1. Introduction
Sulfate, as a common anion in the water environment, is widely distributed in various natural environments and plays an important role in biogeochemical cycles. However, as a constant component in the water environment, its pollution problem is often neglected. In recent years, with the development of industrialization and urbanization, sulfate contamination in the water environment has become increasingly prominent, receiving more and more attention from managers and researchers [
1].
The increasing concentration of sulfate in the water environment not only threatens human health and ecological balance [
2], but may also affect carbonate weathering, erosion processes, and global carbon cycle evolution [
3,
4]. Previous studies have shown that, when the human body take in excessive sulfate, it will cause several diseases, e.g., diarrhea, dehydration, and gastrointestinal disorders, etc. [
5]. Sulfate in the water environment may be transformed into the toxic substances under certain conditions, resulting in the loss of essential metal elements in aquatic plants and changes in the original eco-hydrological function. Soucek et al. have shown that high concentrations of sulfate will cause the death of freshwater invertebrates [
6]. The highly sulfate concentration of water environment not only influences human life, but also places some constraints on industrial water and irrigation water. Therefore, the WHO and China in Sanitary Standard for Drinking Water Quality limit the sulfate concentration less than 250 mg/L [
7].
Identifying the sources of sulfate contamination accurately is the premise of controlling the sulfate pollution in the water environment. Dissolved sulfate in water environment is primarily derived from both natural and anthropogenic sources [
8]. Natural sources include dissolution of sulfate minerals (e.g., gypsum), oxidation of sulfide minerals (e.g., pyrite), precipitation and volcanic activity, etc. Anthropogenic sources contain sewage infiltration, fertilizers, synthetic detergents, industrial wastewater and mining drainage, and so on. In addition, groundwater over-exploitation will accelerate the sulfate pollution [
5,
9,
10]. The diversity of sulfate sources and its effects on the ecological environment are attracting more and more researchers’ attention to distinguish the sulfate sources and determine the mechanisms of sulfur and oxygen isotopic variations of SO
42− in different water and the control factors. Therefore, study on the sources of sulfate in water environment is of great significance to water environment safety.
The traditional method to trace the sulfate sources is combining the geological background of the study area with the hydrochemical characteristics, which is simple, but the accuracy is poor. With the advancement of the science and technology, scholars have found that the SO
42− from different sources has particular δ
34S and δ
18O values and thus it has been widely used to identify sulfate sources and the processes of sulfur biogeochemical cycles [
11,
12,
13,
14].
Based on previous researches, the author reviews the current research progress of identifying the sources of sulfate pollution in water environment by using the δ34S and δ18O isotope technology. The main contents are as follows: (1) The fractionation mechanism of sulfur and oxygen isotope of sulfate is introduced, (2) the ranges of typical values of δ34S and δ18O from different potential sources of sulfate are complied, (3) the research advances of sulfate isotope technology in domestic and abroad, which was used to trace the sources of sulfate contamination in water environment are summarized, and (4) the future traceability techniques of sulfate in water environment are prospected.
2. Stable Sulfur and Oxygen Isotopes and the Kinetic Isotope Fractionation
There are four natural stable isotopes of sulfur: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The sulfur isotope composition is usually characterized by the relative abundance of 32S and 34S, using the troilite (FeS) (CDT) from the Canyon Diablo in United States as a standard. Oxygen possesses three stable isotopes: 16O (99.759%), 17O (0.037%), and 18O (0.204%). The traditional reference is Vienna Standard Mean Ocean Water (VSMOW). The sulfur isotope fractionation mechanism is mainly divided into equilibrium isotope fractionation and kinetic isotope fractionation.
The stable isotope ratio is usually expressed as
δ, which is the stable isotope ratio relative to the standard, the expression is as follows:
where R is the isotope ratio, which is the
34S/
32S or
18O/
16O of the sample or standard.
Due to the different ability of different substances to enrich S and O, isotope fractionation often exists when the state of matter changes. The fractional degree is often expressed by the isotopic fractionation coefficient:
where R
A, R
B represent A, B material isotope ratio, respectively. In addition, the enrichment factor is defined as:
The kinetic isotope fractionation mainly occurs in the unidirectional chemical and biochemical processes. Among them, the unidirectional chemical reaction is common in the precipitation, dissolution and adsorption/desorption processes of sulfate minerals and the oxidation of reduced sulfur (S0, HS−, H2S, FeS2). However, the sulfur isotope fractionation of these processes is relatively small. On the contrary, the microbial reduction of sulfate during biochemical processes will result in larger sulfur isotope fractionation.
3. Pretreatment Technology
The pretreatment technology of sulfur and oxygen isotope samples mainly include graphite reduction method, fluorination method, high temperature pyrolysis method, chemical precipitation method, triacid method, and flame heating method, etc.
3.1. Graphite-Reduction Method
The graphite-reduction method, proposed firstly by Rafter in 1967, is the traditional oxygen determination method in sulfate and is widely used all over the world. The BaSO
4 is reduced with graphite at 1100 °C to produce CO
2 and CO. The CO is determined directly or converted to CO
2, and then analyzed by mass spectrometry (MAT-253EM, Key Laboratory of Isotope Geology of Ministry of Land and Resources, Institute of Mineral Resources, CAGS, Beijing, China). The method is simple and the results are more accurate, and the test precision is ±0.2‰ [
15].
3.2. Fluorination Method
The method comprises the following steps: the sulfate is reacted with a strong oxidizing agent such as fluorine gas or fluorine halide (e.g., BrF
5) under high temperature to generate O
2; the generated O
2 is converted into CO
2 at 700 °C in a graphite furnace; then, the oxygen isotope composition is measured, and the analytical uncertainty is about ±0.17‰ [
16]. This method can also directly measure the oxygen isotope composition in O
2, which can simultaneously determine the δ
18O and δ
17O value [
17,
18]. Among them, when using F
2 as oxidant, because of its low purity, a small amount of oxygen, inconvenience in operation and poor safety, this method has less application. While BrF
5 has strong oxidation properties, it is liquid at normal temperature, and its thermal stability is well, so it is used as the oxidant in determining the oxygen isotope values widely. However, compared with the graphite reduction method, the analysis process is relatively complicated, and the oxygen yield is low, and the measured δ
18O value still needs to be corrected.
3.3. High-Temperature Pyrolysis Method
The samples are pyrolytically decomposed at 1400 °C in the presence of nickelized graphite to produce CO, and the volatile product is separated by a gas chromatography column to directly measure the δ
18O value in CO. This method is simple and convenient, and it can be used to determine the δ
18O values in inorganic and organic samples on-line. However, for some carbonate samples, the CO production rate is low, the measurement results are inaccurate, sulfates of 50–100 μg O can be analyzed for δ
18O, and the standard deviation is better than ±0.5‰ [
19].
