Paraquat Preferentially Induces Apoptosis of Late Stage Effector Lymphocyte and Impairs Memory Immune Response in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, PQ Treatments and KLH Immunization
2.2. Flow Cytometry and Antibodies
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. In Vitro KLH Stimulation
2.5. In Vitro PQ Treatment
2.6. Statistics
3. Results
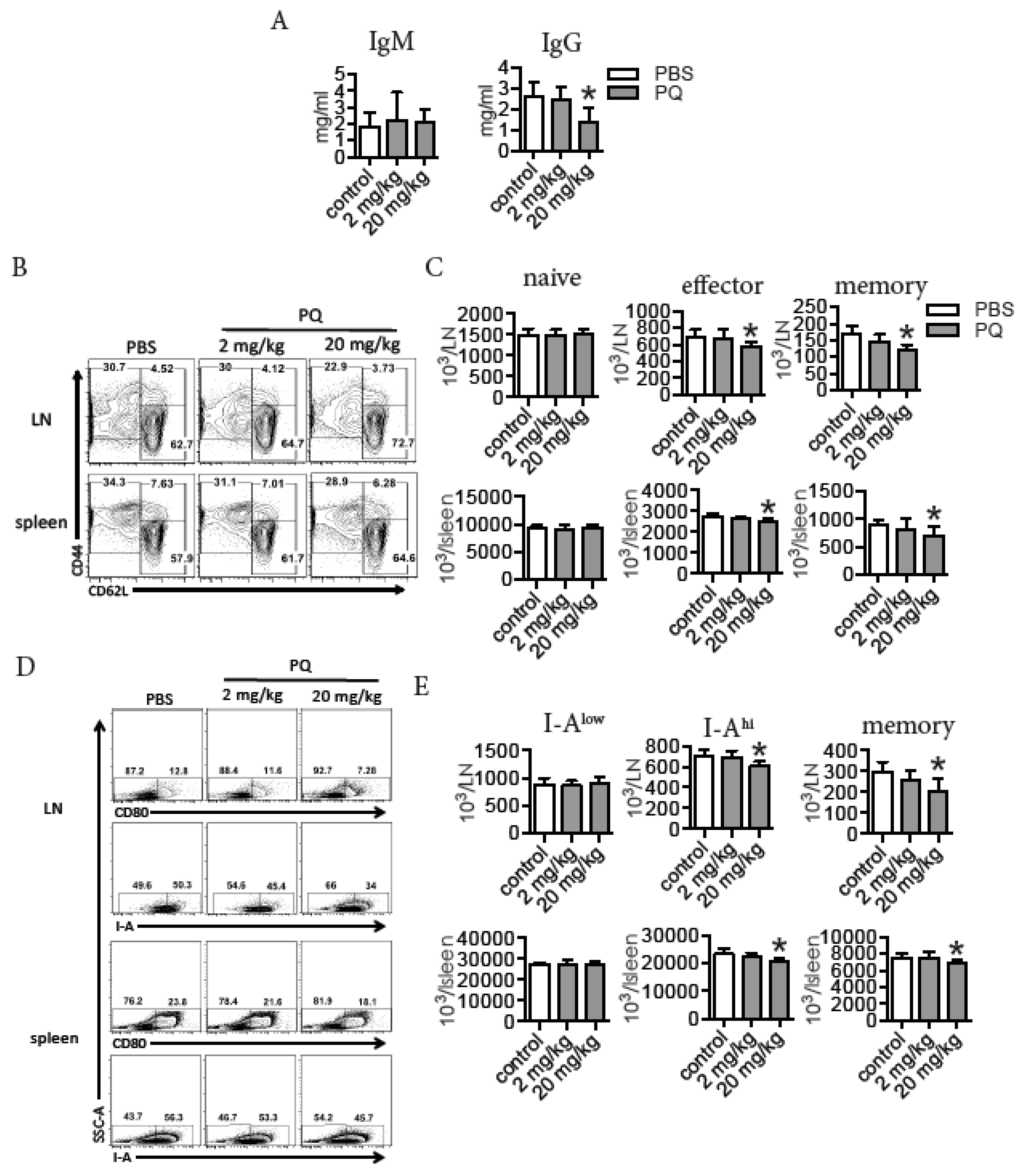
3.1. PQ Reduced the Level of Serum IgG and Selectively Decreased the Number of Effector and Memory Lymphocytes during Steady State
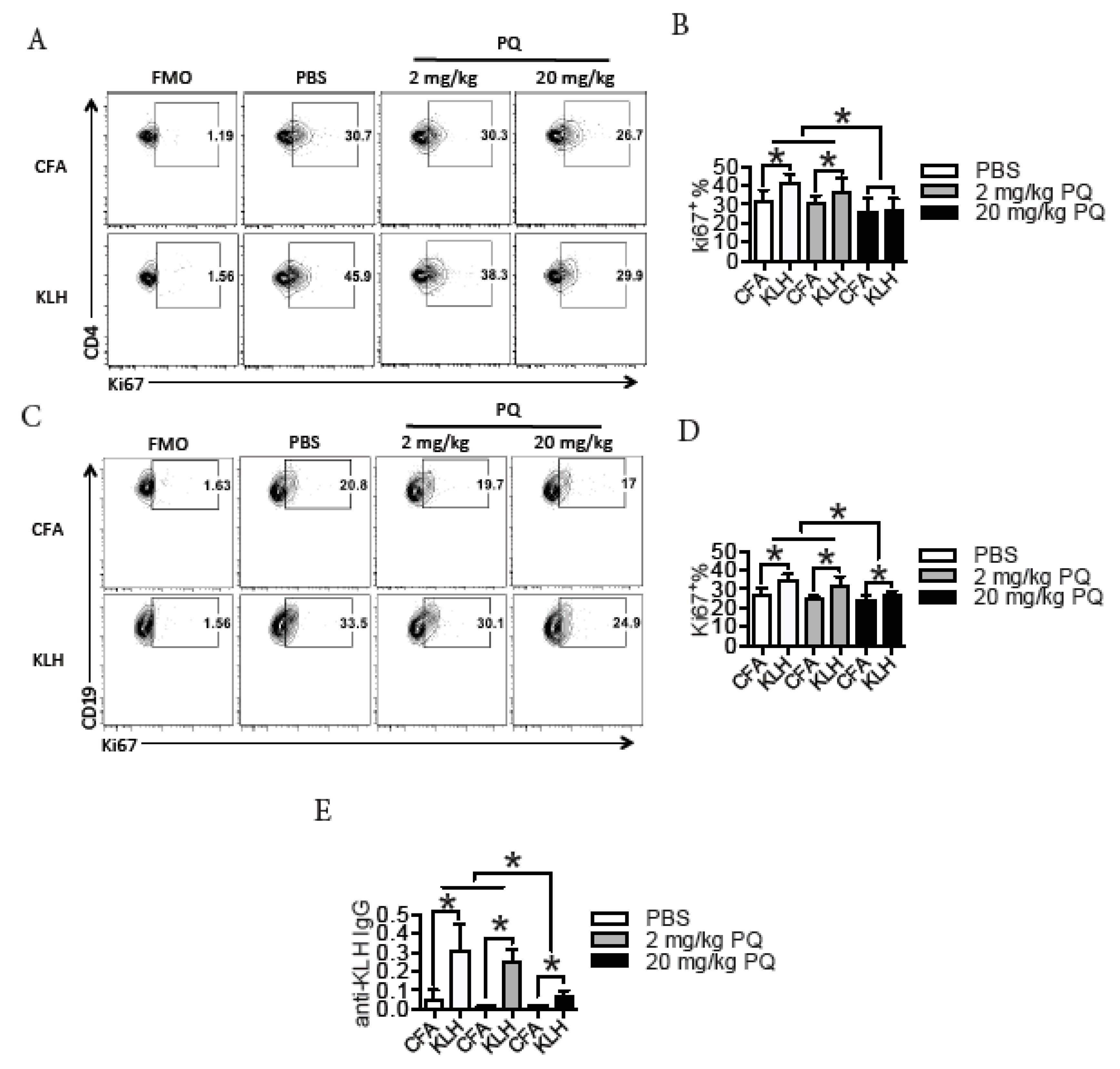
3.2. PQ Impaired Memory Immune Response to KLH
3.3. PQ Did Not Influence the Proliferation of CD4 T Cells and B Cells during the Primary Immune Response to KLH
3.4. PQ Did Not Influence the Expression of Co-Stimulatory Molecules between CD4 T Cells and Antigen Presenting Cells (APC) during the Primary Immune Response to KLH
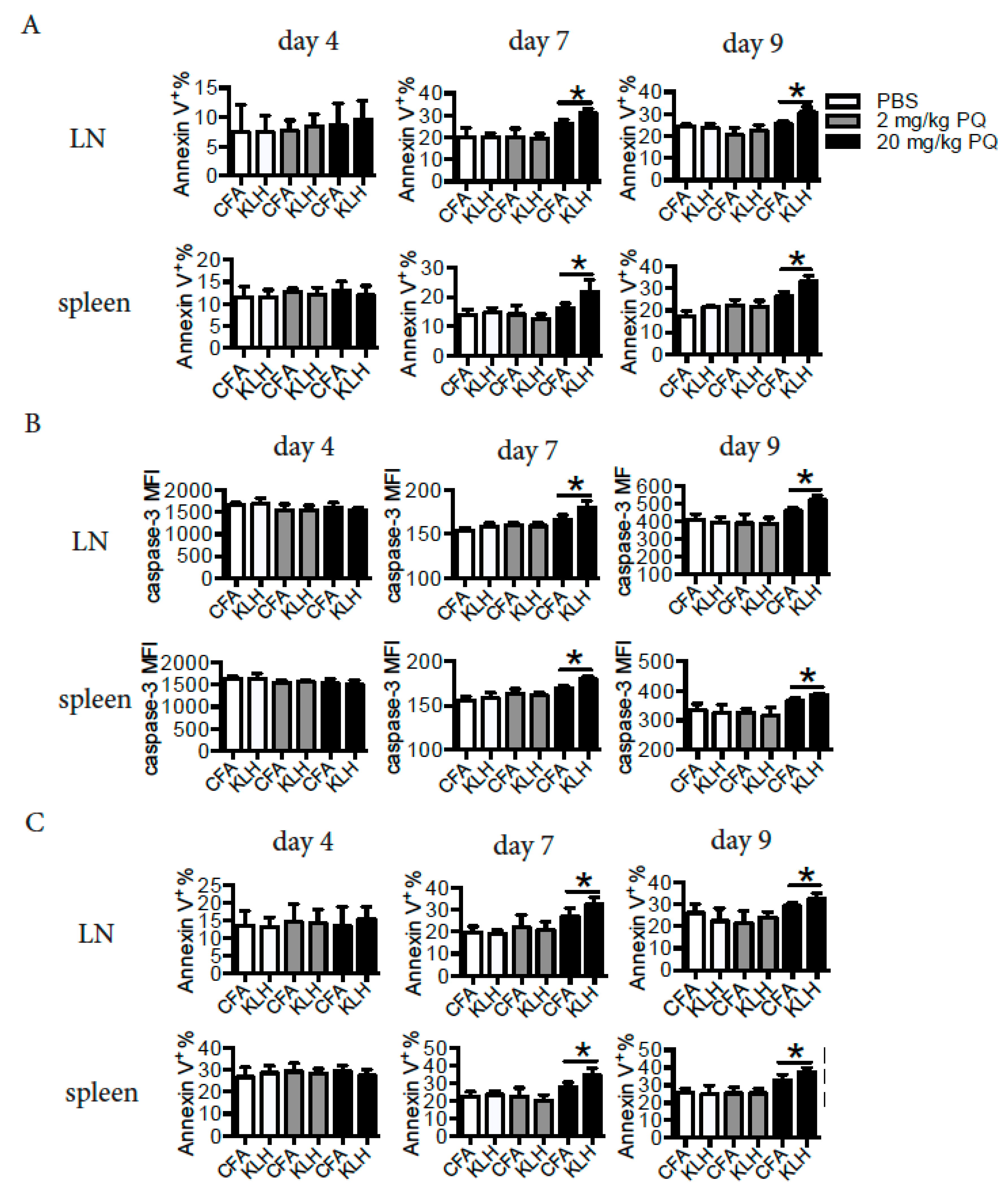
3.5. PQ Induced Apoptosis of CD4 T Cells and B Cells at Early Stage, but not at Late Stage of the Primary Immune Response to KLH
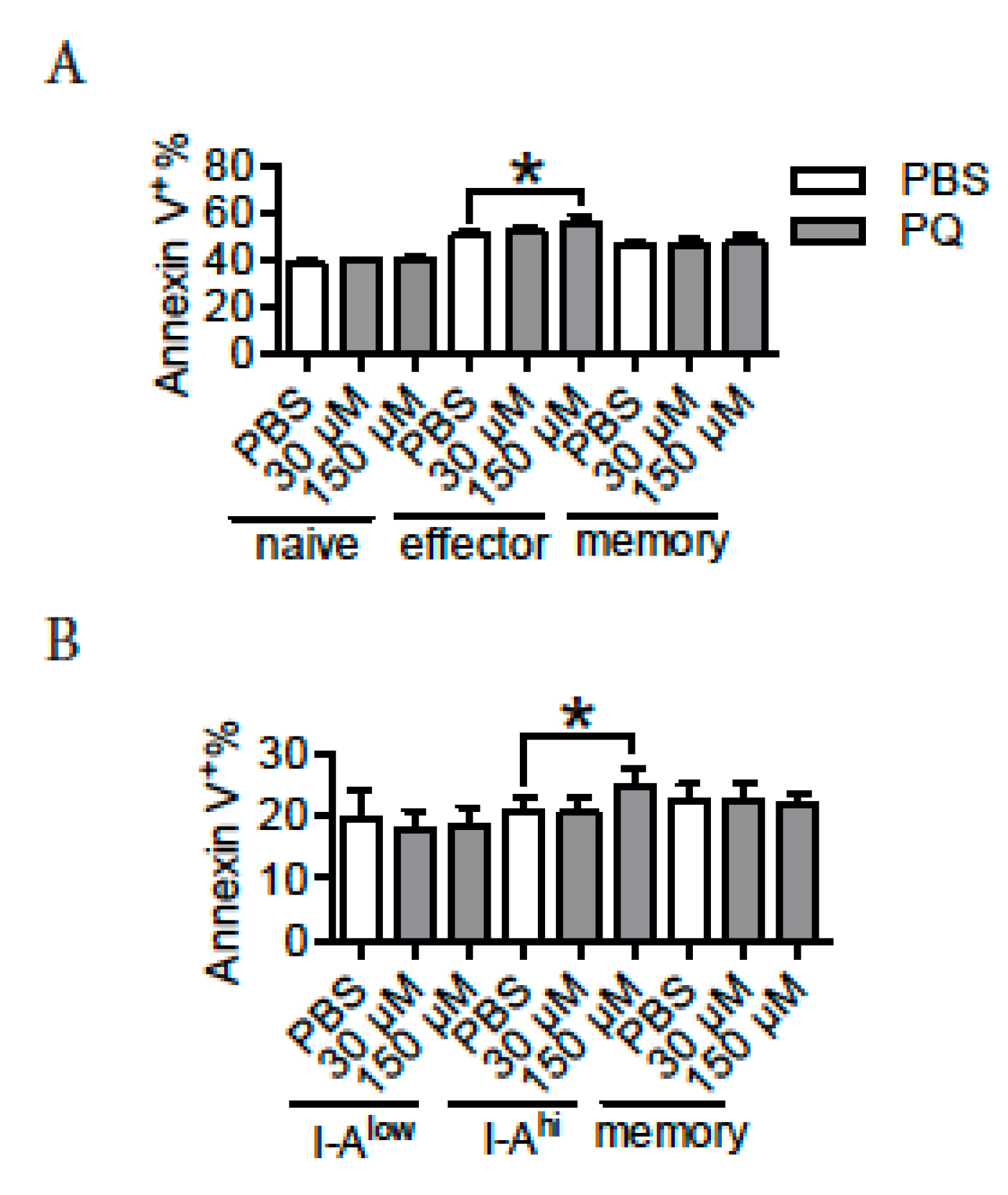
3.6. PQ Preferentially Promoted the Apoptosis of Effector CD4 T Cells and Activated B Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dinis-Oliveira, R.J.; Duarte, J.A.; Sanchez-Navarro, A.; Remiao, F.; Bastos, M.L.; Carvalho, F. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 2008, 38, 13–71. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Fukushima, T. Mechanism of cytotoxicity of paraquat. II. Organ specificity of paraquat-stimulated lipid peroxidation in the inner membrane of mitochondria. Exp. Toxicol. Pathol. 1993, 45, 375–380. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Q.; Jian, X.; Wang, H.; He, X.; Gao, B.; Wang, K.; Kan, B. A new sight for paraquat poisoning from immunology. Immunopharmacol. Immunotoxicol. 