Acute Toxicity of Divalent Mercury Ion to Anguilla japonica from Seawater and Freshwater Aquaculture and Its Effects on Tissue Structure
Abstract
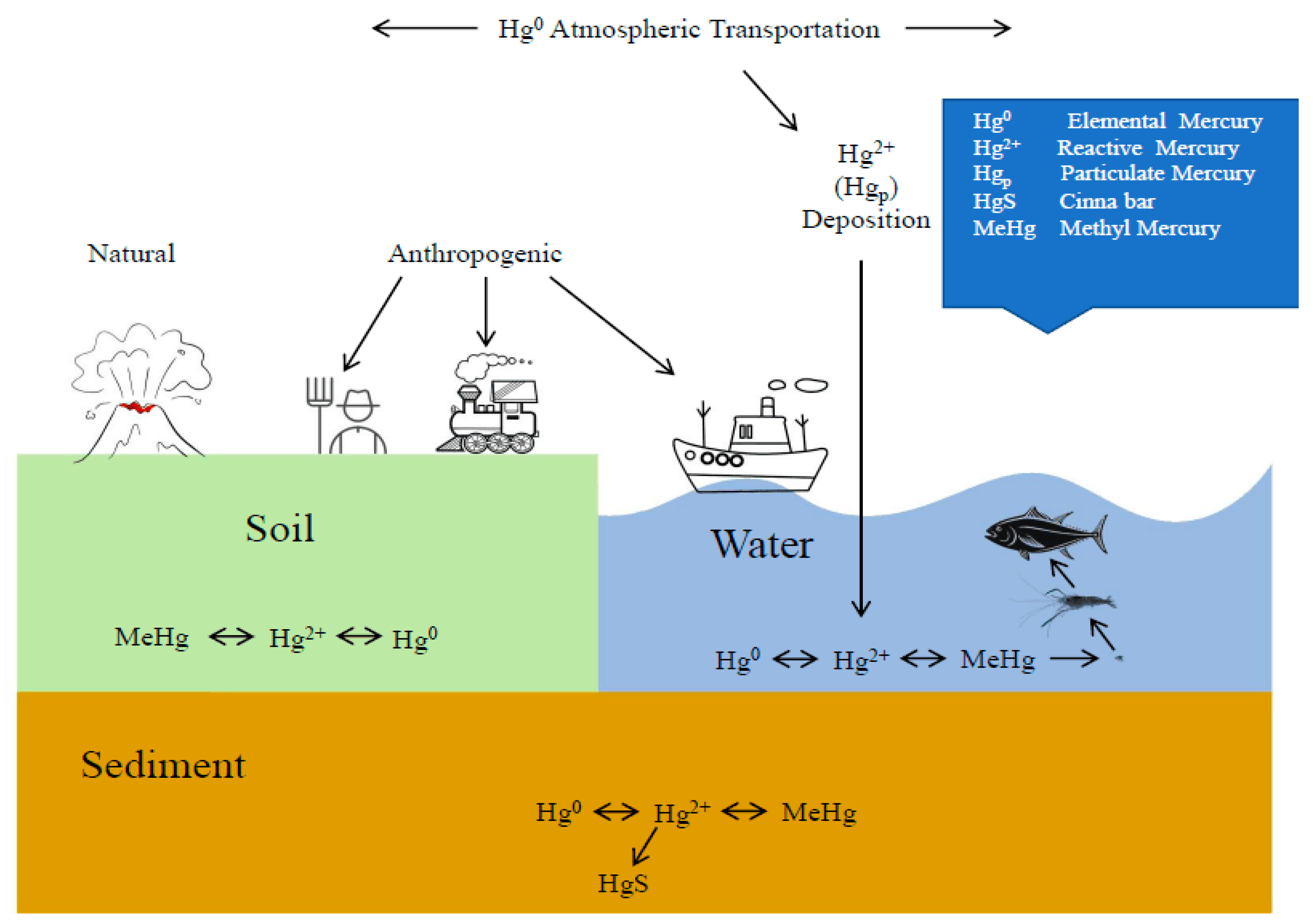
:1. Introduction
2. Materials and Method
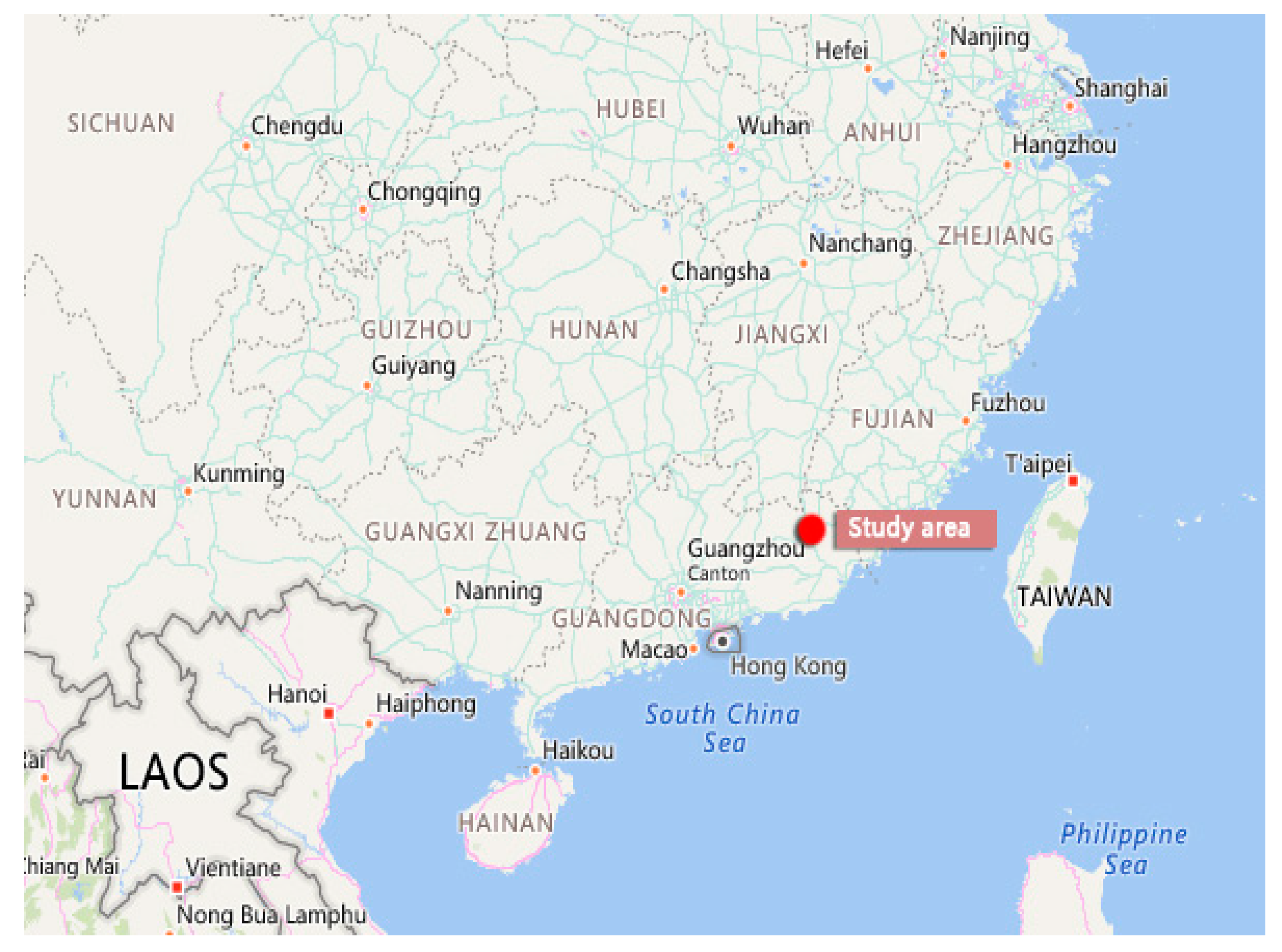
2.1. Test Animals, Experimental Conditions, and Toxic Reagent
2.2. Acute Toxicity Experiment