3.4. Chemical Precipitation Method
The collected water samples (approximately 1.5 L) are filtered through 0.45 μm cellulose-acetate membrane filters, and then acidified to pH ≤ 2 with HCl. The SO
42− of samples is precipitated as BaSO
4 by adding excess 10% BaCl
2. The obtained barite was rinsed with deionized water to remove Cl
−, filtered and dried for 2 h at 850 °C. The δ
34S and δ
18O in BaSO
4 was determined by the element analyzer (Carlo Erba 1108, School of Environmental Studies, China University of Geosciences, Wuhan, China) and isotope mass spectrometer (Delta C Finningan Mat, School of Environmental Studies, China University of Geosciences, Wuhan, China) and the analytical precision for δ
34S is better than ±0.2‰ [
20]. In order to improve the purity of barite, the prepared BaSO
4 is dissolved and reprecipitated by DTPA reagent (diethylenetriaminepentaacetic acid, [(HO
2CCH
2)
2NCH
2CH
2]
2-NCH
2CO
2H). The purified BaSO
4 is purified and analyzed by isotope ratio mass spectrometry (Finnigan MAT 253, Louisiana State University, Baton Rouge, LA, USA), and the test precision of δ
18O is ±0.5‰ [
21,
22]. The dissolution and reprecipitation (DDARP) method can remove the nitrates and other impurities in BaSO
4, which makes the determination of oxygen isotope more accurate.
3.5. Triacid Method
For this method, the mixed solution of HCl, HI and H
3PO
2 are used to react with the sulfate minerals to obtain Ag
2S, which is oxidized to SO
2 directly, and the δ
34S value is determined by the mass spectrometry (MAT-230C, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, China). The chemical process is complicated and inconvenient to operate [
23]. This method is mainly suitable for sulfate minerals.
3.6. The Flame Heating Method
In the flame heating method, sulfate mineral samples are semi-melted by Na
2CO
3-ZnO, and the sulfate samples are transformed into BaSO
4. Then BaSO
4 and SiO
2 are heated by the flame in a vacuum to generate SO
3, which is following reduced by copper to form SO
2 to determine the δ
34S values. This method generates a large amount of harmful gas during the combustion process which is dangerous, and consumes a large amount of quartz tubes. In order to eliminate the drawbacks of the method, BaSO
4 is mixed with SiO
2 and V
2O
5 in a tube furnace under high-temperature heating at a vacuum environment to obtain SO
2 for δ
34S measurements [
24]. The test accuracy (MAT 251 EM, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, China) is better than ±0.2‰ [
25].
At present, the pretreatment method commonly used for the determination of sulfate isotope is chemical precipitation method which is combined with elemental analyzer and stable isotope mass spectrometer, the sulfur and oxygen isotope values of sulfate can be determined. The graphite reduction method, the fluorination method, and the pyrolysis method can determine the oxygen isotope values of the sulfate samples, and the triacid method and the flame heating method are used to determine the sulfur isotope value in the sulfate samples.
4. Sulfur and Oxygen Isotope Values of Sulfate from Different Sources
Sulfate in groundwater mainly originates from the atmosphere, pedosphere, lithosphere and anthropogenic sources of pollution. The SO42− from the atmosphere, biosphere and anthropogenically sources can enter the aquifer through the infiltration and recharge processes, while the lithosphere sulfur can enter the aquifer as a result of the water-rock interaction. Each source has its own sulphur and oxygen isotope characteristics.
4.1. δ34S Values of Sulfate Sources
The pollution status of sulfate in various water bodies is relatively common. Moreover, due to the smaller isotope fractionation (except bacterial reduction) during the biogeochemical cycle of sulfur, sulfur isotopes of sulfate are widely used to identify the pollution sources of sulfate in water environment. Previous studies showed that δ
34S values originating from atmospheric deposition are between −3–12‰, the typical δ
34S values for fertilizers are situated between −7–21‰, from detergent are −3.2–25.8‰ [
26], from evaporites are −14–35‰, and from pyrite ranges from −15‰ to 4‰ [
27].
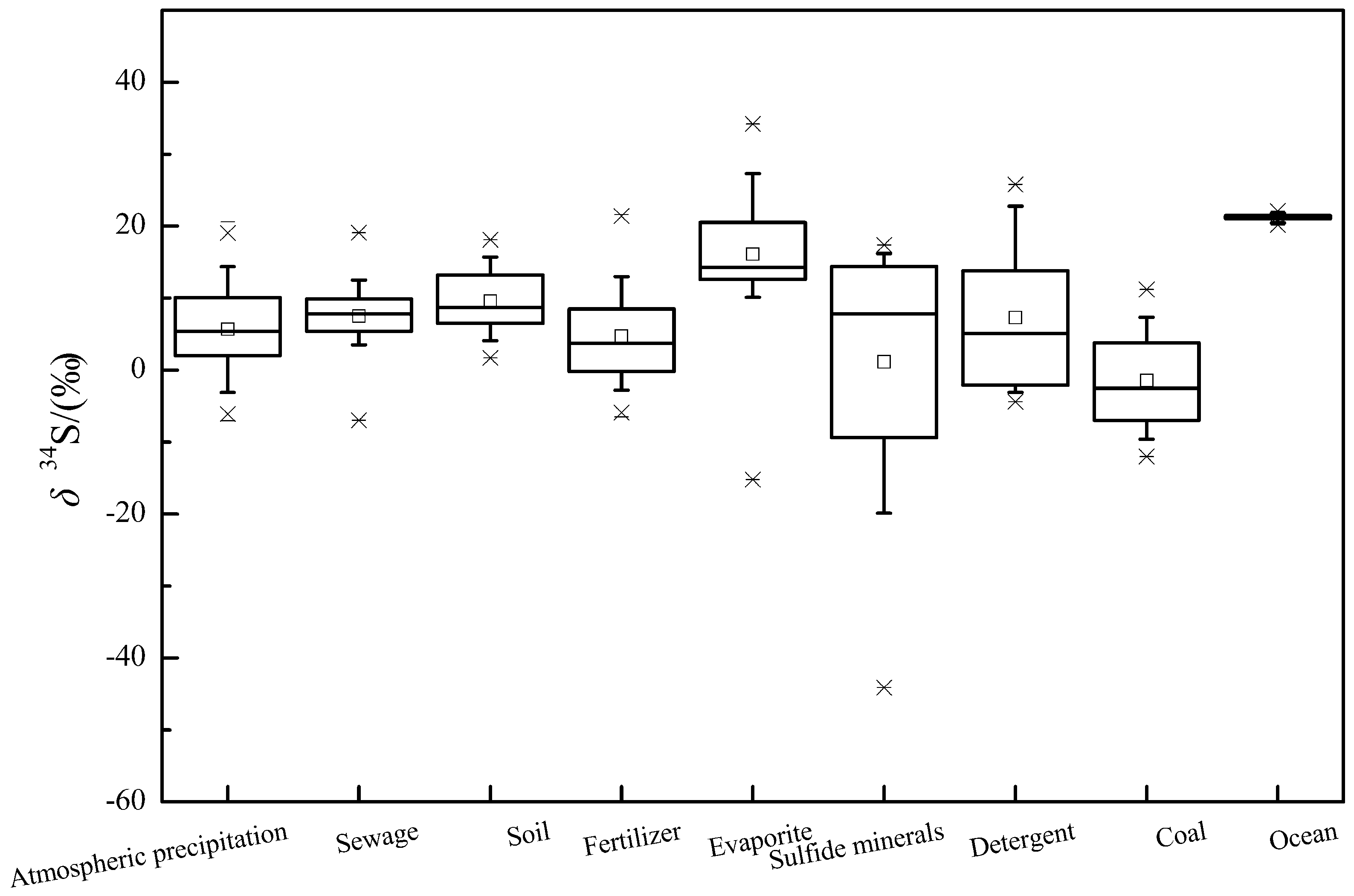
In recent years, researches on the use of sulfur isotope to trace the source of sulfate pollution have emerged. In this paper, nearly 50 literatures about sulfur isotopes of sulfate were collected, and the ranges of δ
34S-SO
42− values from different sources were summarized (
Figure 1).
