Modelling of Indicator Escherichia coli Contamination in Sentinel Oysters and Estuarine Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Environmental Data Collection
2.3. Enumeration of Total Coliforms, Fecal Coliforms, E. coli and V. parahaemolyticus
2.4. Determination of Salmonella spp. and Shigella spp.
2.5. Determination of Heavy Metals
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, L.; He, B.; Jiang, G.; Chen, D.; Yao, Z. Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci. Total Environ. 2004, 324, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Brands, D.A.; Inman, A.E.; Gerba, C.P.; Maré, C.J.; Billington, S.J.; Saif, L.A.; Levine, J.F.; Joens, L.A. Prevalence of Salmonella spp. in Oysters in the United States. Appl. Environ. Microbiol. 2005, 71, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, R.S.; Chu, E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit. Rev. Microbiol. 2016, 42, 548–572. [Google Scholar] [CrossRef]
- McLaughlin, J.B.; DePaola, A.; Bopp, C.A.; Martinek, K.A.; Napolilli, N.P.; Allison, C.G.; Murray, S.L.; Thompson, E.C.; Bird, M.M.; Middaugh, J.P. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N. Engl. J. Med. 2005, 353, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Raszl, S.M.; Froelich, B.A.; Vieira, C.R.; Blackwood, A.D.; Noble, R.T. Vibrio parahaemolyticus and Vibrio vulnificus in South America: Water, seafood and human infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Bach, L.; Arai, T. Monitoring heavy metal contamination using rocky oyster (Saccostrea glomerate) in Haiphong-Halong coastal area, North Vietnam. Int. J. Environ. Res. 2015, 9, 1373–1378. [Google Scholar]
- Muñoz Sevilla, N.P.; Villanueva-Fonseca, B.P.; Góngora-Gómez, A.M.; García-Ulloa, M.; Domínguez-Orozco, A.L.; Ortega-Izaguirre, R.; Campos Villegas, L.E. Heavy metal concentrations in diploid and triploid oysters (Crassostrea gigas) from three farms on the north-central coast of Sinaloa, Mexico. Environ. Monit. Assess. 2017, 189, 536. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.I.; Vieira, R.H.; Menezes, F.G.; Fonteles-Filho, A.A.; Torres, R.C.; Sant’Anna, E.S. Bacteria of fecal origin in mangrove oysters (Crassostrea rhizophorae) in the Cocó River estuary, Ceará State, Brazil. Braz. J. Microbiol. 2004, 35, 126–130. [Google Scholar] [CrossRef]
- Jozić, S.; Šolić, M.; Krstulović, N. The accumulation of the indicator bacteria Escherichia coli in mussels (Mytilus galloprovincialis) and oysters (Ostrea edulis) under experimental conditions. Acta. Adriatica. 2012, 53, 353–361. [Google Scholar]
- Forcelini, H.C.D.L.; Kolm, H.E.; Absher, T.M. Escherichia coli in the surface waters and in oysters of two cultivations of Guaratuba Bay-Paraná-Brazil. Braz. Arch. Biol. Technol. 2013, 56, 319–326. [Google Scholar] [CrossRef]
- Haendiges, J.; Jones, J.; Myers, R.A.; Mitchell, C.S.; Butler, E.; Toro, M.; Gonzalez-Escalona, N. A nonautochthonous US strain of Vibrio parahaemolyticus isolated from Chesapeake Bay oysters caused the outbreak in Maryland in 2010. Appl. Environ. Microbiol. 2016, 82, 3208–3216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tirado, M.; Clarke, R.; Jaykus, L.; McQuatters-Gollop, A.; Frank, J. Climate change and food safety: A review. Food Res. Int. 2010, 43, 1745–1765. [Google Scholar] [CrossRef]
- Garreis, M.J. Sanitary Surveys of Growing Waters. In Environmental Indicators and Shellfish Safety; Hackney, C.R., Pierson, M.D., Eds.; Chapman and Hall: New York, NY, USA, 1994; pp. 289–330. [Google Scholar]
- Commission Regulation (EC) No. 2073/2005 on Microbiological Criteria for Foodstuffs. European Commission 2005. Available online: https://eurlex.europa.eu/eli/reg/2005/2073/oj (accessed on 1 April 2018).
- U.S. Food and Drug Administration (FDA). National Shellfish Sanitation Program (NSSP), Guide for the Control of Molluscan Shellfish. Available online: https://www.fda.gov/food/guidanceregulation/federalstatefoodprograms/ucm2006754.htm (accessed on 31 January 2019).