2018, 40, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Won, J.H.; Ahn, K.H.; Back, M.J.; Fu, Z.; Jang, J.M.; Ha, H.C.; Jang, Y.J.; Kim, D.K. Paraquat reduces natural killer cell activity via metallothionein induction. J. Immunotoxicol. 2015, 12, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ye, Y.; Lv, L.; Zhu, C.; Ye, S. FTY720 attenuates paraquat-induced lung injury in mice. Int. Immunopharmacol. 2014, 21, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, L.; Chen, L.; Lu, X.; Zhu, C. Increased toll-like receptor 9 expression is associated with the severity of paraquat-induced lung injury in mice. Hum. Exp. Toxicol. 2015, 34, 430–438. [Google Scholar] [CrossRef]
- Harchegani, A.L.; Hemmati, A.A.; Nili-Ahmadabadi, A.; Darabi, B.; Shabib, S. Cromolyn Sodium Attenuates Paraquat-Induced Lung Injury by Modulation of Proinflammatory Cytokines. Drug Res. 2017, 67, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Wang, Y.; Zhao, M. NLRP3 inflammasome activation regulated by NF-kappaB and DAPK contributed to paraquat-induced acute kidney injury. Immunol. Res. 2017, 65, 687–698. [Google Scholar] [CrossRef]
- Junbo, Z.; Yongtao, Y.; Hongbo, L.; Fenshuang, Z.; Ruyun, L.; Chun’ai, Y. Experimental study of sucralfate intervention for paraquat poisoning in rats. Environ. Toxicol. Pharmacol. 2017, 53, 57–63. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, Y.T.; Hung, D.Z.; Su, C.C.; Chen, K.L. Paraquat induces lung alveolar epithelial cell apoptosis via Nrf-2-regulated mitochondrial dysfunction and ER stress. Arch. Toxicol. 2012, 86, 1547–1558. [Google Scholar] [CrossRef]
