Release Inventory Method-Focusing on Paint Manufacturing Process. International Journal of Environmental Research and Public Health, 16(18), 3260. https://doi.org/10.3390/ijerph16183260





