Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sites
2.2. Sampling and Analysis
2.3. Statistical Analyses
3. Results
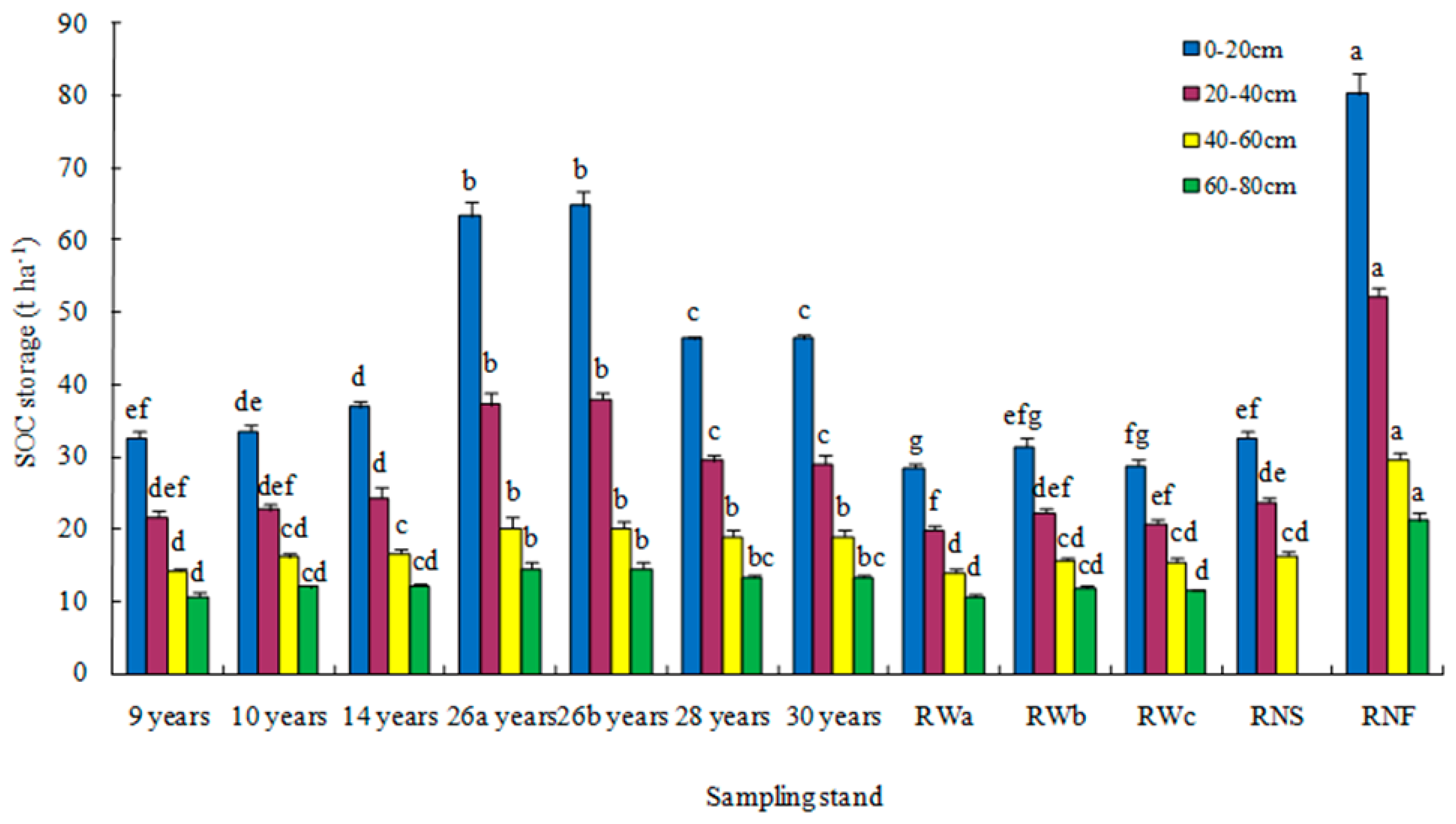
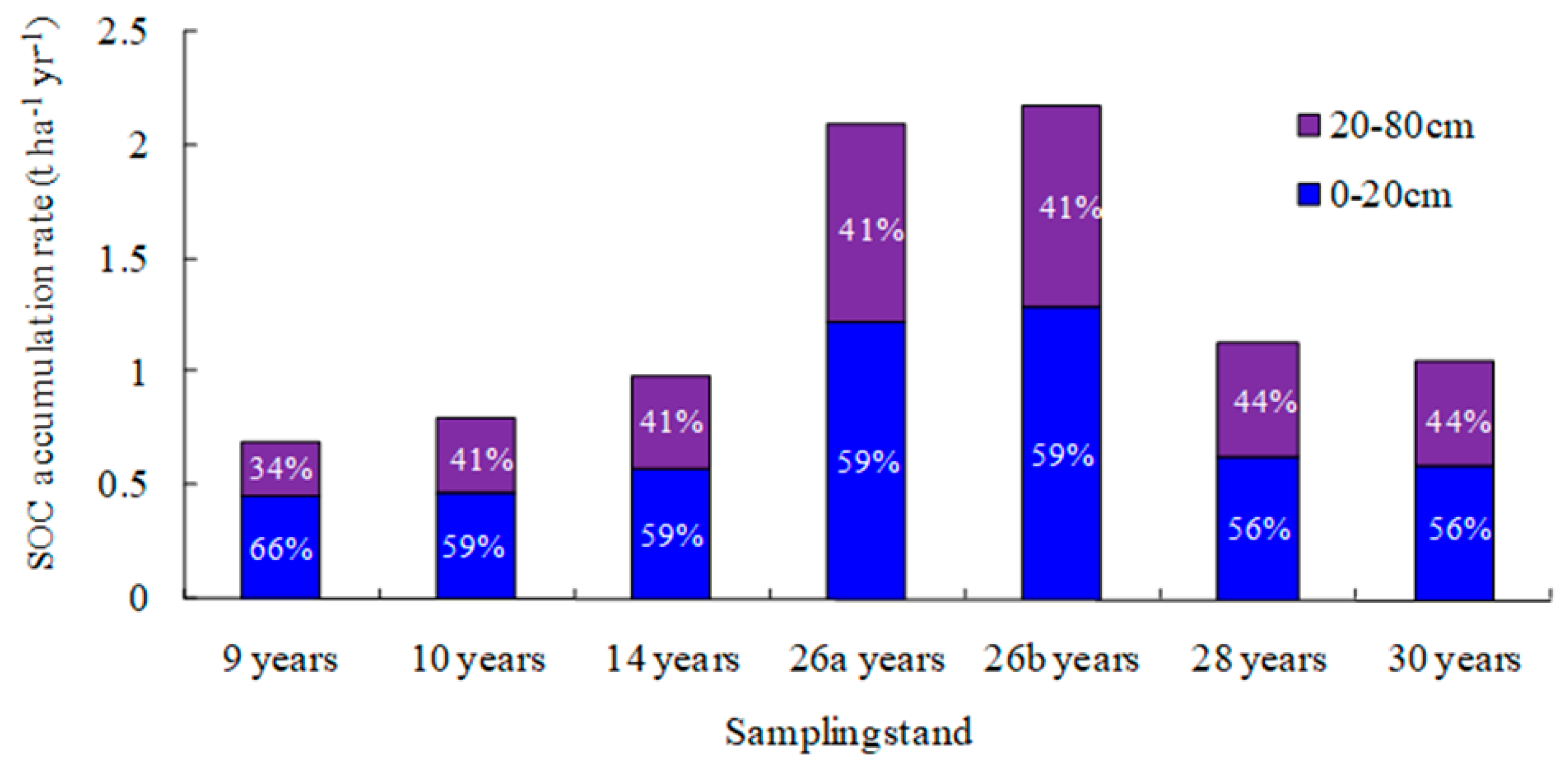
3.1. Changes in Soil Organic Carbon Sequestration with Depth
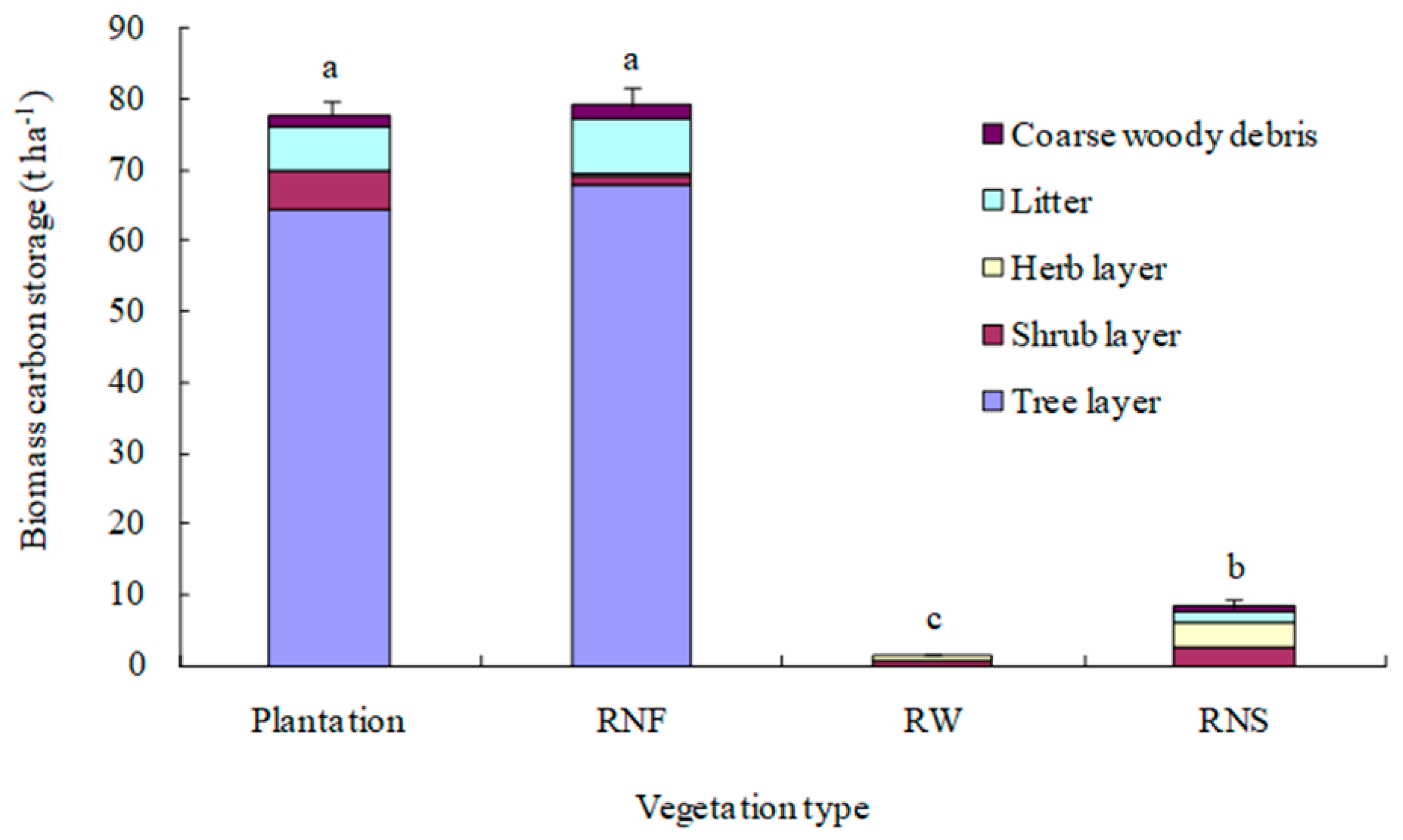
3.2. Biomass Carbon Storage
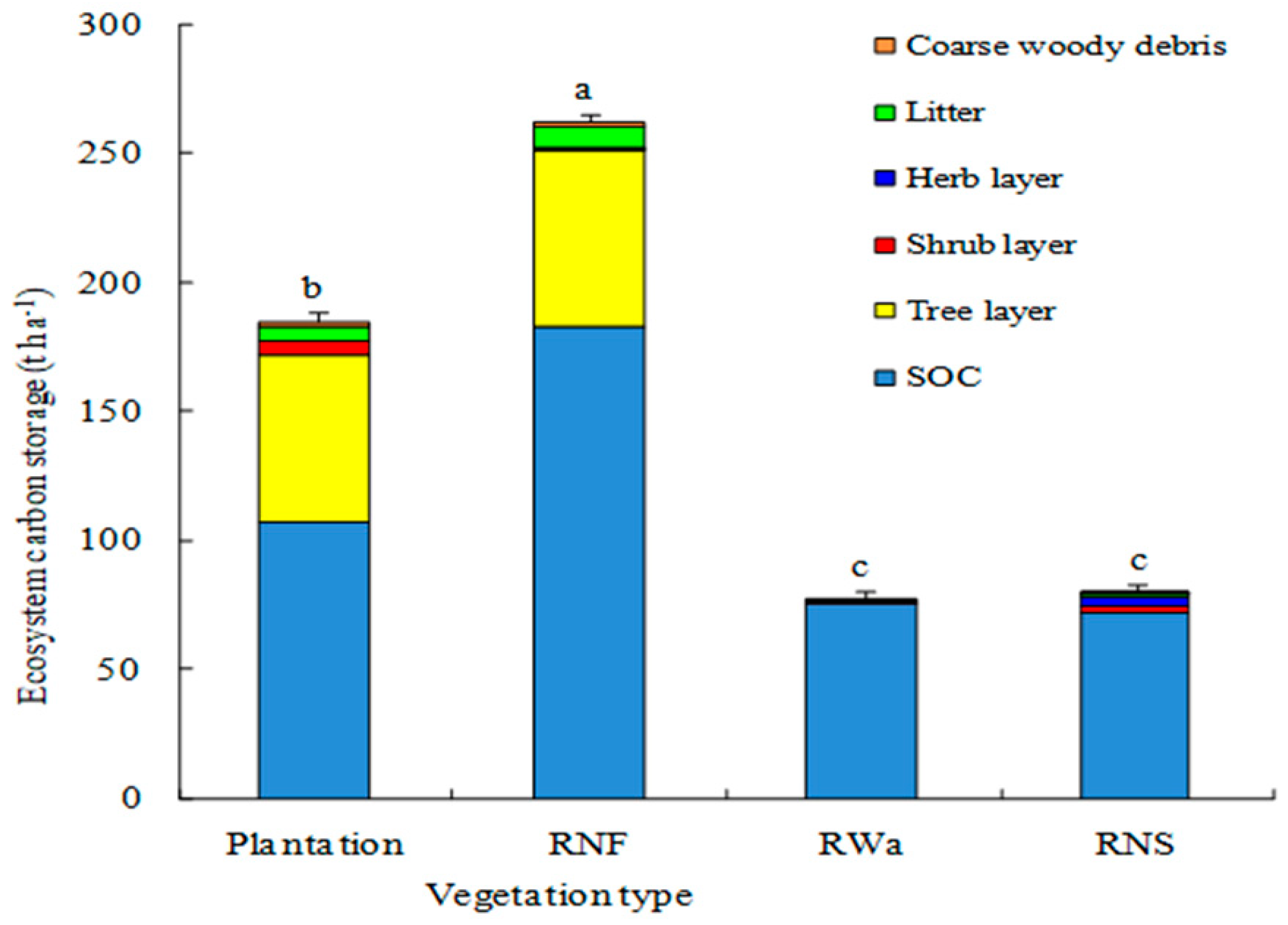
3.3. Total Ecosystem Carbon Sequestration
4. Discussion
4.1. Total Soil Organic Carbon Accumulation in the Soil Profile After 30 Years of Reforestation
4.2. Deep Soil Organic Carbon Accumulation After 30 Years of Reforestation
4.3. Total Biomass Carbon Sequestration
4.4. Total Ecosystem Carbon Pool
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.D.; Yao, X.D.; Wang, W. Variation of soil carbon pools in Pinus sylvestris plantations of different ages in north China. Acta Ecol. Sinica 2018, 38, 248–254. [Google Scholar] [CrossRef]
- Huang, X.F.; Zhou, Y.C.; Zhang, Z.M. Carbon Sequestration Anticipation Response to land use change in a mountainous karst basin in China. J. Environ. Manag. 2018, 228, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Veloso, M.G.; Dieckow, J.; Zanatta, J.A.; Bayer, C.; Higa, R.C.V.; Brevilieri, R.C.; Comerford, N.B.; Stoppe, A.M. Reforestation with loblolly pine can restore the initial soil carbon stock relative to a subtropical natural forest after 30 years. Eur. J. Forest Res. 2018, 137, 593–604. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Yu, S.Q.; Liu, S.P.; Wang, X.L.; Zhang, Y.; Liu, T.; Zhou, L.X.; Zhang, W.X.; Fu, S.L. Reforestation makes a minor contribution to soil carbon accumulation in the short term: Evidence from four subtropical plantations. Forest Ecol. Manag. 2017, 384, 400–405. [Google Scholar] [CrossRef]