2.3. Preparation of Tissue Sections and Data Processing
3. Results and Analysis
3.1. Acute Toxicity of Divalent Mercury Ion to Anguilla japonica
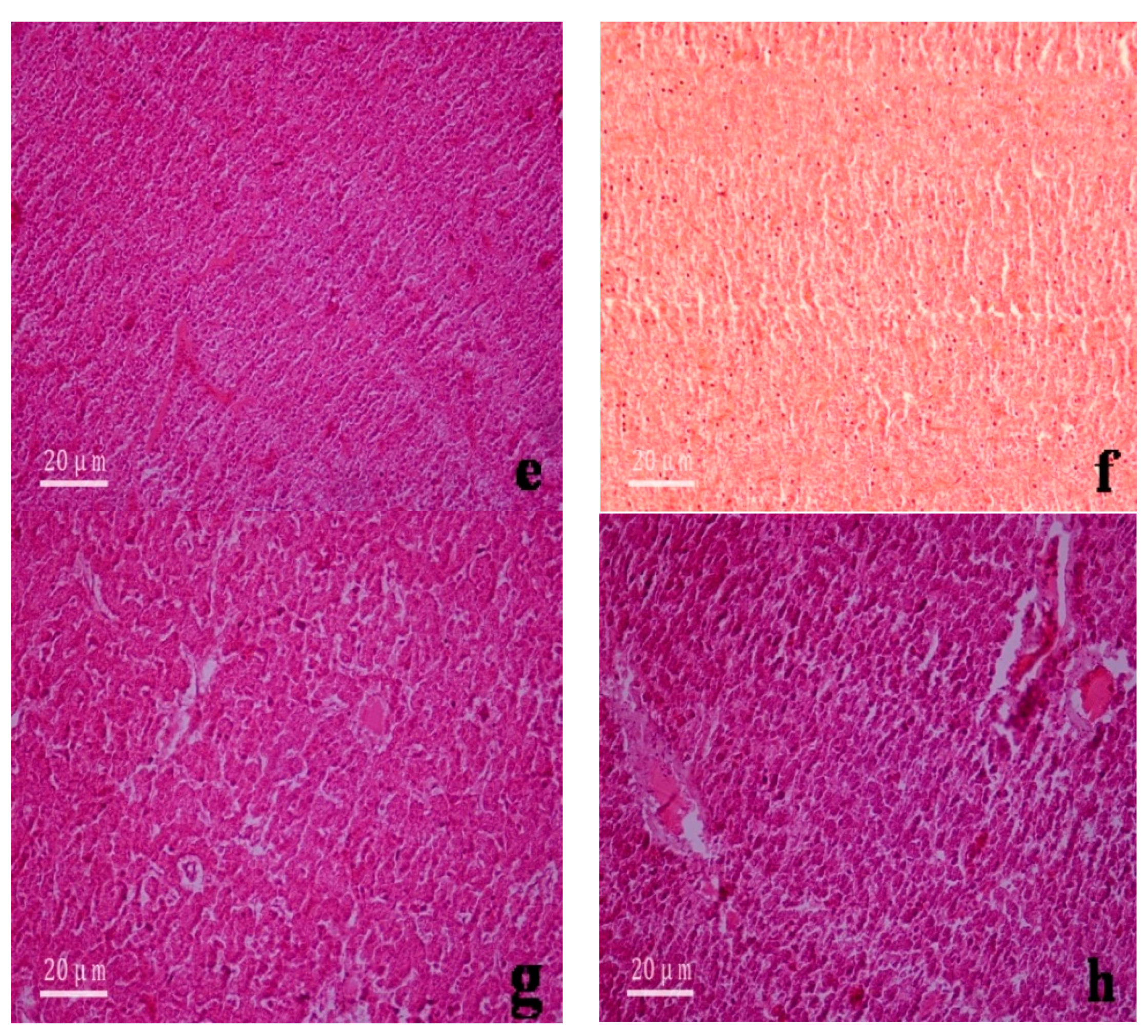
3.2. Histological Changes to the Gills and Liver of Anguilla japonica
4. Discussion
4.1. Toxicity and Median Lethal and Safety Concentrations of Divalent Mercury Ion to Anguilla japonica
4.2. Effects of Divalent Mercury Ion on the Structure of the Gill and Liver Tissue of Anguilla japonica
5. Conclusions
- The design method, process and results used in this experiment will provide some reference for the follow-up eel experiment.
- This paper has a certain reference significance for the staff engaged in aquaculture, scientific research and other fields in the treatment of aquaculture wastewater.