- Chang, X.; Lu, W.; Dou, T.; Wang, X.; Lou, D.; Sun, X.; Zhou, Z. Paraquat inhibits cell viability via enhanced oxidative stress and apoptosis in human neural progenitor cells. Chem. Biol. Interact. 2013, 206, 248–255. [Google Scholar] [CrossRef]
- Toygar, M.; Aydin, I.; Agilli, M.; Aydin, F.N.; Oztosun, M.; Gul, H.; Macit, E.; Karslioglu, Y.; Topal, T.; Uysal, B.; et al. The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum. Exp. Toxicol. 2015, 34, 198–204. [Google Scholar] [CrossRef]
- Hu, X.; Shen, H.; Wang, Y.; Zhang, L.; Zhao, M. Aspirin-triggered resolvin D1 alleviates paraquat-induced acute lung injury in mice. Life Sci. 2019, 218, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Riahi, B.; Rafatpanah, H.; Mahmoudi, M.; Memar, B.; Fakhr, A.; Tabasi, N.; Karimi, G. Evaluation of suppressive effects of paraquat on innate immunity in Balb/c mice. J. Immunotoxicol. 2011, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Nishimoto, S.; Sugahara, T.; Akiyama, K.; Kakinuma, Y. Oral administration of paraquat perturbs immunoglobulin productivity in mouse. J. Toxicol. Sci. 2010, 35, 257–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Li, Y.; Niu, D.; Li, Y.; Li, X. Immunological effects of paraquat on common carp, Cyprinus carpio L. Fish Shellfish Immunol. 2014, 37, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Wu, M.; Zhang, C.; Che, Y.; Li, W.; Li, X. Serum immune responses in common carp (Cyprinus carpio L.) to paraquat exposure: The traditional parameters and circulating microRNAs. Fish Shellfish Immunol. 2018, 76, 133–142. [Google Scholar] [CrossRef]
- Riahi, B.; Rafatpanah, H.; Mahmoudi, M.; Memar, B.; Brook, A.; Tabasi, N.; Karimi, G. Immunotoxicity of paraquat after subacute exposure to mice. Food Chem. Toxicol. 2010, 48, 1627–1631. [Google Scholar] [CrossRef]