- Ministry for Primary Industries, New Zealand Government Animal Products Notice (Specifications for Bivalve Molluscan Shellfish for Human Consumption) 2006. Available online: https://www.mpi.govt.nz/dmsdocument/20999/send (accessed on 31 April 2019).
- Jeamsripong, S.; Chuanchuen, R.; Atwill, E.R. Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand. Int. J. Environ. Res. Public Health 2018, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Weagant, S.D.; Grant, M.A.; Burkhardt, W.; Shellfish, M.; Water, B. BAM: Enumeration of Escherichia coli and the Coliform Bacteria. Bacteriological Analytical Manual (BAM), 2002. Available online: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm064948.htm (accessed on 29 March 2019).
- Kaysner, C.A.; DePaola, A. Bacteriological Analytical Manual (BAM): Vibrio. 2004. Available online: https:// www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm070830.htm (accessed on 31 March 2019).
- Andrews, W.H.; Jacobson, A. BAM: Shigella. Available online: https://www.fda.gov/food/ foodscienceresearch/laboratorymethods/ucm070789.htm (accessed on 31 January 2018).
- Andrews, W.H.; Wang, H.; Jacobson, A.; Hammack, T. BAM: Salmonella. 2007. Available online: https:// www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm (accessed on 1 January 2019).
- Grimont, P.A.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; WHO Collaborating Centre for Reference and Research on Salmonella: Paris, France, 2007. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; AOAC: Washington, DC, USA, 1975. [Google Scholar]
- Scopel, C.O.; Harris, J.; McLellan, S.L. Influence of Nearshore Water Dynamics and Pollution Sources on Beach Monitoring Outcomes at Two Adjacent Lake Michigan Beaches. J. Great Lakes Res. 2006, 32, 543–552. [Google Scholar] [CrossRef]
- Lunestad, B.T.; Frantzen, S.; Svanevik, C.S.; Roiha, I.S.; Duinker, A. Time trends in the prevalence of Escherichia coli and enterococci in bivalves harvested in Norway during 2007–2012. Food Control. 2016, 60, 289–295. [Google Scholar] [CrossRef][Green Version]
- Cann, K.F.; Thomas, D.R.; Salmon, R.L.; Wyn-Jones, A.P.; Kay, D. Extreme water-related weather events and waterborne disease. Epidemiol. Infect. 2013, 141, 671–686. [Google Scholar] [CrossRef]
- Nichols, G.; Lane, C.; Asgari, N.; Verlander, N.Q.; Charlett, A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J. Water Health 2009, 7, 1–8. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Pachepsky, Y.; Hill, R.L.; Shelton, D.R.; Whelan, G. Escherichia coli survival in waters: Temperature dependence. Water Res. 2013, 47, 569–578. [Google Scholar] [CrossRef]