- This paper has a certain reference value for researchers engaged in the field of environment and food safety and managers of relevant government functional departments when they study, deal with unexpected environmental disasters and make emergency decision-making deployment.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Starr, L.D. Mercury Pollution in an Urban and Suburban Lacustrine System in Summit County, Ohio. Master’s Thesis, University of Akron, Akron, OH, USA, 2017. [Google Scholar]
- Wang, X. Theory and Practice of Water Environmental Characteristic Pollutant Screening in Watershed; China Environmental Publishing House: Beijing, China, December 2014. [Google Scholar]
- Akihiro, O.; Yohei, S.; Yoshiaki, Y.; Katsumi, T. Accumulation of hyaluronan in reared Japanese eel Anguilla japonica during early ontogeny. Aquaculture 2018, 497, 220–225. [Google Scholar] [CrossRef]
- Okamoto, O.K.; Shao, L.; Woodland Hastings, J.; Colepicolo, P. Acute and chronic effects of toxic metals on viability, encystment and bioluminescence in the dinoflagellate Gonyaulax polyedra. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 123, 75–83. [Google Scholar] [CrossRef]
- Chen, S.; Sun, T.; Zhou, Q. Interactions between microorganisms and heavy metals and their applications. J. Appl. Ecol. 2002, 13, 239–242. [Google Scholar]
- Eisler, R.; Hennekey, R.J. Acute toxicities of Cd2+, Cr+6, Hg2+, Ni2+ and Zn2+ to estuarine macrofauna. Arch. Environ. Contam. Toxicol. 1977, 6, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, B.; Gao, F. Ecological Risk Assessment and Warning of Heavy Metals in the Watershed; Chemical Industry Press: Beijing, China, January 2017. [Google Scholar]
- Zhang, X.; Shao, J.; Chen, A.; Shang, C.; Hu, X.; Luo, S.; Lei, M.; Peng, L.; Zeng, Q. Effects of cadmium on calcium homeostasis in the white-rot fungus Phanerochaete chrysosporium. Ecotoxicol. Environ. Saf. 2018, 157, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zheng, Z.; Dai, B.; Fang, J.; Li, D. Acute Toxicity of Cadmium to Allogynogenetic Silver Crucian Carp. Chin. J. Fish. 2018, 31, 36–39. [Google Scholar]
- Yang, L.; Chen, Z.; Cui, D.; Luo, X.; Liang, B.; Yang, L.; Liu, T.; Wang, A.; Luo, S. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem. Eng. J. 2019, 359, 894–901. [Google Scholar] [CrossRef]
- Zou, L.; Shao, P.; Zhang, K.; Yang, L.; You, D.; Shi, H.; Spyros, G.P.; Lai, W.; Liang, D.; Luo, X. Tannic acid-based adsorbent with superior selectivity for lead(II) capture: Adsorption site and selective mechanism. Chem. Eng. J. 2019, 364, 160–166. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Li, X.; Zhang, X.; Zhao, F.; Li, Z. Acute Toxicity of Four Common Aquatic Drugs to Young Percocypris pingi. Guizhou Agric. Sci. 2018, 46, 87–90. [Google Scholar]
- Folmar, L.C. Inhibition of rat brain ATPase PNPase and ATP-32P exchange by chlorinated-dipheny ethanes and cyclodiene insecticides. Bull. Environ. Contam. Toxicol. 1978, 20, 481–486. [Google Scholar] [CrossRef]
- Narbonne, J.F. Polychlorinated biophenyls: Effect of diet level on ATPase activity in rats. Bull. Environ. Contam. Toxicol. 1978, 20, 184–190. [Google Scholar] [CrossRef]
- Huang, W.; Cao, L.; Shan, X.; Lin, L.; Dou, S. Toxicity testing of waterborne mercury with red sea bream (Pagrus major) embryos and larvae. Bull. Environ. Contam. Toxicol. 2011, 86, 398–405. [Google Scholar] [CrossRef]
- Wang, C.F.; Fang, Z.Q. Acute toxicity of mercury and selenium to swordtails and evaluation of the safety concentrations. Environ. Sci. Technol. 2005, 28, 32–34. [Google Scholar]
- China Aquatic Society. National Aquatic Technology Promotion Station. In China Fisheries Statistics Yearbook; Fisheries and Fisheries Administration Bureau, Ministry of Agriculture and Rural Areas, Eds.; China Agricultural Press: Beijing, China, 2018. [Google Scholar]