- Hassuneh, M.R.; Albini, M.A.; Talib, W.H. Immunotoxicity induced by acute subtoxic doses of paraquat herbicide: Implication of shifting cytokine gene expression toward T-helper (T(H))-17 phenotype. Chem. Res. Toxicol. 2012, 25, 2112–2116. [Google Scholar] [CrossRef]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef]
- Schluns, K.S.; Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 2003, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2011, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shao, C.; Wu, Q.; Wu, Q.; Huang, M.; Zhou, Z. Pyrrolidine dithiocarbamate attenuates paraquat-induced lung injury in rats. J. Biomed. Biotechnol. 2009, 2009, 619487. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lou, D.; Cai, Q.; Chang, X.; Wang, X.; Zhou, Z. Characterization of paraquat-induced miRNA profiling response in hNPCs undergoing proliferation. Int. J. Mol. Sci. 2014, 15, 18422–18436. [Google Scholar] [CrossRef]
- Dou, T.; Yan, M.; Wang, X.; Lu, W.; Zhao, L.; Lou, D.; Wu, C.; Chang, X.; Zhou, Z. Nrf2/ARE Pathway Involved in Oxidative Stress Induced by Paraquat in Human Neural Progenitor Cells. Oxid. Med. Cell. Longev. 2016, 2016, 8923860. [Google Scholar] [CrossRef]
- Lou, D.; Wang, Q.; Huang, M.; Zhou, Z. Does age matter? Comparison of neurobehavioral effects of paraquat exposure on postnatal and adult C57BL/6 mice. Toxicol. Mech. Methods 2016, 26, 667–673. [Google Scholar] [CrossRef]
- Huang, M.; Yang, H.; Zhu, L.; Li, H.; Zhou, J.; Zhou, Z. Inhibition of connective tissue growth factor attenuates paraquat-induced lung fibrosis in a human MRC-5 cell line. Environ. Toxicol. 2016, 31, 1620–1626. [Google Scholar] [CrossRef]
- Ortiz, M.S.; Forti, K.M.; Suarez Martinez, E.B.; Munoz, L.G.; Husain, K.; Muniz, W.H. Effects of Antioxidant N-acetylcysteine Against Paraquat-Induced Oxidative Stress in Vital Tissues of Mice. Int. J. Sci. Basic Appl. Res. 2016, 26, 26–46. [Google Scholar]