- Dolman, A.; Valentini, R.; Freibauer, A. The Continental-Scale Greenhouse Gas Balance of Europe; Springer Science & Business Media: New York, NY, USA, 2008; pp. 153–189. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv. Agron. 2005, 88, 35–66. [Google Scholar]
- Harrison, R.B.; Footen, P.W.; Strahm, B.D. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. Forest Sci. 2011, 57, 67–76. [Google Scholar]
- Liu, S.; Bliss, N.; Sundquist, E.; Huntington, T.G. Modeling carbon dynamics in vegetation and soil under the impact of soil erosion and deposition. Glob. Biogeochem. Cycle 2003, 17, 1–24. [Google Scholar] [CrossRef]
- Shi, S.W.; Zhang, W.; Zhang, P.; Yu, Y.Q.; Ding, F. A synthesis of change in deep soil organic carbon stores with reforestation of agricultural soils. Forest Ecol. Manag. 2013, 296, 53–63. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.; Paul, E.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Lewis, T.; Verstraten, L.; Hogg, B.; Wehr B., J.; Swift, S.; Tindale, N.; Menzies, N.W.; Dalal, R.C.; Bryant, P.; Francis, B.; Smith, T.E. Reforestation of agricultural land in the tropics: The relative contribution of soil, living biomass and debris pools to carbon sequestration. Sci. Total Environ. 2019, 649, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, D.S.; Song, M.W. Biomass and carbon storage of Eucalyptus and Acacia plantations in the Pearl River Delta, South China. Forest Ecol. Manag. 2012, 277, 90–97. [Google Scholar] [CrossRef]
