Prognostic Value of the Modified Atherogenic Index of Plasma during Body Mass Reduction in Polish Obese/Overweight People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. Description of Dietary and Physical Activity Recommendations
2.3. Classification of Patients into Study Groups
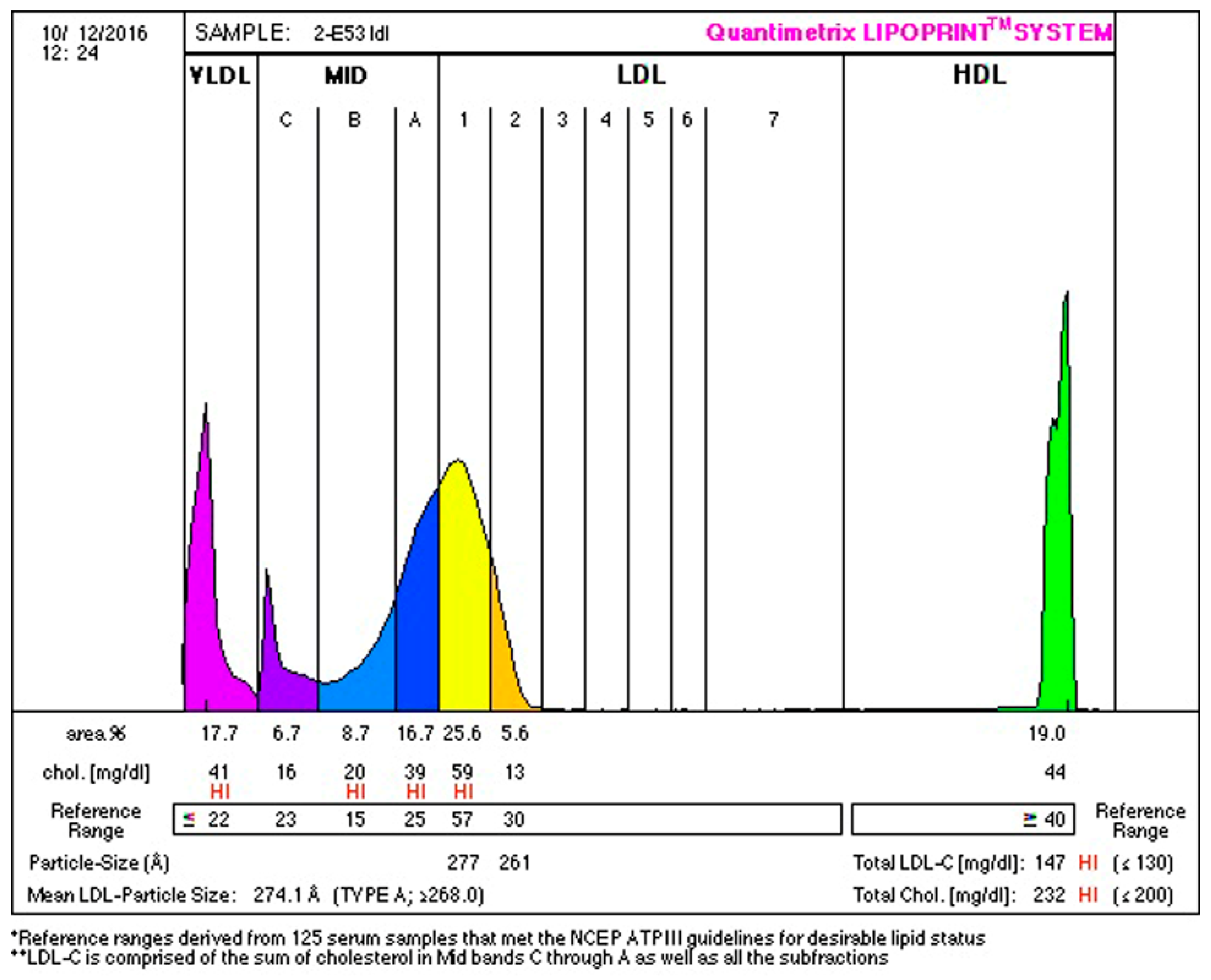
2.4. Biochemical Examination
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Pearson-Stuttard, J.; Bandosz, P.; Rehm, C.D.; Afshin, A.; Peñalvo, J.L.; Whitsel, L.; Danaei, G.; Micha, R.; Gaziano, T.; Lloyd-Williams, F.; et al. Comparing the effectiveness of mass media campaigns with price reductions targeting fruit and vegetable intake on US cardiovascular disease mortality and race disparities. Am. J. Clin. Nutr. 2017, 106, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Anyżewska, A.; Wawrzyniak, A.; Woźniak, A.; Krotki, M.; Górnicka, M. Nutritional assessment in Polish men with cardiovascular diseases. Rocz. Panstw. Zakl. Hig. 2013, 64, 211–215. [Google Scholar] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART Study): Case-control study. Lancet 2004, 64, 937–952. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed]
- Bangalore, S.; Fayyad, R.; Laskey, R.; DeMicco, D.A.; Messerli, F.H.; Waters, D.D. Body-weight fluctuations and outcomes in coronary disease. N. Engl. J. Med. 2017, 376, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Joint WHO/FAO Export Consultations on Diet, Nutrition and the Prevention of Chronic Diseases 2002; Raport No. 916; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Bandosz, P.; O’Flaherty, M.; Drygas, W.; Rutkowski, M.; Koziarek, J.; Wyrzykowski, B.; Bennett, K.; Zdrojewski, T.; Capewell, S. Decline in mortality from coronary heart disease in Poland after socio-economic transformation: Modeling study. BMJ 2012, 344, d8136. [Google Scholar] [CrossRef]
- Drygas, W.; Niklas, A.A.; Piwońska, A.; Piotrowski, W.; Flotyńska, A.; Kwaśniewska, M.; Nadrowski, P.; Puch-Walczak, A.; Szafraniec, K.; Bielecki, W.; et al. Multi-centre National Population Health Examination Survey (WOBASZ II study): Assumptions, methods, and implementation. Kardiol. Polska 2016, 74, 681–690. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- General Statistical Office. Branch Yearbooks, Demographic Year-Book of Poland; General Statistical Office: Warsaw, Poland, 2012. [Google Scholar]