In this paper, the main pollution sources of sulfate are roughly divided into atmospheric deposition, soil, fertilizer, evaporite, sulfide mineral, detergent and coal. Among them, the isotopic composition of sulfur in atmospheric deposition is mainly affected by natural and anthropogenic activities (such as the burning of fossil fuels). In China, studies have shown that atmospheric precipitation exhibited significant spatial distribution characteristics, which the precipitation in the North part of the Yangtze River is mainly enriched in the heavier sulfur isotopes and the values are mostly positive, while the South part is the opposite [
35]. As shown in
Figure 1, the typical δ
34S values range of the atmospheric precipitation (10% and 90% of the Box-whisker Plot) is between −3.2–14.4‰, with a mean value of 5.7‰.
The δ
34S values of sewage and agricultural fertilizes are significantly affected by the geological conditions, local human activities and the different sources of fertilizer raw materials [
8,
12]. The typical δ
34S values for sewage and fertilizes are situated between 2–12.5‰ and −3.2–13‰, with an average of 7.4‰ and 4.7‰, respectively (
Figure 1).
The sulfur isotope composition in soil is mainly affected by the type of sulfur-containing substances in the soil, biological processes(mineralization of organic sulfur and dissimilatory sulfate reduction) and abiotic effects(migration and transformation of sulfides) [
61]. During the dissimilatory microbial sulfate reduction, the sulfate-reducing bacteria are more inclined to utilize the lighter isotopes which resulting in the enrichment of δ
34S in the residual sulfate, while the reduced products are enriched in δ
32S [
24,
62,
63,
64]. According to
Figure 1, the typical range of δ
34S in soil is between 4.1–13.6‰, with a mean value of 9.6‰.
When the stratum contains evaporites (such as gypsum), the water body usually has a high SO
42− concentration and δ
34S value during the water-rock interaction process, and the δ
34S value in the gypsum is positive [
65,
66]. The isotope compositions based on the geological age [
67]. For sulfide minerals, SO
42− formed by oxidation generally has a negative δ
34S value, which is affected by the oxygen isotope composition of oxygen sources (H
2O and O
2) and its contribution during oxidation [
65,
68]. According to
Figure 1, the typical δ
34S values for evaporites and sulfide minerals ranges from 9.5‰ to 28.3‰ and −25‰ to 16.2‰, with mean values of 16.1‰ and 1.15‰, respectively.
The sulfur isotope composition in detergents is primarily attributed to different sources of the raw materials that provide S. Generally powder detergents have a relatively higher δ
34S value [
12]. It is known from
Figure 1, the typical δ
34S values for detergents ranges from −3.2‰ to 22.8‰, with a mean value of 7.3‰. The value of sulfur isotope in modern oceans is relatively narrow, with a typical range of 20.5–21.7‰ (the average is 21.24‰).
Due to the different genesis of coal in different regions, the sulfur isotope composition is quite different. In China, coal in the Northern regions has a relatively positive δ
34S value and lower sulfur content, while the South is opposite [
60]. As shown in
Figure 1, the typical values of δ
34S in coal range from −9.9‰ to 7.3‰, with a mean value of −1.5‰.
4.2. δ18O Values of Sulfate Sources
Since when the main sources of sulfate in the water environment are traced by the single δ
34S isotope, there will be overlaps in the range of δ
34S values from different sulfate sources, such as atmospheric precipitation and sulfide oxidation sources, sulfate bacterial reduction processes and gypsum dissolution, etc. [
28,
69]. Therefore, utilizing the single δ
34S value has constrains on identifying the main sources of sulfate in water environment. In order to trace the sulfate sources accurately, researchers gradually have begun to use the sulfate oxygen isotope to trace the sources together.
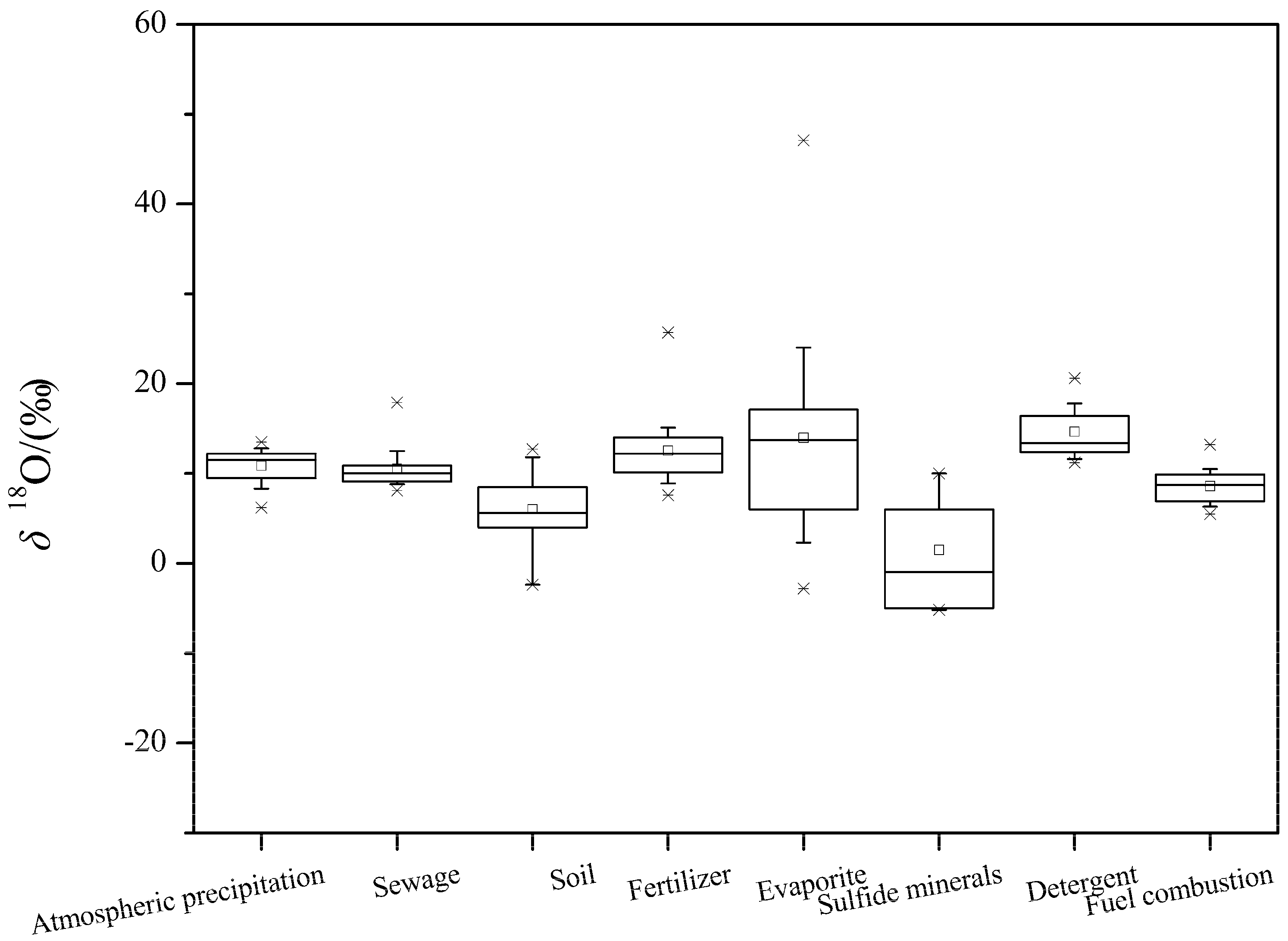
The oxygen isotopic composition of sulfate is mainly affected by the weathered zone, the oxidation reaction pathway, sulfate bacteria reduction and the isotopic composition of local water [
24]. At present, the researches on the oxygen isotopes of sulfate pollution sources are relatively few, which mainly focused on atmospheric deposition, soil, chemical fertilizers, and detergents. Previous studies showed that the δ
18O values of sulfate in chemical fertilizers are 7.7–16.5‰, δ
18O values in detergents are 11.2–20.6‰, and δ
18O values in atmospheric deposition are 5–17‰ [
26]. The δ
18O values in sulfide oxidation are from −5–4‰, while in gypsum it ranges from 14.5‰ to 32.5‰ [
69].