- Javad-Mousavi, S.A.; Hemmati, A.A.; Mehrzadi, S.; Hosseinzadeh, A.; Houshmand, G.; Rashidi Nooshabadi, M.R.; Mehrabani, M.; Goudarzi, M. Protective effect of Berberis vulgaris fruit extract against Paraquat-induced pulmonary fibrosis in rats. Biomed. Pharmacother. 2016, 81, 329–336. [Google Scholar] [CrossRef]
- Heo, Y.; Zhang, Y.; Gao, D.; Miller, V.M.; Lawrence, D.A. Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE 2011, 6, e20912. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, Y.; Li, Q.; Shao, Y.; Yu, X.; Cong, W.; Jia, X.; Qu, W.; Cheng, L.; Xue, P.; et al. Developmental exposure to mercury chloride impairs social behavior in male offspring dependent on genetic background and maternal autoimmune environment. Toxicol. Appl. Pharmacol. 2019, 370, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kil, L.P.; de Bruijn, M.J.; van Nimwegen, M.; Corneth, O.B.; van Hamburg, J.P.; Dingjan, G.M.; Thaiss, F.; Rimmelzwaan, G.F.; Elewaut, D.; Delsing, D.; et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 2012, 119, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Bemark, M.; Hazanov, H.; Stromberg, A.; Komban, R.; Holmqvist, J.; Koster, S.; Mattsson, J.; Sikora, P.; Mehr, R.; Lycke, N.Y. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat. Commun. 2016, 7, 12698. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, C.; Meyer, T.; Durek, P.; Maul, J.; Preiss, J.; Jacobs, J.F.M.; Thiel, A.; Radbruch, A.; Ullrich, R.; Dorner, T. Simultaneous Presence of Non- and Highly Mutated Keyhole Limpet Hemocyanin (KLH)-Specific Plasmablasts Early after Primary KLH Immunization Suggests Cross-Reactive Memory B Cell Activation. J. Immunol. 