- Wei, X.H.; Li, Q.L.; Liu, Y.Q.; Liu, S.R.; Guo, X.M.; Zhang, L.; Niu, D.K.; Zhang, W.Y. Restoring ecosystem carbon sequestration through afforestation: A sub-tropic restoration case study. Forest Ecol. Manag. 2013, 300, 60–67. [Google Scholar] [CrossRef]
- Xie, J.S.; Guo, J.F.; Yang, Z.J.; Huang, Z.Q.; Chen, G.S.; Yang, Y.S. Rapid accumulation of carbon on severely eroded red soils through reforestation in subtropical China. Forest Ecol. Manag. 2013, 300, 53–59. [Google Scholar] [CrossRef]
- Peng, S.L.; Chen, A.Q.; Fang, H.D.; Wu, J.L.; Liu, G.C. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.L.; Tang, Y. Impacts of woody species composition, species diversity and community structure in dry-hot valley of the Jinsha River, southwestern China. J. Mt. Sci. 2016, 13, 2182–2191. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Wei, J.; Lui, W.G.; Cheng, J.M.; Li, W.J. Dynamics of soil organic carbon storage following restoration of grassland on Yunwu Mountain. Acta Ecol. Sin. 2011, 31, 271–275. [Google Scholar] [CrossRef]
- Toenshoff, C.; Stuelpnagel, R.; Joergensen, R.G.; Wachendorf, C. Carbon in plant biomass and soils of poplar and willow plantations—Implications for SOC distribution in different soil fractions after reconversion to arable land. Plant Soil 2013, 367, 407–417. [Google Scholar] [CrossRef]
- Guedes, B.S.; Olsson, B.A.; Karltunc, E. Effects of 34-year-old Pinus taeda and Eucalyptus grandis plantations on soil carbon and nutrient status in former miombo forest soils. Glob. Ecol. Conserv. 2016, 8, 190–202. [Google Scholar] [CrossRef]
- Tang, G.Y.; Li, K. Tree species controls on soil carbon sequestration and carbon stability following 20 years of reforestation in a valley-type savanna. Forest Ecol. Manag. 2013, 291, 13–19. [Google Scholar] [CrossRef]