- Dobiásová, M. AIP-atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Despres, J.P. Body fat distribution and risk of cardiovascular disease. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, H. Obesity and target organ damage: The heart. Int. J. Obes. 2002, 26, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European guidelines for the prevention of diseases of the heart and vessels in clinical practice for 2012. (Europejskie wytyczne dotyczące zapobiegania chorobom serca i naczyń w praktyce klinicznej na 2012 rok.). Kardiol. Polska 2012, 1, 49–51. [Google Scholar]
- WHO. Waist Circumference and Waist–Hip Ratio; Report of a WHO Expert Consultation, 8–11 December 2008; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Roever, L.; Veloso, F.C.; Resende, E.S. Visceral Fat, Atherosclerosis and Coronary Artery Disease. Intern. Med. 2015, 5, 3. [Google Scholar] [CrossRef]
- Hiuge-Shimizu, A.; Kishida, K.; Funahashi, T.; Okutsu, M.; Kametani, R.; Kobayashi, H.; Nozaki, Y.; Nomura, A.; Yokoi, H.; Yoshizumi, T.; et al. Coexistence of visceral fat and multiple risk factor accumulations is strongly associated with coronary artery disease in Japanese (the vacation-j study). J. Atheroscler. Thromb. 2012, 19, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Speaker, K.; Fleshner, M. Interleukin-1 beta: A potential link between stress and development of visceral obesity. BMC Physiol. 2012, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Cortes-Bergoderi, M. Obesity and the Heart. Rev. Esp. Cardiol. 2011, 64, 140–149. [Google Scholar] [CrossRef]
- Reaven, G. Insulin Resistance and coronary heart disease in nondiabetic individuals. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1754–1759. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Hamułka, J.; Głąbska, D.; Guzek, D.; Białkowska, A.; Sulich, A. Intake of Saturated Fatty Acids Affects Atherogenic Blood Properties in Young, Caucasian, Overweight Women Even without Influencing Blood Cholesterol. Int. J. Environ. Res. Public Health 2018, 15, 2530. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Moore, H.J.; Douthwaite, W.; Skeaff, C.M.; Summerbell, C.D. Effect of reducing total fat intake on body weight: Systematic review and meta-analysis of randomized controlled trials and cohort studies. BMJ 2012, 345, 7666. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Joint, F.A.O. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO Food and Nutrition Paper 91; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Nishida, C.; Uauy, R. WHO scientific update on health consequences of trans fatty acids: Introduction. Eur. J. Clin. Nutr. 2009, 63, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015.
- Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012.
- Lavie, C.J.; Milani, R.V.; Ventura, H.O. Obesity and cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Lau, D. The obesity epidemic and its impact on hypertension. Can. J. Cardiol. 2012, 28, 326–333. [Google Scholar] [CrossRef] [PubMed]



| Nutrient | % of Total Energy Intake | Note |
|---|---|---|
| fats | 25–35% | limiting intake of saturated fatty acids to less than 10% of total energy intake, and intake of 3–6% mono- and polyunsaturated fatty acids in the form of vegetable oils and fish oils |
| carbohydrates | 45–65% | limiting intake of free sugars to less than 10% of total energy intake |
| proteins | 10–15% | animal and vegetable sources |
| Group I n = 16 | Group II n = 36 | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| AIP (regular formula)_1 | 0.18 ± 0.21 | 0.37 ± 0.27 | 0.011 * |
| AIP (regular formula)_2 | 0.23 ± 0.21 | 0.22 ± 0.25 | ns |
| delta AIP (regular formula) | 0.05 ± 0.10 | −0.16 ± 0.14 | ≤0.001 *** |
| AIP (new formula)_1 | 0.65 ± 0.26 | 1.02 ± 0.37 | ≤0.001 *** |
| AIP (new formula)_2 | 0.75 ± 0.29 | 0.73 ± 0.30 | ns |
| delta AIP (new formula) | 0.09 ± 0.08 | −0.29 ± 0.18 | ≤0.001 *** |
| Group I n = 16 | Group II n = 36 | p | |
|---|---|---|---|
| Age (years) | 49.06 ± 10.07 | 47.42 ± 11.05 | ns |
| Height (cm) | 166.31 ± 6.78 | 167.14 ± 9.09 | ns |
| Weight_1 (kg) | 95.91 ± 22.11 | 102.46 ± 20.37 | ns |
| Weight_2 (kg) | 87.94 ± 18.79 | 92.24 ± 19.30 | ns |
| % change in weight | −7.84 ± 4.84 | −8.89 ± 7.56 | ns |
| Waist circumference_1 (cm) | 106.75 ± 18.10 | 111.03 ± 13.26 | ns |
| Waist circumference_2 (cm) | 97.88 ± 14.59 | 99.78 ± 14.54 | ns |
| % change in waist circumference | −7.88 ± 6.35 | −10.25 ± 5.80 | ns |
| Hip circumference _1 (cm) | 120.06 ± 18.78 | 122.31 ± 11.77 | ns |
| Hip circumference _2 (cm) | 113.31 ± 13.88 | 113.83 ± 11.40 | ns |
| % change in hip circumference | −5.06 ± 5.38 | −6.92 ± 5.09 | ns |
| WHR_1 | 0.89 ± 0.11 | 0.91 ± 0.08 | ns |
| WHR_2 | 0.86 ± 0.09 | 0.88 ± 0.09 | ns |
| % change in WHR | −2.75 ± 5.46 | −3.69 ± 4.97 | ns |
| ICO_1 | 0.64 ± 0.10 | 0.66 ± 0.08 | ns |
| ICO_2 | 0.59 ± 0.09 | 0.60 ± 0.08 | ns |
| % change in ICO | −7.88 ± 6.35 | −10.22 ± 5.82 | ns |
| BMI_1 | 34.64 ± 7.66 | 36.57 ± 5.86 | ns |
| BMI_2 | 31.73 ± 6.68 | 32.91 ± 5.85 | ns |
| % change in BMI | −8.13 ± 4.53 | −10.00 ± 5.66 | ns |
| VFA _1 | 139.53 ± 39.71 | 141.14 ± 32.25 | ns |
| VFA _2 | 117.95 ± 31.81 | 118.79 ± 32.33 | ns |