In this paper, approximately 30 literatures about oxygen isotopes of sulfate were summarized, including the atmospheric precipitation, sewage discharge, soil, chemical fertilizer, evaporites, sulfide minerals, detergents, and fuel combustion sources. The results are shown in
Figure 2.
The δ
18O values in atmospheric deposition are mainly affected by natural and anthropogenic activities (such as fuel combustion), and have a relatively positive value of δ
18O [
28]. As shown in
Figure 2, the typical δ
18O value of atmospheric deposition is situated between 7.7–12.8‰ (average 11‰); the typical δ
18O value of fuel combustion ranges from 5.5‰ to 10.5‰, with an average value of 8.6‰. The typical δ
18O values for soil S, evaporites and sulfide minerals are situated between −2.4–11.8‰, 1.1–24‰, and −5.17–6‰, with an average of 6.01‰, 13.98‰, and 1.47‰, respectively (
Figure 2).
The typical δ18O values of sulfate from sewage, fertilizers and detergent (powder and liquid) fall into the ranges of 8.2–12.5‰, 8.8–15.1‰, and 11.2–17.8‰, respectively, while the average value is 10.5‰, 12.5‰, and 14.7‰, respectively.
5. Research Advances in the Application of Sulfur and Oxygen Stable Isotopes in the Identification of Sulfate Sources in Water Environments
The stable isotope techniques for the identification of sulfate sources at home and abroad have been studied for more than 40 years. It has gone through the processes from studying the mechanisms of sulfate isotope fractionation to identify sulfate sources qualitatively and quantitatively. In the early research stage, scholars mainly relied on the traditional hydrochemistry techniques to analyze and discuss the sources and pollution mechanism of sulfate. With the development of science and technology, stable isotope technology developed gradually, and sulfate sulfur isotope technology was widely used to trace the sources of sulfate in water environment [
9,
52,
53,
78]. Mizota et al. [
45] found that the δ
34S values of soils and fertilizers were generally higher in areas of the Southern Hemisphere than the Northern Hemisphere which were due to the differences in the relative contribution of S from marine aerosols and anthropogenic activities. Otero et al. [
8] used sulfate sulfur isotopes to study the effects of potassium mining on groundwater salinization in Llobregat Basin. The results showed that the main sources of sulfate pollution were tailings water and fertilizers. Yang et al. [
79] investigated the main sources of sulfate in the Ordos Cretaceous Groundwater Basin and the results showed that the sulfate in groundwater was mainly derived from gypsum and mirabilite in the stratum, followed by sulfide in the stratum and a small amount from organic sulfur. Hosono et al. [
46] used complex isotope (H, O, N, S, Sr) techniques to identify the main sources of sulfate in different water bodies from different regions. The main sources of sulfate in karst groundwater in Guiyang were studied by using sulfur and chlorine isotopes and the results showed that the main sources of sulfate in groundwater were the dissolution of gypsum, the influence of coal-bearing stratum, the dry and wet atmospheric deposition, and the organic sulfur oxidation in soil [
9,
78].
Since there may be overlaps in the δ
34S values of sulfate from different sources, therefore, researchers began to pay more attention to simultaneous determination of sulfate sulfur and oxygen isotopes in the sulfate sources identification [
28,
69]. Dowuona et al. [
71] traced the sulfate sources in Southern Saskatchewan (Canada) by using sulfate sulphur and oxygen isotopes, and the results showed that the sulfate in this region was derived from sulfides. Yang et al. [
70] studied the main sulfate sources in the the Ordos Cretaceous Groundwater Basin by using the S and O isotopes. It was identified that the sulfate in shallow groundwater was mainly derived from atmospheric precipitation, sulfides oxidation, and sulfate minerals dissolution, while in the deep groundwater, was the dissolution of sulfate minerals. Li et al. [
76] used a dual isotopic approach to trace sulfate sources in Changjiang Estuary, China, and the results indicated that atmospheric deposition, dissolution of evaporate and oxidation of sulfide minerals were the main sources of water sulfate in this area. The δ
34S and δ
18O values of the groundwater sulfate in the Caldas da Rainha Spas indicated that the sulfate were the result of water-rock interaction with evaporitic rocks (e.g., gypsum and anhydrite) [
80]. Al-Charideh et al. [
81] traced the main sources of sulfate in carbonate aquifer system in Aleppo basin (North Syria) based on sulfate sulfur and oxygen isotopes. Xiao et al. suggested that the high SO
42− concentrations in the geothermal water resulted mainly from the dissolution of gypsum according to the δ
34S and δ
18O values [
82].