- Campos, C.J.; Cachola, R.A. Faecal coliforms in bivalve harvesting areas of the Alvor lagoon (Southern Portugal): Influence of seasonal variability and urban development. Environ. Monit. Assess. 2007, 133, 31–41. [Google Scholar] [CrossRef]
- Atidégla, S.C.; Huat, J.; Agbossou, E.K.; Saint-Macary, H.; Glèlè Kakai, R. Vegetable Contamination by the Fecal Bacteria of Poultry Manure: Case Study of Gardening Sites in Southern Benin. Int. J. Food Sci. Nutr. 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mritunjay, S.K.; Kumar, V. A study on prevalence of microbial contamination on the surface of raw salad vegetables. Biotech 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.; Pedersen, G.K. Jensen’s Operator Inequality. Bull. London Math. Soc. 2003, 35, 553–564. [Google Scholar] [CrossRef]
- Chase, J.A.; Atwill, E.R.; Partyka, M.L.; Bond, R.F.; Oryang, D. Inactivation of Escherichia coli O157:H7 on romaine lettuce when inoculated in a fecal slurry matrix. J. Food Prot. 2017, 80, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, X.; Carabez, J.; Ragosta, G.; Fernandez, K.L.; Wang, E.; Thiptara, A.; Antaki, E.; Atwill, E.R. Cross-sectional survey of indicator and pathogenic bacteria on vegetables sold from Asian vendors at farmers’ markets in northern California. J. Food Prot. 2015, 78, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Navratil, S.; Gregory, A.; Bauer, A.; Srinath, I.; Szonyi, B.; Nightingale, K.; Anciso, J.; Jun, M.; Han, D.; et al. Multifactorial effects of ambient temperature, precipitation, farm management, and environmental factors determine the level of generic Escherichia coli contamination on preharvested spinach. Appl. Environ. Microbiol. 2015, 81, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; McDonald, M.; Lundberg, D.S.; Dangl, J.L.; Jojic, V. Learning microbial interaction networks from metagenomic count data. J. Comput. Biol. 2016, 23, 526–535. [Google Scholar] [CrossRef]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef]
- Zhang, X.; Mallick, H.; Tang, Z.; Zhang, L.; Cui, X.; Benson, A.K.; Yi, N. Negative binomial mixed models for analyzing microbiome count data. BMC Bioinformat. 2017, 18, 4. [Google Scholar] [CrossRef]
- Mok, J.S.; Lee, T.S.; Kim, P.H.; Lee, H.J.; Ha, K.S.; Shim, K.B.; Lee, K.J.; Jung, Y.J.; Kim, J.H. Bacteriological quality evaluation of seawater and oysters from the Hansan-Geojeman area in Korea, 2011–2013: Impact of inland pollution sources. Springerplus 2016, 5, 1412. [Google Scholar] [CrossRef]
- Hood, M.A.; Ness, G.E.; Blake, N.J. Relationship among fecal coliforms, Escherichia coli, and Salmonella spp. in shellfish. Appl. Environ. Microbiol. 1983, 45, 122–126. [Google Scholar] [PubMed]

| Parameter | Med | SD | Min | Max | Pooled Oyster (p-Value 1) | Estuarine Water (p-Value 1) | ||
|---|---|---|---|---|---|---|---|---|
| Model A 2 | Model B 3 | Model C 2 | Model D 3 | |||||