- Guo, Y.; Liu, Y.; Zeng, G.; Hu, X.; Li, X.; Huang, D.; Liu, Y.; Yin, Y. A restoration-promoting integrated floating bed and its experimental performance in eutrophication remediation. J. Environ. Sci. 2014, 26, 1090–1098. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Zeng, G.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Li, J.; Liu, C.; et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Tan, X.; Zeng, G.; Gong, J.; Lai, C.; Niu, Q.; Tang, Y. Enhancement of Detoxification of Petroleum Hydrocarbons and Heavy Metals in Oil-Contaminated Soil by Using Glycine-β-Cyclodextrin. Int. J. Environ. Res. Public Health 2019, 16, 1155. [Google Scholar] [CrossRef]
- Hu, L.; Wan, J.; Zeng, G.; Chen, A.; Chen, G.; Huang, Z.; He, K.; Cheng, M.; Zhou, C.; Xiong, W.; et al. Comprehensive evaluation of the cytotoxicity of CdSe/ZnS quantum dots in Phanerochaete chrysosporium by cellular uptake and oxidative stress. Environ. Sci. Nano 2017, 4, 2018–2029. [Google Scholar] [CrossRef]
- Hu, K.; Zeng, D.; Xu, Z.; Wei, D.; Ma, X.; Zhang, X.; Hu, K.; Yan, X. Operation effect of in-situ ecological restoration demonstration project of water environment in Tonghu area. Water Supply Drain. China 2015, 31, 15–19. [Google Scholar]
- Dou, J. Statistical Analysis and Modeling of China’s Fishery Economic Output. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2017. [Google Scholar]
- Anthony, R. Animals and Their Moral Standing: A Philosophical Exploration of the Relationship between Animals and Human Beings in Agriculture. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, August 2003. [Google Scholar]
- National Key Laboratory of Pharmaceutical Biotechnology, Nanjing University. An Overview of the Management of Ethical Review in Animal Experiments. Summary of the 11th Symposium on Management and Development of Public Platform for Life Sciences in China; National Key Laboratory of Pharmaceutical Biotechnology, Nanjing University: Nanjing, China, 2018. [Google Scholar]
- Kuang, W.H.; Zhang, D.Y.; Huang, X.Y. Study on residual rules of heavy metal ions Cd, Hg, and Pb in eels. Sci. Bull. 2007, 23, 689–692. [Google Scholar]
- Wu, B.; Fei, L. Modern Environmental Testing Technologies. Master’s Thesis, Chinese Environmental Science Press, Beijing, China, 1999; pp. 252–254. [Google Scholar]
- Zhou, Y.; Zhang, Z. Methods for Testing Toxicity in Biological Organisms; China Agriculture Press: Beijing, China, 1989. [Google Scholar]
- Heath, A.G. Water Pollution and Fish Physiology; CRC Press: Boca Raton, FL, USA, 1987; pp. 181–196. [Google Scholar]
- Richmonds, C.; Dutta, H.M. Histopathological changes induced by malathion in the gills of Bluegill Lepomis macrochirus. Bull. Environ. Contam. Toxicol. 1989, 43, 123–130. [Google Scholar] [CrossRef]
- Alazemi, B.M.; Lewis, J.W.; Andrews, E.B. Gill damage in the freshwater fish Gnathonemus petersii (family: Mormyridae) exposed to selected pollutants: An ultrastructural study. Environ. Technol. 1996, 17, 225–238. [Google Scholar] [CrossRef]
- Fanta, E.; Rios, F.S.; Romão, S.; Vianna, A.C.; Freiberger, S. Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicol. Environ. Saf. 2003, 54, 119–130. [Google Scholar] [CrossRef]
- Cengiz, E.I.; Unlu, E. Sublethal effects of commercial deltamethrin on the structure of the gill, liver and gut tissues of mosquitofish, Gambusia affinis: A microscopic study. Environ. Toxicol. Pharmacol. 2006, 21, 246–253. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, G.; Xu, J.; Zhong, A. Acute and joint toxicities of Cr6+, Zn2+, and Hg2+ to postlarvae of Litopenaeus vannamei. J. Mar. Fish. Res. 2005, 26, 6–12. [Google Scholar]
- Gao, S.Y.; Zou, D.L.; Li, H.M. Acute toxicity of mercury, cadmium, zinc, and manganese to postlarvae of Marsupenaeus japonicus. Mar. Sci. Bull. 1999, 18, 93–96. [Google Scholar]
- Chen, C.; Xie, J.; Zhuo, L. Acute toxicity of cadmium to Carassius auratus auratus. J. Quanzhou Normal Univ. 2006, 24, 104–107. [Google Scholar]