With the deepening of research, scholars have generalized models of the sulfate sources contribution rate in different water environments based on the principle of mass balance. Miao et al. [
74] used stable isotope techniques, combined with geochemical and hydrogeological data, to explore that groundwater sulfate sources at a mining site (The Monument Valley site in Arizona, AZ, USA) and calculate the sources contribution rates by using the quantitative models. Samborska et al. [
73] found that nearly 50% of the sulfate was derived from the weathering of sulfide minerals, the second largest source is atmospheric precipitation (accounting for 30%), and the rest comes from the dissolution and evaporation of sulfate minerals by utilizing the models. The sources apportionment contribution model of sulfate sulphur and oxygen isotopes can be generalized to [
83]:
Among them, i represents different pollution sources, δ34Si and δ18Oi represent the δ34S and δ18O values of sulfate in the pollution source i, and fi represents the contribution rate of different pollution sources.
6. Research Deficiency and Prospect
As a constant component in water environment, the problem of excessive sulfate concentration is often neglected. With the continuous development of the economy and society, the problem of sulfate pollution in the water environment has become increasingly prominent, which has been widely concerned by scholars. Comprehensive analysis of traceability of sulfate contamination at home and abroad shows that although the traceability technology for sulfate pollution in groundwater has been developed, the accuracy of traceability still needs to be further improved. There are mainly several deficiencies in the following aspects: First, previous studies on sulfate pollution in water environment are mainly based on the hydrochemistry theory, or only the application of the sulfate δ
34S isotope with poor accuracy of tracing results, and mainly concentrated in the qualitative researches. In the later stage, researches on the sulfate contamination traceability gradually developed into dual and multiple isotopes traceability, while the comprehensive tracing method and quantitative research are not mature. Second, previous researches on sulfate pollution mainly focus on surface water and rainwater, and there are relatively few studies on groundwater sulfate pollution, especially in areas where human activities are relatively intensive. Third, the determination of the end element values of potential sulfate pollution sources is mostly based on the data in the literature, and it lacks the actual measured values in the study area, which affects the accuracy of the traceability results. Therefore, in the future research, the source apportionment of sulfate in the water environment should be studied by using multiple stable isotope techniques, such as, δD and δ
18O of H
2O and
87Sr/
86Sr of Sr [
84], combined with hydrochemical evolution theory and multivariate statistical techniques. At the same time, a source apportionment model should be established to quantitatively study the contribution of various sulfate sources to sulfate contamination in water environment, which can provide data support for scientific prevention and control of sulfate pollution in water environment.
7. Conclusions
The source apportionment of sulfate contamination in water environments has gone through the process of relying on the hydrogeochemical theory to the application of stable isotope techniques. In this paper, the application of stable isotope techniques to trace the sources of sulfate pollution at home and abroad is reviewed. In this paper, we have summarized the pretreatment methods of sulfur and oxygen isotopes in sulfate, which mainly include graphite reduction, fluorination, high temperature pyrolysis, chemical precipitation, triacid method, and flame heating method. The ranges of sulfur and oxygen isotopes values from potential sulfates sources were calculated. Furthermore, we have reviewed the research advances in the application of stable isotopes to identify sulfates sources in the water environment, which was developed from the qualitative method to quantitative method, and from single isotope to multiple isotope method. Due to the limitations of early technical conditions, only the δ34S isotope was used to identify the sulfate sources in water environment, and then the δ34S and δ18O double isotopes were developed to trace the sources. In recent years, the traceability researches have gradually evolved from qualitative to quantitative, and the accuracy has been improved gradually. However, due to the complexity of sulfate pollution sources and the isotope fractionation, using sulfur and oxygen isotopes alone cannot accurately identify the sources of sulfate pollution in water environment. Therefore, in order to provide a guarantee for the accurate identification of sulfate sources in the water environment the multi-isotopic traceability technology, combined with hydrochemistry and multivariate statistical analysis methods, and a source apportionment model are developed.
Author Contributions
H.W. and Q.Z. conceived the research ideas and undertook the research design. H.W. collected the data and wrote the manuscript. Q.Z. revised the manuscript and gave modification advises.
Funding
This research was funded by the Fundamental Research Funds of the Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences, grant number SK201707, the projects of China Geological Survey, grant number DD20190331, and the open funds of state key laboratory of urban and region ecology of China, grant number SKLURE2019-2-3.
Acknowledgments
The authors gratefully acknowledge the editor and anonymous reviewers for their valuable comments on this manuscript. The authors also appreciate the financial support from the different organizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, Y.H.; Su, C.L.; Liu, W.J.; Zhu, Y.P.; Li, J.X. Identification of sulfate sources in the groundwater system of Zaozhuang: Evidences from isotopic and hydrochemical characteristics. Environ. Sci. 2016, 37, 4690–4699, (In Chinese with English abstract). [Google Scholar]
- Geurts, J.J.M.; Sarneel, J.M.; Willers, B.J.C.; Roelofs, J.G.M.; Verhoeven, J.T.A.; Lamers, L.P.M. Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef]