- Tang, G.Y.; Li, K.; Sun, Y.; Zhang, C. Dynamics and stabilization of soil organic carbon after nineteen years of reforestation in valley-type savannah in southwest China. Soil Use Manag. 2013, 29, 48–56. [Google Scholar] [CrossRef]
- Yan, M.F.; Zhang, W.J.; Zhang, Z.Y.; Wang, L.; Ren, H.R.; Jiang, Y.; Zhang, X.S. Responses of soil C stock and soil C loss to land restoration in Ili River Valley, China. Catena 2018, 171, 469–474. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Zhu, G.Y.; Liu, Y.L.; Chen, L.; Shangguan, Z.P. Changes of soil carbon in five land use stages following 10 years of vegetation succession on the Loess Plateau, China. Catena 2018, 171, 185–192. [Google Scholar] [CrossRef]
- Binkley, D.; Kaye, J.; Barry, M.; Ryan, M.G. First-rotation changes in soil carbon and nitrogen in a plantation in Hawaii. Soil Sci. Soc. Am. J. 2004, 68, 1713–1719. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Bradley, K.L. Soil carbon and nitrogen accumulation and vertical distribution across a 74-year chronosequence. Soil Sci. Soc. Am. J. 2009, 73, 2096–2104. [Google Scholar] [CrossRef]
- McLauchlan, K. The nature and longevity of agricultural impacts on soil carbon and nutrients: A review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Jonard, M.; Nicolas, M.; Coomes, D.A.; Caignet, I.; Saenger, A.; Ponette, Q. Forest soils in France are sequestering substantial amounts of carbon. Sci. Total Environ. 2017, 574, 616–628. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; He, X.Y.; Su, Y.R.; Wang, K.L. Soil carbon and nitrogen accumulation following agricultural abandonment in a subtropical karst region. Appl. Soil Ecol. 2018, 132, 169–178. [Google Scholar] [CrossRef]