- Zhou, L.; Chen, X.; Qin, D. Toxicity of four heavy metals to embryos and postlarvae of Misgurnus anguillicaudatus. J. Xiamen Fish. Coll. 1994, 16, 11–19. [Google Scholar]
- Guan, H.H.; Lin, Y.H.; Liu, W. Mercury conversion inside Cyprinus carpio and damages to its gill tissues. J. Dali Fish. Coll. 2004, 19, 58–61. [Google Scholar]
- Jian, X. Mercury Pollution Control Technologies and Countermeasures, 1st ed.; Metallurgical Industry Press: Beijing, China, 2013; pp. 8–16. [Google Scholar]
- Urieta, I.; Jalon, M.; Eguileor, I. Food surveillance in the Basque Country (Spain). II. Estimation of the dietary intake of organochlorine pesticides, heavy metals, arsenic, aflatoxin M1, iron and zinc through the Total Diet Study, 1990/91. Food Addit. Contam. 1996, 13, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Sanzo, J.M.; Dorronsoro, M.; Amiano, P.; Amurrio, A.; Aguinagalde, F.X.; Azpiri, M.A. Estimation and validation of mercury intake associated with fish consumption in an EPIC cohort of Spain. Public Health Nutr. 2002, 4, 981–988. [Google Scholar] [CrossRef]

| Hg2+ Mass Concentration | Mortality Rate (%) | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| Seawater | ||||
| 0.891250938 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 10 |
| 1.122018454 | 10 | 20 | 20 | 20 |
| 1.258925412 | 10 | 20 | 20 | 30 |
| 1.412537545 | 30 | 30 | 40 | 50 |
| 1.584893192 | 50 | 50 | 60 | 60 |
| 1.77827941 | 50 | 60 | 60 | 70 |
| 1.995262315 | 70 | 80 | 80 | 90 |
| 2.238721139 | 100 | 100 | 100 | 100 |
| Freshwater | ||||
| 0.891250938 | 0 | 0 | 0 | 0 |
| 1 | 0 | 10 | 20 | 20 |
| 1.122018454 | 20 | 30 | 30 | 40 |
| 1.258925412 | 30 | 30 | 50 | 50 |
| 1.412537545 | 50 | 50 | 60 | 70 |
| 1.584893192 | 60 | 70 | 70 | 90 |
| 1.77827941 | 80 | 80 | 100 | 100 |
| 1.995262315 | 100 | 100 | 100 | 100 |
| 2.238721139 | 100 | 100 | 100 | 100 |
| Hg2+ | Experiment Duration | Regression Equation for Probability Unit—Concentration Logarithm | LC50 | Safety Concentration |
|---|---|---|---|---|
| (h) | (mg/L) | |||
| Seawater | 24 | y = 10.228 × −2.190 | 1.637 | 0.01442 |
| 48 | y = 9.640 × −1.867 | 1.562 | ||
| 72 | y = 9.557 × −1.765 | 1.530 | ||
| 96 | y = 9.213 × −1.464 | 1.442 | ||
| Freshwater | 24 | y = 11.523 × −1.782 | 1.428 | 0.01228 |
| 48 | y = 10.112 × −1.404 | 1.377 | ||
| 72 | y = 10.733 × −1.165 | 1.284 | ||
| 96 | y = 12.231 × −1.091 | 1.228 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Liu, Y.; Zhang, T.; Li, J.; Wang, X.; Zhang, W.; Zeng, G.; Liu, S.; Guan, L. Acute Toxicity of Divalent Mercury Ion to Anguilla japonica from Seawater and Freshwater Aquaculture and Its Effects on Tissue Structure. Int. J. Environ. Res. Public Health 2019, 16, 1965. https://doi.org/10.3390/ijerph16111965
Tang Y, Liu Y, Zhang T, Li J, Wang X, Zhang W, Zeng G, Liu S, Guan L. Acute Toxicity of Divalent Mercury Ion to Anguilla japonica from Seawater and Freshwater Aquaculture and Its Effects on Tissue Structure. International Journal of Environmental Research and Public Health. 2019; 16(11):1965. https://doi.org/10.3390/ijerph16111965
Chicago/Turabian StyleTang, Yuanqiang, Yunguo Liu, Tao Zhang, Jiang Li, Xiaohua Wang, Wei Zhang, Guangming Zeng, Shaobo Liu, and Lei Guan. 2019. "Acute Toxicity of Divalent Mercury Ion to Anguilla japonica from Seawater and Freshwater Aquaculture and Its Effects on Tissue Structure" International Journal of Environmental Research and Public Health 16, no. 11: 1965. https://doi.org/10.3390/ijerph16111965
APA StyleTang, Y., Liu, Y., Zhang, T., Li, J., Wang, X., Zhang, W., Zeng, G., Liu, S., & Guan, L. (2019). Acute Toxicity of Divalent Mercury Ion to Anguilla japonica from Seawater and Freshwater Aquaculture and Its Effects on Tissue Structure. International Journal of Environmental Research and Public Health, 16(11), 1965. https://doi.org/10.3390/ijerph16111965