- Tostevin, R.; Turchyn, A.V.; Farquhar, J.; Johnston, D.T.; Eldridge, D.L.; Bishop, J.K.B.; McIlvin, M. Multiple sulfur isotope constraints on the modern sulfur cycle. Earth Planet. Sci. Lett. 2014, 396, 14–21. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhou, A.; Zhang, B. Sulfur and oxygen isotope compositions of dissolved sulfate in the Yangtze River during high water period and its sulfate source tracing. Earth Sci. J. China Univ. Geosci. 2014, 39, 1547–1554. [Google Scholar]
- Man, K.; Ma, Z.M.; Xu, X.J. Research on the mechanism of sulfate pollution of groundwater in Jiaozuo area. Appl. Mech. Mater. 2014, 665, 436–439. [Google Scholar] [CrossRef]
- Soucek, D.J.; Kennedy, A.J. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ. Toxicol. Chem. 2005, 24, 1204–1210. [Google Scholar] [CrossRef]
- GB5749-2006. Standards of Drinking Water Quality; Minister of Health of the People’s Republic of China: Beijing, China, 2006. [Google Scholar]
- Otero, N.; Soler, A. Sulphur isotopes as tracers of the influence of potash mining in groundwater salinisation in the Llobregat Basin (NE Spain). Water Res. 2002, 36, 3989–4000. [Google Scholar] [CrossRef]
- Liu, C.Q.; Lang, Y.C.; Satake, H.; Wu, J.; Li, S.L. Identification of anthropogenic and natural inputs of sulfate and chloride into the karstic ground water of Guiyang, SW China: Combined δ37Cl and δ34S approach. Environ. Sci. Technol. 2008, 42, 5421–5427. [Google Scholar] [CrossRef]
- Otero, N.; Canals, À.; Soler, A. Using dual-isotope data to trace the origin and processes of dissolved sulphate: A case study in Calders stream (Llobregat basin, Spain). Aquat. Geochem. 2007, 13, 109–126. [Google Scholar] [CrossRef]
- Crowley, S.F.; Mccarthy, M.D.B.; Bottrell, S.H.; Ward, J.; Young, B. δ34S of lower carboniferous anhydrite, cumbria and its implications for barite mineralization in the Northern Pennines. J. Geol. Soc. 1997, 154, 597–600. [Google Scholar] [CrossRef]
- Hosono, T.; Nakano, T.; Igeta, A.; Tayasu, I.; Tanaka, T.; Yachi, S. Impact of fertilizer on a small watershed of Lake Biwa: Use of sulfur and strontium isotopes in environmental diagnosis. Sci. Total Environ. 2007, 384, 342–354. [Google Scholar] [CrossRef]
- Yang, X.Q.; Li, H.M.; Li, L.X.; Ma, Y.B.; Chen, J.; Liu, M.J.; Yao, T.; Chen, W.S.; Yao, L.D. Characteristics of fluid inclusion, S, H and O isotope of iron deposit in Anshan-Benxi Area, Liaoning Province. Acta Geol. Sin. 2014, 88, 1917–1931, (In Chinese with English abstract). [Google Scholar]
- Jambrina-Enriquez, M.; Recio, C.; Armenteros, I. Biogeochemical characterization of a Mediterranean shallow lake using stable isotopes: Laguna del Cristo (NW Iberian Peninsula). Environ. Earth Sci. 2018, 77, 49. [Google Scholar] [CrossRef]
- Wan, D.F.; Li, Y.H.; Qin, Y. Carbon reduction method for oxygen isotope composition of sulfates. Miner. Deposits 2011, 30, 749–753, (In Chinese with English abstract). [Google Scholar]
- Wasserman, M.D.; Rye, R.O.; Bethke, P.M.; Arribas, A., Jr. Methods for separation and total stable isotope analysis of alunite. Blood 1992, 48, 843–853. [Google Scholar]
- Bao, H.; Thiemens, M.H.; Farquhar, J.; Campbell, D.A.; Lee, C.C.; Heine, K.; Loope, D.B. Anomalous 17O compositions in massive sulphate deposits on the Earth. Nature 2000, 406, 176–178. [Google Scholar] [CrossRef]
- Bao, H.M.; Thiemens, M.H. Generation of O2 from BaSO4 using a CO2−Laser fluorination system for simultaneous analysis of δ18O and δ17O. Anal. Chem. 2000, 72, 4029–4032. [Google Scholar] [CrossRef]
- Kornexl, B.E.; Gehre, M.; Hofling, R.; Werner, R.A. On-line δ18O measurement of organic and inorganic substances. Rapid Commun. Mass Spectrom. 1999, 13, 1685–1693. [Google Scholar] [CrossRef]
- Li, R.; Xiao, Q.; Liu, W.; Guo, F.; Pan, M.C.; Yu, S. Using δ34S-SO42− and δ15N-NO3−, δ18O-NO3− to trace the sources of sulfur and nitrate in Lihu Lake underground water, Guangxi, China. Environ. Sci. 2015, 2877–2886. [Google Scholar] [CrossRef]
- Bao, H.M. Purifying barite for oxygen isotope measurement by dissolution and reprecipitation in a chelating solution. Anal. Chem. 2006, 78, 304–309. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhou, A.G.; Gan, Y.Q.; Yu, T.T.; Wang, D.; Liu, Y.D. Controls on the δ34S and δ18O of dissolved sulfate in the Quaternary aquifers of the North China Plain. J. Hydrol. 2011, 400, 312–322. [Google Scholar] [CrossRef]
- Bai, R.M.; Li, J.C. Thermal decomposition of barium sulfate for preparation of sulfur dioxide used in sulfur isotope analysis. Rock Miner. Anal. 1998, 17, 40–43, (In Chinese with English abstract). [Google Scholar]
- Fritz, P.; Basharmal, G.M.; Drimmie, R.J.; Ibsen, J.; Qureshi, R.M. Oxygen isotope exchange between sulphate and water during bacterial reduction of sulphate. Chem. Geol. 1989, 79, 99–105. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.H.; Zhou, X.; Jia, J.Y.; Zhou, Q.P.; Li, Y.F. Characteristics of hydraulic connection and sulfate contamination within the groundwater system of Yangzhou-Taizhou-Jingjiang area. Acta Geosci. Sin. 2014, 25, 183–190. [Google Scholar]
- Zhang, D.; Li, X.D.; Zhao, Z.Q.; Liu, C.Q. Using dual isotopic data to track the sources and behaviors of dissolved sulfate in the western North China Plain. Appl. Geochem. 2015, 52, 43–56. [Google Scholar] [CrossRef]
- Cortecci, G.; Dinelli, E.; Bencini, A.; Adorni-Braccesi, A.; La Ruffa, G. Natural and anthropogenic SO4 sources in the Arno river catchment, northern Tuscany, Italy: A chemical and isotopic reconnaissance. Appl. Geochem. 2002, 17, 79–92. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, X.Y.; Li, C.J. Sources of riverine sulfate in Yellow River and its tributaries determined by sulfur and oxygen isotopes. Adv. Water Sci. 2013, 24, 418–426, (In Chinese with English abstract). [Google Scholar]
- Vitòria, L.; Otero, N.; Albert Soler, A.; Canals, À. Fertilizer characterization: Isotopic data (N, S, O, C, and Sr). Environ. Sci. Technol. 2004, 38, 3254–3262. [Google Scholar] [PubMed]
- Querol, X.; Alastuey, A.; Chaves, A.; Spiro, B.; Plana, F.; Lopez-Soler, A. Sources of natural and anthropogenic sulphur around the Teruel power station, NE Spain. Inferences from sulphur isotope geochemistry. Atmos. Environ. 2000, 34, 333–345. [Google Scholar] [CrossRef]