- Blanco, J.A. Forests may need centuries to recover their original productivity after continuous intensive management: An example from Douglas-fir stands. Sci. Total Environ. 2012, 437, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.H.; Wang, D.J.; Zhang, Y.H.; Jiao, Z.; Chen, D. Dynamics and vertical distribution patterns of fine root weights of different aged leucaena leucocephala stand in debris flow source area. Sci. Soil Water Conserv. 2010, 8, 41–46. [Google Scholar]
- Ussiri, D.A.N.; Lal, R.; Jacinthe, P.A. Soil properties and carbon sequestration of afforested pastures in reclaimed mine soils of Ohio. Soil Sci. Soc. Am. J. 2006, 70, 1797–1806. [Google Scholar] [CrossRef]
- Jones, D.; Nguyen, C.; Finlay, R. Carbon flow in the rhizosphere: Carbon trading at the soil-root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, X.Y.; Li, Y.Q.; Cui, J.Y. Restoration of shrub communities elevates organic carbon in arid soils of northwestern China. Soil Biol. Biochem. 2012, 47, 123–132. [Google Scholar]
- Block, R.; Rees, K.; Knight, J. A review of fine root dynamics in Populus plantations. Agroforest Syst. 2006, 67, 73–84. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Veldkamp, E. The role of dissolved organic carbon, dissolved organic nitrogen, and dissolved inorganic nitrogen in a tropical wet forest ecosystem. Ecosystems 2005, 8, 339–351. [Google Scholar] [CrossRef]
- Harrison-Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil organic matter and texture affect responses to dry/wet cycles: Changes in soil organic matter fractions and relationships with C and N mineralization. Soil Biol. Biochem. 2014, 74, 50–60. [Google Scholar] [CrossRef]