| 1-day environmental data | ||||||||
| Maximum wind gust (m/s) | 6.2 | 2.4 | 5.2 | 12.9 | 0.001 | <0.0001 | 0.968 | 0.313 |
| Current wind speed (m/s) | 0.8 | 0.4 | 0.0 | 1.5 | 0.002 | 0.066 | 0.713 | 0.383 |
| Precipitation (mm) | 2.4 | 28.8 | 0.0 | 87.8 | <0.0001 | 0.001 | <0.0001 | <0.0001 |
| Temperature (°C) | 28.0 | 1.5 | 25.2 | 29.7 | 0.020 | <0.0001 | 0.008 | <0.0001 |
| Relative humidity (%) | 83.0 | 7.8 | 74.0 | 97.0 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 7-day environmental data | ||||||||
| Maximum wind gust (m/s) | 6.6 | 1.9 | 5.6 | 11.4 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Current wind speed (m/s) | 1.0 | 0.4 | 0.6 | 1.8 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Precipitation (mm) | 19.1 | 24.4 | 0.0 | 79.8 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Temperature (°C) | 27.3 | 1.0 | 26.1 | 29.6 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Relative humidity (%) | 86.1 | 4.9 | 78.3 | 92.7 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Parameter | Pooled Oyster (p-Value 1) | Estuarine Water (p-Value 1) | ||
|---|---|---|---|---|
| Model A 2 | Model B 3 | Model C 2 | Model D 3 | |
| Concentrations of bacteria 4 | ||||
| TC concentration | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| FC concentration | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| VP concentration | 0.224 | 0.116 | 0.012 | 0.650 |
| The present of Salmonella | ||||
| No (reference) | - | - | - | - |
| Yes | <0.0001 | <0.0001 | * | * |
| The present of Shigella | ||||
| No (reference) | - | - | - | - |
| Yes | <0.0001 | 0.006 | <0.0001 | <0.0001 |
| Heavy metal (ppm) | ||||
| Mn | 0.020 | 0.002 | 0.729 | 0.343 |
| Cd | 0.393 | 0.718 | <0.0001 | <0.0001 |
| Pb | <0.0001 | 0.382 | <0.0001 | <0.0001 |
| Factor | Coefficient | 95% CI 1 | p-Value 1 |
|---|---|---|---|
| Model A: No log-transformation | |||
| Intercept | 16.11 | 7.38 to 24.83 | <0.0001 |
| Precipitation prior 7 days (mm) | 2.29 × 10−2 | 1.36 × 10−3 to 3.22 × 10−4 | <0.0001 |
| Temperature (°C) | −0.28 | −0.54 to −0.02 | 0.036 |
| Salmonella present in sample | |||
| No | 0.0 | - | - |
| Yes | 0.62 | 0.01 to 1.23 | 0.046 |
| Model B: Log-transformation | |||
| Intercept | 7.36 | 5.04 to 9.67 | <0.0001 |
| Precipitation prior 7 days (mm) | 1.56 × 10−2 | 8.72 × 10−3 to 2.26 × 10−2 | <0.0001 |
| Temperature (°C) | −0.16 | −0.23 to −0.09 | <0.0001 |
| Salmonella present in sample | |||
| No | 0.0 | - | - |
| Yes | 0.42 | 0.07 to 0.78 | 0.019 |
| Factor | Coefficient | 95% CI 1 | p-Value 1 |
|---|---|---|---|
| Model C: No-log transformation | |||
| Intercept | 6.40 | 3.30 to 9.49 | <0.0001 |
| Concentration of FC (MPN/100 mL) | 8.75 × 10−4 | 5.88 × 10−4 to 1.16 × 10−3 | <0.0001 |