2018, 200, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, Z.; Zhang, P.; Zhao, Y.; Yu, X.; Xue, P.; Shao, Y.; Li, Q.; Jia, X.; Zhang, Q.; et al. Mercury impact on hematopoietic stem cells is regulated by IFNgamma-dependent bone marrow-resident macrophages in mice. Toxicol. Lett. 2018, 295, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front. Immunol. 2013, 4, 82. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- Jang, Y.J.; Won, J.H.; Back, M.J.; Fu, Z.; Jang, J.M.; Ha, H.C.; Hong, S.; Chang, M.; Kim, D.K. Paraquat Induces Apoptosis through a Mitochondria-Dependent Pathway in RAW264.7 Cells. Biomol. Ther. 2015, 23, 407–413. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, J.; Tai, W.; Deng, S.; Li, T.; Wu, W.; Pu, L.; Fan, D.; Lei, W.; Zhang, T.; et al. High-Dose Paraquat Induces Human Bronchial 16HBE Cell Death and Aggravates Acute Lung Intoxication in Mice by Regulating Keap1/p65/Nrf2 Signal Pathway. Inflammation 2019, 42, 471–484. [Google Scholar] [CrossRef]
- Hu, X.; Chen, L.; Li, T.; Zhao, M. TLR3 is involved in paraquat-induced acute renal injury. Life Sci. 2019, 223, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Ayala, T.; Anderica-Romero, A.C.; Pedraza-Chaverri, J. New insights into antioxidant strategies against paraquat toxicity. Free Radic. Res. 2014, 48, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol. 2018, 39, 489–502. [Google Scholar] [CrossRef]
- Zhan, Y.; Carrington, E.M.; Zhang, Y.; Heinzel, S.; Lew, A.M. Life and Death of Activated T Cells: How Are They Different from Naive T Cells? Front. Immunol. 2017, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Polo, R.A.; Niso-Santano, M.; Ortiz-Ortiz, M.A.; Gomez-Martin, A.; Moran, J.M.; Garcia-Rubio, L.; Francisco-Morcillo, J.; Zaragoza, C.; Soler, G.; Fuentes, J.M. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2007, 97, 448–458. [Google Scholar] [CrossRef] [PubMed]