- Zhang, H.B.; Hu, A.Q.; Lu, C.Z.; Zhang, G.X. Sulfur isotopic composition of acid deposition in South China Regions and its environmental significance. China Environ. Sci. 2002, 22, 165–169, (In Chinese with English abstract). [Google Scholar]
- Shanley, J.B.; Mayer, B.; Mitchell, M.J.; Michel, R.L.; Bailey, S.W.; Kendall, C. Tracing sources of streamwater sulfate during snowmelt using S and O isotope ratios of sulfate and 35S activity. Biogeochemistry 2005, 76, 161–185. [Google Scholar] [CrossRef]
- Shanley, J.B.; Mayer, B.; Mitchell, M.J.; Bailey, S.W. Seasonal and event variations in δ34S values of stream sulfate in a Vermont forested catchment: Implications for sulfur sources and cycling. Sci. Total Environ. 2008, 404, 262–268. [Google Scholar] [CrossRef]
- Li, X.D.; Masuda, H.; Kusakabe, M.; Yanagisawa, F.; Zeng, H.A. Degradation of groundwater quality due to anthropogenic sulfur and nitrogen contamination in the Sichuan Basin, China. Geochem. J. 2006, 40, 475–482. [Google Scholar] [CrossRef]
- Hong, Y.T.; Zhang, H.B.; Zhu, Y.X.; Piao, H.C.; Jiang, H.B.; Liu, D.P. Characters of sulfur isotopic composition of precipitation in China. Adv. Nat. Sci. 1994, 4, 741–745. (In Chinese) [Google Scholar]
- Bai, L.; Wang, Z.L. Sulfur isotope geochemistry of atmospheric precipitation in Xi’an and Xianyang, Shanxi Province, China. Geochimica 2009, 38, 273–281, (In Chinese with English abstract). [Google Scholar]
- Xiao, H.Y.; Liu, C.Q. Sources of nitrogen and sulfur in wet deposition at Guiyang, southwest China. Atmos. Environ. 2002, 36, 5121–5130. [Google Scholar] [CrossRef]
- Yao, W.H.; Chen, Y.P.; Liu, J.; Yao, W.X.; Chen, H.; Yin, X.F.; Wen, X.F. The research on the environmental significance of atmospheric sulfur isotopic composition in Hengyang. Res. Environ. Sci. 2003, 16, 3–5, (In Chinese with English abstract). [Google Scholar]
- van Stempvoort, D.R.; Fritz, P.; Reardon, E.J. Sulfate dynamics in upland forest soils, central and southern Ontario, Canada: Stable isotope evidence. Appl. Geochem. 1992, 7, 159–175. [Google Scholar] [CrossRef]
- Bottrell, S.; Tellam, J.; Bartlett, R.; Hughes, A. Isotopic composition of sulfate as a tracer of natural and anthropogenic influences on groundwater geochemistry in an urban sandstone aquifer, Birmingham, UK. Appl. Geochem. 2008, 23, 2382–2394. [Google Scholar] [CrossRef]
- Wu, Q.; Han, G. Sulfur isotope and chemical composition of the rainwater at the Three Gorges Reservoir. Atmos. Res. 2015, 155, 130–140. [Google Scholar] [CrossRef]
- Lang, Y.C.; Liu, C.Q.; Satake, H.; Wu, J.H.; Li, S.L. δ37Cl and δ34S variations of Cl− and SO42− in groundwater and surface water of Guiyang area, China. Adv. Earth Sci. 2008, 23, 151–159, (In Chinese with English abstract). [Google Scholar]
- Otero, N.; Soler, A.; Canals, À. Controls of δ34S and δ18O in dissolved sulphate: Learning from a detailed survey in the Llobregat River (Spain). Appl. Geochem. 2008, 23, 1166–1185. [Google Scholar] [CrossRef]
- Moncaster, S.J.; Bottrell, S.H.; Tellam, J.H.; Lloyd, J.W.; Konhauser, K.O. Migration and attenuation of agrochemical pollutants: Insights from isotopic analysis of groundwater sulphate. J. Contam. Hydrol. 2000, 43, 147–163. [Google Scholar] [CrossRef]
- Mizota, C.; Sasaki, A. Sulfur isotope composition of soils and fertilizers: Differences between Northern and Southern hemispheres. Geoderma 1996, 71, 77–93. [Google Scholar] [CrossRef]
- Hosono, T.; Siringan, F.; Yamanaka, T.; Yu, U.; Onodera, S.I.; Nakano, T.; Taniguchi, M. Application of multi-isotope ratios to study the source and quality of urban groundwater in Metro Manila, Philippines. Appl. Geochem. 2010, 25, 900–909. [Google Scholar] [CrossRef]
- Bartlett, R.; Bottrell, S.H.; Sinclair, K.; Thornton, S.; Fielding, I.D.; Hatfield, D. Lithological controls on biological activity and groundwater chemistry in Quaternary sediments. Hydrol. Process. 2010, 24, 726–735. [Google Scholar] [CrossRef]
- Hosono, T.; Wang, C.H.; Umezawa, Y.; Nakano, T.; Onodera, S.I.; Nagata, T.; Yoshimizu, C.; Tayasu, I.; Taniguchi, M. Multiple isotope (H, O, N, S and Sr) approach elucidates complex pollution causes in the shallow groundwaters of the Taipei urban area. J. Hydrol. 2011, 397, 23–36. [Google Scholar] [CrossRef]
- Hosono, T.; Delinom, R.; Nakano, T.; Kagabu, M.; Shimada, J. Evolution model of δ34S and δ18O in dissolved sulfate in volcanic fan aquifers from recharge to coastal zone and through the Jakarta urban area, Indonesia. Sci. Total Environ. 2011, 409, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.W.; Liang, Y.P. The use of 34S in the analysis of sulfate contamination on karst groundwater in Yangquan. West-China Explor. Eng. 2006. (In Chinese) [Google Scholar] [CrossRef]
- Lin, Y.T.; Cao, S.X.; Xiong, S.J.; Song, H.B. Anhydrite and salt brine of trissic sequence in Sichuan Basin: Composition and implication of sulfur isotope. Geol. Chem. Miner. 1997, 171–176, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.H.; Liang, Y.P.; Wang, W.T.; Han, X.R.; Hou, G.C. A practical use of 34S in the investigation of karst groundwater resource in North China. Carsol. Sin. 2009, 28, 235–241, (In Chinese with English abstract). [Google Scholar]
- Huo, J.G.; Zhao, C.H.; Liang, Y.P.; Wang, T.L.; Tang, C.L.; Wang, W.T.; Shen, H.Y. Characteristic and cause analysis in the runoff-drainage area of Niangziguan Spring. Geol. Sci. Technol. Inf. 2015, 147–152, (In Chinese with English abstract). [Google Scholar]
- Wang, L.C.; Liu, C.L.; Fei, M.M.; Shen, L.J.; Zhang, H. Sulfur isotopic composition of sulfate and its geological significance of the Yunlong formation in the Lanping Basin, Yunnan Province. China Min. Mag. 2014, 23, 57–65, (In Chinese with English abstract). [Google Scholar]
- Li, X.B.; Huang, Z.L.; Li, W.B.; Zhang, Z.L.; Yan, Z.F. Sulfur isotopic compositions of the Huize super-large Pb–Zn deposit, Yunnan Province, China: Implications for the source of sulfur in the ore-forming fluids. J. Geochem. Explor. 2006, 89, 227–230. [Google Scholar] [CrossRef]
- Brown, C.J.; Schoonen, M.A.A.; Candela, J.L. Geochemical modeling of iron, sulfur, oxygen and carbon in a coastal plain aquifer. J. Hydrol. 2000, 237, 147–168. [Google Scholar] [CrossRef]