| Precipitation prior 7 days (mm) | 2.81 × 10−2 | 2.28 × 10−2 to 3.33 × 10−2 | 0.030 |
| Temperature (°C) | −0.11 | −0.20 to −0.01 | <0.0001 |
| Model D: Log transformation | |||
| Intercept | 2.95 | 0.89 to 5.01 | 0.005 |
| Concentration of FC (logMPN/100 mL) | 2.43 × 10−4 | 1.47 × 10−4 to 3.40 × 10−4 | <0.0001 |
| Precipitation prior 7 days (mm) | 1.99 × 10−2 | 1.45 × 10−2 to 2.53 × 10−2 | <0.0001 |
| Temperature (°C) | −0.07 | −0.13 to −2.63 × 10−2 | 0.041 |
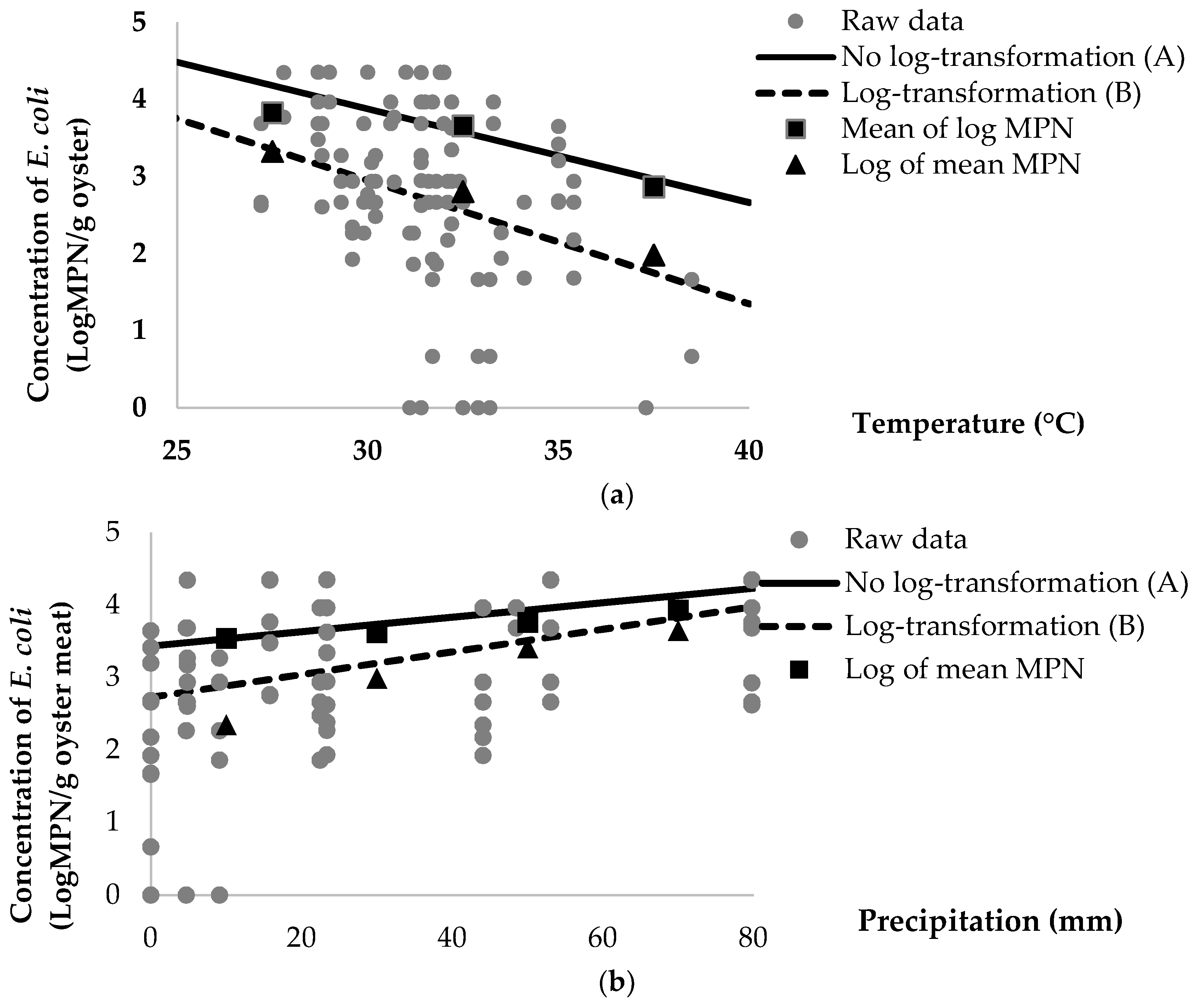
| Sample | Parameter | Number of Sample (%) | Mean of Log (MPN/g or MPN/100 mL) | Log of Mean (MPN/g or MPN/100 mL) | Log Difference 1 |
|---|---|---|---|---|---|
| Pooled oyster | Temperature | ||||
| 25.0–29.9 | 36 (25.0%) | 3.82 | 3.32 | 0.50 | |
| 30.0–34.9 | 90 (62.5%) | 3.65 | 2.80 | 0.85 | |
| 35.0–39.9 | 18 (12.5%) | 2.86 | 1.98 | 0.88 | |
| Total | 144 (100%) | 3.66 | 2.83 | 0.83 | |
| Estuarine water | Temperature | ||||
| 25.0–29.9 | 20 (20.8%) | 2.63 | 1.59 | 1.04 | |
| 30.0–34.9 | 68 (70.8%) | 2.25 | 1.52 | 0.73 | |
| 35.0–39.9 | 8 (8.30%) | 1.32 | 0.64 | 0.68 | |
| Total | 96 (100%) | 2.34 | 1.46 | 0.88 | |
| Pooled oyster | Precipitation | ||||
| 0–19.9 | 72 (50.0%) | 3.54 | 2.35 | 1.19 | |
| 20.0–39.9 | 24 (16.7%) | 3.62 | 2.99 | 0.63 | |
| 40.0–59.9 | 36 (25.0%) | 3.76 | 3.41 | 0.35 | |
| 60.0–79.9 | 12 (8.3%) | 3.93 | 3.65 | 0.28 | |
| Total | 144 (100%) | 3.66 | 2.83 | 0.83 | |
| Estuarine water | Precipitation | ||||
| 0–19.9 | 48 (50.0%) | 1.38 | 0.88 | 0.50 | |
| 20.0–39.9 | 16 (16.7%) | 1.84 | 1.64 | 0.20 | |
| 40.0–59.9 | 24 (25.0%) | 2.84 | 2.24 | 0.60 | |
| 60.0–79.9 | 8 (8.3%) | 2.37 | 2.31 | 0.06 | |
| Total | 96 (100%) | 2.34 | 1.46 | 0.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeamsripong, S.; Atwill, E.R. Modelling of Indicator Escherichia coli Contamination in Sentinel Oysters and Estuarine Water. Int. J. Environ. Res. Public Health 2019, 16, 1971. https://doi.org/10.3390/ijerph16111971
Jeamsripong S, Atwill ER. Modelling of Indicator Escherichia coli Contamination in Sentinel Oysters and Estuarine Water. International Journal of Environmental Research and Public Health. 2019; 16(11):1971. https://doi.org/10.3390/ijerph16111971
Chicago/Turabian StyleJeamsripong, Saharuetai, and Edward R. Atwill. 2019. "Modelling of Indicator Escherichia coli Contamination in Sentinel Oysters and Estuarine Water" International Journal of Environmental Research and Public Health 16, no. 11: 1971. https://doi.org/10.3390/ijerph16111971
APA StyleJeamsripong, S., & Atwill, E. R. (2019). Modelling of Indicator Escherichia coli Contamination in Sentinel Oysters and Estuarine Water. International Journal of Environmental Research and Public Health, 16(11), 1971. https://doi.org/10.3390/ijerph16111971