- Shi, J.; Cui, L.L. Soil carbon change and its affecting factors following reforestation in China. Landscape Urban Plan 2010, 98, 75–85. [Google Scholar] [CrossRef]
- Prest, D.; Kellman, L.; Lavigne, M.B. Mineral soil carbon and nitrogen still low three decades following clearcut harvesting in a typical Acadian Forest stand. Geoderma 2014, 214–215, 62–69. [Google Scholar] [CrossRef]
- Liu, F.Y.; Li, K.; Ma, J.M. Ecological study on several man-made forests of introduced species in Jinshajiang dry-hot valley. Resour. Environ. Yangtze Basin 2008, 17, 468–474. [Google Scholar]
- Li, B.; Tang, G.Y.; Li, K.; Gao, C.J.; Liu, F.Y.; Wang, X.F. Vegetation biomass allocation and its spatial distribution after 20 years ecological restoration in a dry-hot valley in Yuanmou, Yunnan Province of Southwest China. Chin. J. Appl. Ecol. 2013, 24, 1479–1486. [Google Scholar]
- Li, Y.; Bao, W.K.; Bongers, F.; Chen, B.; Chen, G.K.; Guo, K.; Jiang, M.X.; Lai, J.S.; Lin, D.M.; Liu, C.J.; et al. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ. 2019, 654, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Gu, F.X.; Liu, Y.C.; Li, C. Variations of carbon stock with forest types in subalpine region of southwestern China. Forest Ecol. Manag. 2013, 300, 88–95. [Google Scholar] [CrossRef]
| Sites | Stands | Slope aspect (°) | Slope (°) | Altitude (m) | Woody species richness | Woody species heterogeneity |
|---|---|---|---|---|---|---|
| Tuobuka town, Dongchuan municipality, Yunnan (N26°25′12″; E103°04′43″)(MAT: 22 ℃; MAP: 700 mm) | 9 years old mixed plantation (9 years) | NE80 | 20 | 895 | ST = 3; SS = 4 | H’T = 0.15; H’S = 0.27 |