| % change in VFA | −13.69 ± 15.99 | −15.26 ± 15.47 | ns |
| BFM (body fat mass)1 | 38.46 ± 15.28 | 41.03 ± 14.06 | ns |
| BFM (body fat mass)2 | 32.21 ± 10.34 | 34.26 ± 12.36 | ns |
| % change in BFM | −8.36 ± 17.77 | −16.47 ± 21.61 | ns |
| Group I n = 16 | Group II n = 36 | p | |
|---|---|---|---|
| TC_1 (mg/dL) | 194.63 ± 40.50 | 231.58 ± 46.61 | 0.008 ** |
| TC_2 (mg/dL) | 217.58 ± 39.57 | 226.43 ± 66.26 | ns |
| % change in TC | +13.75 ± 18.73 | −1.64 ± 19.19 | 0.010 ** |
| TG_1 (mg/dL) | 90.19 ± 38.26 | 152.46 ± 116.99 | 0.044 * |
| TG_2 (mg/dL) | 110.63 ± 45.75 | 111.84 ± 76.16 | ns |
| % change in TG | +27.50 ± 33.66 | −20.11 ± 28.13 | ≤0.001 *** |
| HDL-C_1 (mg/dL) | 56.56 ± 10.42 | 55.32 ± 9.53 | ns |
| HDL-C_2 (mg/dL) | 61.97 ± 9.90 | 60.75 ± 11.69 | ns |
| % change in HDL-C | +10.75 ± 11.94 | +10.50 ± 15.12 | ns |
| LDL-C_1 (mg/dL) | 120.06 ± 33.51 | 144.37 ± 33.18 | 0.019 * |
| LDL-C_2 (mg/dL) | 137.07 ± 33.97 | 143.09 ± 58.02 | ns |
| % change in LDL-C | +16.60 ± 32.04 | −0.23 ± 26.45 | ns |
| Group I n = 16 | Group II n = 36 | p | |
|---|---|---|---|
| HDL1_1 | 5.13 ± 2.47 | 2.86 ± 2.40 | 0.003 ** |
| delta HDL1 | −1.50 ± 2.00 | 0.86 ± 2.60 | 0.002 ** |
| % change in HDL1 | −20.60 ± 41.17 | 56.90 ± 127.84 | 0.003 ** |
| HDL2_1 | 8.81 ± 3.04 | 6.69 ± 2.99 | 0.023 * |
| delta HDL2 | 0.19 ± 1.97 | 1.44 ± 2.06 | 0.045 * |
| % change in HDL2 | 3.43 ± 24.80 | 33.53 ± 46.49 | 0.019 * |
| delta HDL3 | 1.56 ± 1.86 | 2.92 ± 1.73 | 0.014 * |
| delta large HDL (1–3) | 0.69 ± 4.66 | 5.08 ± 4.16 | 0.001 ** |
| % change in large HDL (1–3) | 4.33 ± 26.36 | 52.32 ± 55.00 | ≤0.001 *** |
| HDL6_1 | 10.50 ± 1.67 | 12.61 ± 1.84 | ≤0.001 *** |
| HDL7_ | 3.56 ± 0.73 | 4.22 ± 0.93 | 0.015 * |
| delta HDL7 | 0.31 ± 0.70 | −0.39 ± 0.99 | 0.014 * |
| delta HDL8 | 0.25 ± 0.93 | −0.64 ± 1.22 | 0.013 * |
| delta HDL9 | 0.19 ± 0.91 | −0.58 ± 1.11 | 0.018 * |
| delta HDL10 | −0.13 ± 1.93 | −2.17 ± 3.01 | 0.016 * |
| delta small HDL | 0.38 ± 3.65 | −3.53 ± 4.44 | 0.003 ** |
| % change in small HDL | 8.99± 36.70 | −23.68± 31.77 | 0.002 ** |
| VLDL_1 | 32.38 ± 15.10 | 43.19 ± 18.71 | 0.047 * |
| delta VLDL | 2.56 ± 14.07 | −8.69 ± 11.42 | 0.004 ** |
| IDL-B_1 | 11.50 ± 5.49 | 15.39 ± 6.35 | 0.039 * |
| delta LDL1 | 8.81 ± 17.38 | −3.03 ± 17.53 | 0.029 * |
| LDL2_1 | 11.94 ± 11.28 | 21.67 ± 14.20 | 0.019 * |
| delta LDL2 | 4.06 ± 16.27 | −5.36 ± 11.76 | 0.022 * |
| LDL (1–2)1 | 53.69 ± 21.60 | 72.61 ± 25.08 | 0.012 * |
| delta LDL (1–2) | 12.88 ± 28.79 | −8.39 ± 23.77 | 0.007 ** |
| % change in LDL (1–2) | 50.06 ± 89.17 | −5.20 ± 28.69 | 0.027 * |