- Huang, X.W.; Qi, L.; Meng, Y.M.; Chen, D.; Ling, H.D. Origin of siderite mineralization in western Guizhou, SW China: Constrains from REEs, C, O, Sr and S isotopes. Ore Geol. Rev. 2015, 66, 252–265. [Google Scholar] [CrossRef]
- Zhang, H.B.; Chen, Y.W.; Liu, D.P. Study on sulfur source of acid rain using sulfur isotopic trace. Geochimica 1995, 24, 126–133, (In Chinese with English abstract). [Google Scholar]
- Jiang, Y. Sources of sulfur in the Nandong underground river system, southwest China: A chemical and isotopic reconnaissance. Appl. Geochem. 2012, 27, 1463–1470. [Google Scholar] [CrossRef]
- Hong, Y.T.; Zhang, H.B.; Zhu, Y.X. Sulfur isotopic characteristics of coal in China and sulfur isotopic fractionation during coal-burning process. Chin. J. Geochem. 1993, 12, 51–59. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. Fate of elemental sulfur in an intertidal sediment. FEMS Microbiol. Ecol. 1996, 19, 95–103. [Google Scholar] [CrossRef]
- Antler, G.; Turchyn, A.V.; Rennie, V.; Herut, B.; Sivan, O. Coupled sulfur and oxygen isotope insight into bacterial sulfate reduction in the natural environment. Geochim. Cosmochim. Acta 2013, 118, 98–117. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, C. A preliminary study on sulfate reduction bacteria behaviors in groundwater by sulfur and carbon isotopes: A case study in Jiaozuo City, China. Ecotoxicology 2014, 23, 2014–2024. [Google Scholar]
- Aharon, P.; Fu, B. Sulfur and oxygen isotopes of coeval sulfate–sulfide in pore fluids of cold seep sediments with sharp redox gradients. Chem. Geol. 2003, 195, 201–218. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, C.Q.; Liu, X.L.; Bao, L.R. Identification of dissolved sulfate sources and the role of sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J. Asian Earth Sci. 2011, 42, 370–380. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.L.; Zhong, J.; Guo, Q.J.; Lang, Y.C.; Han, X.K. Sulfate sources constrained by sulfur and oxygen isotopic compositions in the upper reaches of the Xijiang River, China. Acta Geochim. 2017, 36, 611–618. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Cook, P.G.; Herczeg, A.L. Environmental Tracers in Subsurface Hydrology, 1st ed.; Springer: Boston, MA, USA, 2000; pp. 195–231. [Google Scholar]
- Ren, K.; Pan, X.D.; Lan, G.J.; Jiao, Y.J.; Zeng, J.; Meng, X.J.; Pang, Y. Sulfate concentrations and source identification in different water bodies of the Chadianqiao underground river basin in central Guizhou. Acta Geol. Sin. 2016, 90, 1922–1932, (In Chinese with English abstract). [Google Scholar]
- Yang, Y.C.; Shen, Z.L.; Wen, D.G.; Hou, G.C.; Zhao, Z.H.; Wang, D.; Li, J.W. Distribution of δ34S and δ18O in SO42− in groundwater from the Ordos Cretaceous groundwater Basin and geological implications. Acta Geosci. Sin. 2010, 84, 432–440. [Google Scholar] [CrossRef]
- Dowuona, G.N.; Mermut, A.R.; Krouse, H.R. Stable isotope geochemistry of sulfate in relation to hydrogeology in southern Saskatchewan, Canada. Appl. Geochem. 1993, 8, 255–263. [Google Scholar] [CrossRef]
- Mayer, B.; Shanley, J.B.; Bailey, S.W.; Mitchell, M.J. Identifying sources of stream water sulfate after a summer drought in the Sleepers River watershed (Vermont, USA) using hydrological, chemical, and isotopic techniques. Appl. Geochem. 2010, 25, 747–754. [Google Scholar] [CrossRef]
- Samborska, K.; Halas, S.; Bottrell, S.H. Sources and impact of sulphate on groundwaters of Triassic carbonate aquifers, Upper Silesia, Poland. J. Hydrol. 2013, 486, 136–150. [Google Scholar] [CrossRef]
- Miao, Z.H.; Carroll, K.C.; Brusseau, M.L. Characterization and quantification of groundwater sulfate sources at a mining site in an arid climate: The Monument Valley site in Arizona, USA. J. Hydrol. 2013, 504, 207–215. [Google Scholar] [CrossRef]
- Tuttle, M.L.W.; Breit, G.N.; Cozzarelli, I.M. Processes affecting δ34S and δ18O values of dissolved sulfate in alluvium along the Canadian River, central Oklahoma, USA. Chem. Geol. 2009, 265, 455–467. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Patra, S.; Wang, F.; Wang, B.; Yue, F. Using a dual isotopic approach to trace sources and mixing of sulphate in Changjiang Estuary, China. Appl. Geochem. 2011, 26, S210–S213. [Google Scholar] [CrossRef]
- Valiente, N.; Carrey, R.; Otero, N.; Gutierrez-Villanueva, M.A.; Soler, A.; Sanz, D.; Castano, S.; Gomez-Alday, J.J. Tracing sulfate recycling in the hypersaline Perola Lake (SE Spain): A combined isotopic and microbiological approach. Chem. Geol. 2017, 473, 74–89. [Google Scholar] [CrossRef]
- Lang, Y.C.; Liu, C.Q.; Li, S.L.; Zhao, Z.Q.; Zhou, Z.H. Tracing natural and anthropogenic sources of dissolved sulfate in a karst region by using major ion chemistry and stable sulfur isotopes. Appl. Geochem. 2011, 26, S202–S205. [Google Scholar] [CrossRef]
- Yang, Y.C.; Shen, Z.L.; Wen, D.G.; Hou, G.C.; Zhao, Z.H.; Wang, D. Hydrochemical characteristics and sources of sulfate in groundwater of the Ordos Cretaceous groundwater basin. Acta Geosci. Sin. 2008, 29, 553–562, (In Chinese with English abstract). [Google Scholar]
- Marques, J.M.; Graça, H.; Eggenkamp, H.G.M.; Neves, O.; Carreira, P.M.; Matias, M.J.; Mayer, B.; Nunes, D.; Trancoso, V.N. Isotopic and hydrochemical data as indicators of recharge areas, flow paths and water-rock interaction in the Caldas da Rainha-Quinta das Janelas thermomineral carbonate rock aquifer (Central Portugal). J. Hydrol. 2013, 476, 302–313. [Google Scholar] [CrossRef]
- Al-Charideh, A. Isotopic evidence to characterize the sources of sulfate ions in the carbonate aquifer system in Aleppo basin (North Syria). Environ. Earth Sci. 2015, 73, 127–137. [Google Scholar] [CrossRef]
- Xiao, Q.; Jiang, Y.J.; Shen, L.C.; Yuan, D.X. Origin of calcium sulfate-type water in the Triassic carbonate thermal water system in Chongqing, China: A chemical and isotopic reconnaissance. Appl. Geochem. 2018, 89, 49–58. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Uncertainty in source partitioning using stable isotopes. Oecologia 2001, 127, 171–179. [Google Scholar] [CrossRef]
- Meghdadi, A.; Javar, N. Quantification of spatial and seasonal variations in the proportional contribution of nitrate sources using a multi-isotope approach and Bayesian isotope mixing model. Environ. Pollut. 2018, 235, 207–222. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).