| Reference wasteland in Tuobuka (RWa) (being wasteland for over 30 years) | NE80 | 20 | 910 | SS = 1 | H’S = 0 | |
| Pisha town, Ningnan county, Sichuan (N27°04′15″; E102.43′42″) (MAT: 20 ℃; MAP: 800 mm) | 26 years old mixed plantation (26a years) | NE75 | 20 | 1273 | ST = 2; SS = 3 | H’T = 0.17; H’S = 0.23 |
| 26 years old mixed plantation (26b years) | NE76 | 19 | 1273 | ST = 1; SS = 3 | H’T = 0; H’S = 0.20 | |
| Reference wasteland in Pisha (RWb) (being wasteland for over 46 years) | NE77 | 22 | 1260 | SS = 1 | H’S = 0 | |
| Reference natural forest (NF) (about 200 years old) | NE45 | 27 | 1230 | ST = 9; SS = 15 | H’T = 1.57; H’S = 2.28 | |
| Hulukou town, Ningnan county, Sichuan (N26°57′24″; E102.53′01″) (MAT: 22 ℃; MAP: 700 mm) | 10 years old mixed plantation (10 years) | NE63 | 18 | 860 | - | - |
| 14 years old mixed plantation (14 years) | NE66 | 19 | 800 | - | - | |
| 28 years old mixed plantation (28 years) | NE60 | 17 | 840 | ST = 1; SS = 3 | H’T = 0; H’S = 0.36 | |
| 30 years old mixed plantation (30 years) | NE70 | 18 | 805 | ST = 3; SS = 5 | H’T = 0.26; H’S = 0.44 | |
| Reference natural recovery shrub grassland (RNS) (about 35 years old) | NE63 | 21 | 840 | SS = 4 | H’S = 0.46 | |
| Reference wasteland in Hulukou (RWc) (being wasteland for over 55 years) | NE35 | 21 | 821 | SS = 1 | H’S = 0 |
| Sites | Stands | pH | Bulk density (g/cm3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | ||
| Tuobuka town | 9 years | 7.6 | 7.5 | 7.9 | 8.0 | 1.52 | 1.57 | 1.59 | 1.61 |
| RWa | 7.9 | 7.8 | 8.0 | 8.0 | 1.57 | 1.59 | 1.60 | 1.61 | |