| Cells | Organs Treatments | LN | Spleen | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS | 2 mg/kg PQ | 20 mg/kg PQ | PBS | 2 mg/kg PQ | 20 mg/kg PQ | ||||||||
| CFA | KLH | CFA | KLH | CFA | KLH | CFA | KLH | CFA | KLH | CFA | KLH | ||
| CD4 T cells | Ki67 (%) | 24.1 ± 5.7 | 39.3 ± 1.9 * | 27.7 ± 3.3 | 39.2 ± 2.9 * | 26.0 ± 3.4 | 39.0 ± 3.0 * | 19.1 ± 2.0 | 26.3 ± 1.2 * | 20.4 ± 1.2 | 27.2 ± 1.9 * | 20.4 ± 1.2 | 26.8 ± 0.9 * |
| OX40 (%) | 18.5 ± 1.6 | 21.8 ± 1.1 * | 19.4 ± 0.9 | 22.5 ± 2.6 * | 19.0 ± 0.6 | 22.2 ± 1.3 * | 10.2 ± 0.9 | 12.2 ± 0.7 * | 9.7 ± 1.1 | 12.4 ± 0.6 * | 10.4 ± 1.1 | 12.3 ± 0.5 * | |
| ICOS (%) | 12.5 ± 1.1 | 25.3 ± 2.7 * | 12.6 ± 1.2 | 24.3 ± 2.7 * | 13.4 ± 1.4 | 26.2 ± 4.1 * | 11.5 ± 0.8 | 14.7 ± 1.0 * | 11.7 ± 0.5 | 14.4 ± 0.8 * | 11.3 ± 1.1 | 14.6 ± 0.8 * | |
| CD154 (%) | 4.5 ± 0.9 | 7.5 ± 2.1 * | 4.8 ± 0.4 | 6.9 ± 1.1 * | 5.1 ± 0.6 | 7.5 ± 1.0 * | 3.3 ± 0.9 | 5.3 ± 0.6 * | 3.6 ± 0.4 | 4.8 ± 0.4 * | 3.7 ± 0.5 | 5.1 ± 0.5 * | |
| B cells | Ki67 (%) | 13.8 ± 1.1 | 16.9 ± 0.9 * | 13.7 ± 1.5 | 16.5 ± 0.5 * | 13.7 ± 1.1 | 17.1 ± 0.7 * | 20.8 ± 2.4 | 26.0 ± 1.4 * | 21.2 ± 1.5 | 25.6 ± 1.2 * | 21.0 ± 2.0 | 26.3 ± 1.0 * |
| I-A (MFI) | 5529 ± 646 | 8621 ± 1209 * | 4899 ± 847 | 7728 ± 794 * | 5683 ± 512 | 7747 ± 693 * | 1263 ± 121 | 1708 ± 144 * | 1151 ± 161 | 1677 ± 79 * | 1106 ± 98 | 1791 ± 166 * | |
| CD40 (MFI) | 1146 ± 202 | 1558 ± 144 * | 952 ± 136 | 1598 ± 163 * | 1035 ± 175 | 1519 ± 113 * | 978 ± 204 | 1403 ± 105 * | 915 ± 213 | 1397 ± 150 * | 990 ± 169 | 1365 ± 86 * | |
| DC | I-A (MFI) | 7191 ± 681 | 9705 ± 564 * | 7393 ± 519 | 9357 ± 411 * | 6882 ± 521 | 9051 ± 444 * | 1413 ± 157 | 2024 ± 116 * | 1423 ± 130 | 1968 ± 168 * | 1401 ± 138 | 1947 ± 75 * |