| Group I n = 16 | Group II n = 36 | p | |
|---|---|---|---|
| HDL6_1 | 11.00 ± 1.90 | 12.53 ± 2.01 | 0.011 * |
| delta HDL10_2 | 0.12 ± 1.80 | 2.25 ± 3.01 | 0.009 ** |
| delta small HDL (8–10) | 0.18 ± 3.45 | 3.68 ± 4.89 | 0.011 * |
| % change in small HDL (8–10) | −1.19 ± 33.99 | 22.41 ± 35.77 | 0.026 * |
| delta VLDL | −1.35 ± 9.90 | 8.81 ± 13.86 | 0.009 ** |
| delta IDL-B | −3.29 ± 8.63 | 1.75 ± 6.02 | 0.017 * |
| delta LDL1 | −10.00 ± 12.98 | 3.89 ± 18.46 | 0.007 ** |
| LDL2_1 | 13.12 ± 11.77 | 21.50 ± 14.18 | 0.039 * |
| delta LDL2 | −5.94 ± 14.70 | 5.08 ± 14.38 | 0.013 * |
| LDL (1–2)_1 | 57.41 ± 24.75 | 73.14 ± 26.74 | 0.046 * |
| delta LDL (1–2) | −15.94 ± 22.33 | 8.97 ± 26.26 | 0.001 ** |
| % change in LDL (1–2) | −51.43 ± 81.46 | 6.34 ± 32.79 | 0.011 * |
| LDL3_1 | 0.76 ± 2.22 | 3.33 ± 6.73 | 0.045 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalejska-Fiolka, J.; Hubková, B.; Birková, A.; Veliká, B.; Puchalska, B.; Kasperczyk, S.; Błaszczyk, U.; Fiolka, R.; Bożek, A.; Maksym, B.; et al. Prognostic Value of the Modified Atherogenic Index of Plasma during Body Mass Reduction in Polish Obese/Overweight People. Int. J. Environ. Res. Public Health 2019, 16, 68. https://doi.org/10.3390/ijerph16010068
Zalejska-Fiolka J, Hubková B, Birková A, Veliká B, Puchalska B, Kasperczyk S, Błaszczyk U, Fiolka R, Bożek A, Maksym B, et al. Prognostic Value of the Modified Atherogenic Index of Plasma during Body Mass Reduction in Polish Obese/Overweight People. International Journal of Environmental Research and Public Health. 2019; 16(1):68. https://doi.org/10.3390/ijerph16010068
Chicago/Turabian StyleZalejska-Fiolka, Jolanta, Beáta Hubková, Anna Birková, Beáta Veliká, Beata Puchalska, Sławomir Kasperczyk, Urszula Błaszczyk, Rafał Fiolka, Andrzej Bożek, Beata Maksym, and et al. 2019. "Prognostic Value of the Modified Atherogenic Index of Plasma during Body Mass Reduction in Polish Obese/Overweight People" International Journal of Environmental Research and Public Health 16, no. 1: 68. https://doi.org/10.3390/ijerph16010068
APA StyleZalejska-Fiolka, J., Hubková, B., Birková, A., Veliká, B., Puchalska, B., Kasperczyk, S., Błaszczyk, U., Fiolka, R., Bożek, A., Maksym, B., Mareková, M., & Birkner, E. (2019). Prognostic Value of the Modified Atherogenic Index of Plasma during Body Mass Reduction in Polish Obese/Overweight People. International Journal of Environmental Research and Public Health, 16(1), 68. https://doi.org/10.3390/ijerph16010068