| Pisha town | 26a years | 5.7 | 5.6 | 5.8 | 5.9 | 1.36 | 1.41 | 1.43 | 1.45 |
| 26b years | 6.8 | 6.7 | 7.0 | 7.0 | 1.33 | 1.39 | 1.42 | 1.44 | |
| RWb | 7.5 | 7.4 | 7.7 | 7.7 | 1.43 | 1.45 | 1.46 | 1.47 | |
| RNF | 5.6 | 5.5 | 5.6 | 5.8 | 1.35 | 1.37 | 1.41 | 1.41 | |
| Hulukou town | 10 years | 7.6 | 7.5 | 7.7 | 8.0 | 1.53 | 1.57 | 1.59 | 1.59 |
| 14 years | 8.0 | 7.9 | 8.0 | 8.0 | 1.47 | 1.55 | 1.58 | 1.59 | |
| 28 years | 7.4 | 7.3 | 7.3 | 7.6 | 1.44 | 1.51 | 1.55 | 1.58 | |
| 30 years | 7.7 | 7.6 | 7.7 | 7.9 | 1.44 | 1.52 | 1.56 | 1.59 | |
| RNS | 7.8 | 7.7 | 8.0 | - | 1.47 | 1.54 | 1.57 | - | |
| RWc | 8.2 | 8.1 | 8.1 | 8.2 | 1.57 | 1.59 | 1.60 | 1.61 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Tang, Y.; Xu, W.; Mou, Z. Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River. Int. J. Environ. Res. Public Health 2019, 16, 1937. https://doi.org/10.3390/ijerph16111937
Gong Z, Tang Y, Xu W, Mou Z. Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River. International Journal of Environmental Research and Public Health. 2019; 16(11):1937. https://doi.org/10.3390/ijerph16111937
Chicago/Turabian StyleGong, Zhilian, Ya Tang, Wenlai Xu, and Zishen Mou. 2019. "Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River" International Journal of Environmental Research and Public Health 16, no. 11: 1937. https://doi.org/10.3390/ijerph16111937
APA StyleGong, Z., Tang, Y., Xu, W., & Mou, Z. (2019). Rapid Sequestration of Ecosystem Carbon in 30-year Reforestation with Mixed Species in Dry Hot Valley of the Jinsha River. International Journal of Environmental Research and Public Health, 16(11), 1937. https://doi.org/10.3390/ijerph16111937