| CD80 (MFI) | 6507 ± 1065 | 8816 ± 476 * | 6788 ± 918 | 8418 ± 635 * | 6799 ± 625 | 8900 ± 626 * | 4943 ± 337 | 5654 ± 271 * | 4861 ± 364 | 5768 ± 239 * | 5142 ± 258 | 6058 ± 589 * | |
| CD40 (MFI) | 1142 ± 105 | 1545 ± 111 * | 1030 ± 185 | 1637 ± 145 * | 1061 ± 200 | 1483 ± 100 * | 695 ± 124 | 1004 ± 157 * | 736 ± 109 | 1125 ± 215 * | 754 ± 151 | 1032 ± 109 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Zhao, Y.; Zhu, T.; Zhang, F.; Chang, X.; Zhang, Y.; Zhou, Z. Paraquat Preferentially Induces Apoptosis of Late Stage Effector Lymphocyte and Impairs Memory Immune Response in Mice. Int. J. Environ. Res. Public Health 2019, 16, 2060. https://doi.org/10.3390/ijerph16112060
Shao Y, Zhao Y, Zhu T, Zhang F, Chang X, Zhang Y, Zhou Z. Paraquat Preferentially Induces Apoptosis of Late Stage Effector Lymphocyte and Impairs Memory Immune Response in Mice. International Journal of Environmental Research and Public Health. 2019; 16(11):2060. https://doi.org/10.3390/ijerph16112060
Chicago/Turabian StyleShao, Yiming, Yifan Zhao, Tingting Zhu, Fen Zhang, Xiuli Chang, Yubin Zhang, and Zhijun Zhou. 2019. "Paraquat Preferentially Induces Apoptosis of Late Stage Effector Lymphocyte and Impairs Memory Immune Response in Mice" International Journal of Environmental Research and Public Health 16, no. 11: 2060. https://doi.org/10.3390/ijerph16112060
APA StyleShao, Y., Zhao, Y., Zhu, T., Zhang, F., Chang, X., Zhang, Y., & Zhou, Z. (2019). Paraquat Preferentially Induces Apoptosis of Late Stage Effector Lymphocyte and Impairs Memory Immune Response in Mice. International Journal of Environmental Research and Public Health, 16(11), 2060. https://doi.org/10.3390/ijerph16112060





