A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Treatments
2.2.1. Chlorpyrifos Exposure
2.2.2. Doxycycline Treatment
2.3. Cell Culture and Treatment
2.4. RNA Sequencing Data and Bioinformatics Analyses
2.5. RNA Extraction and Quantitative Reverse Transcription PCR
2.6. Protein Extraction and Western Blotting
2.7. Statistical and Bioinformatics Analyses
2.8. Data Availability Statement
3. Results
3.1. In Silico Toxicogenomic Analyses of Chlorpyrifos Activity Reveal a Gene Regulatory Network Potentially Involved in Thyroid Carcinogenesis
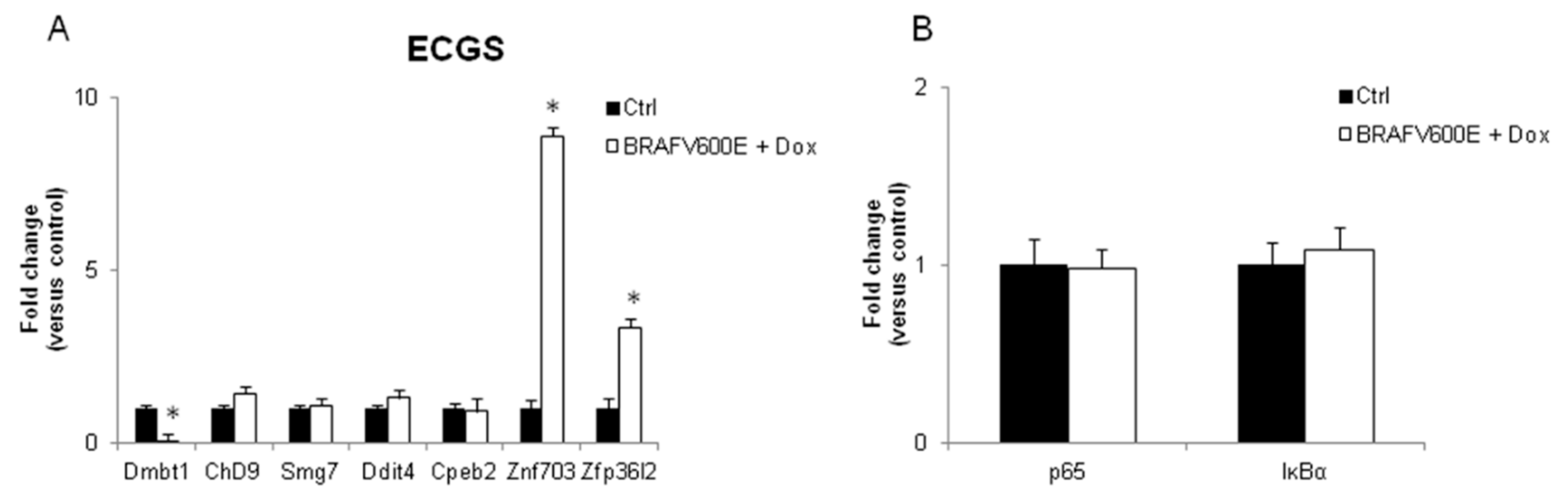
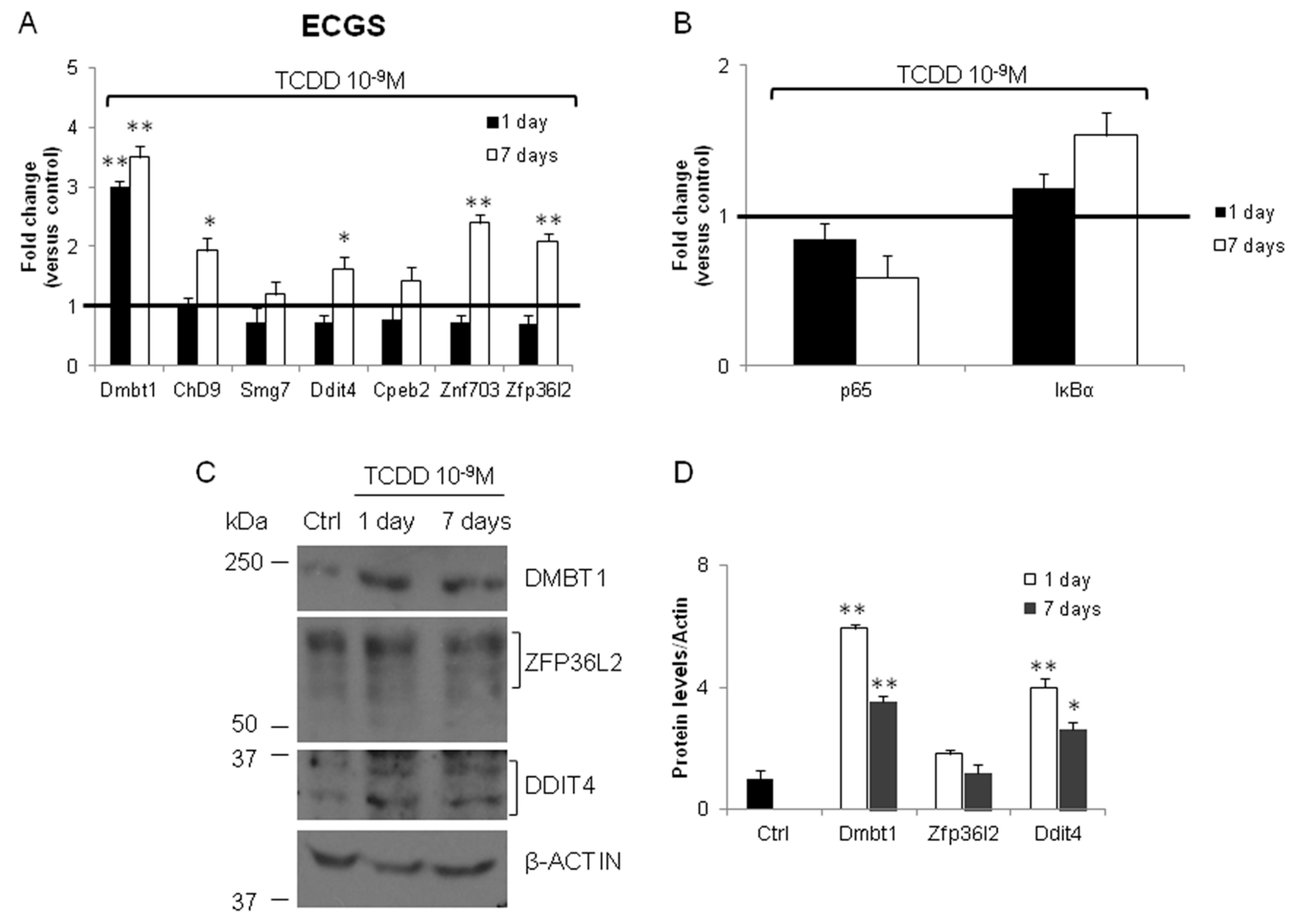
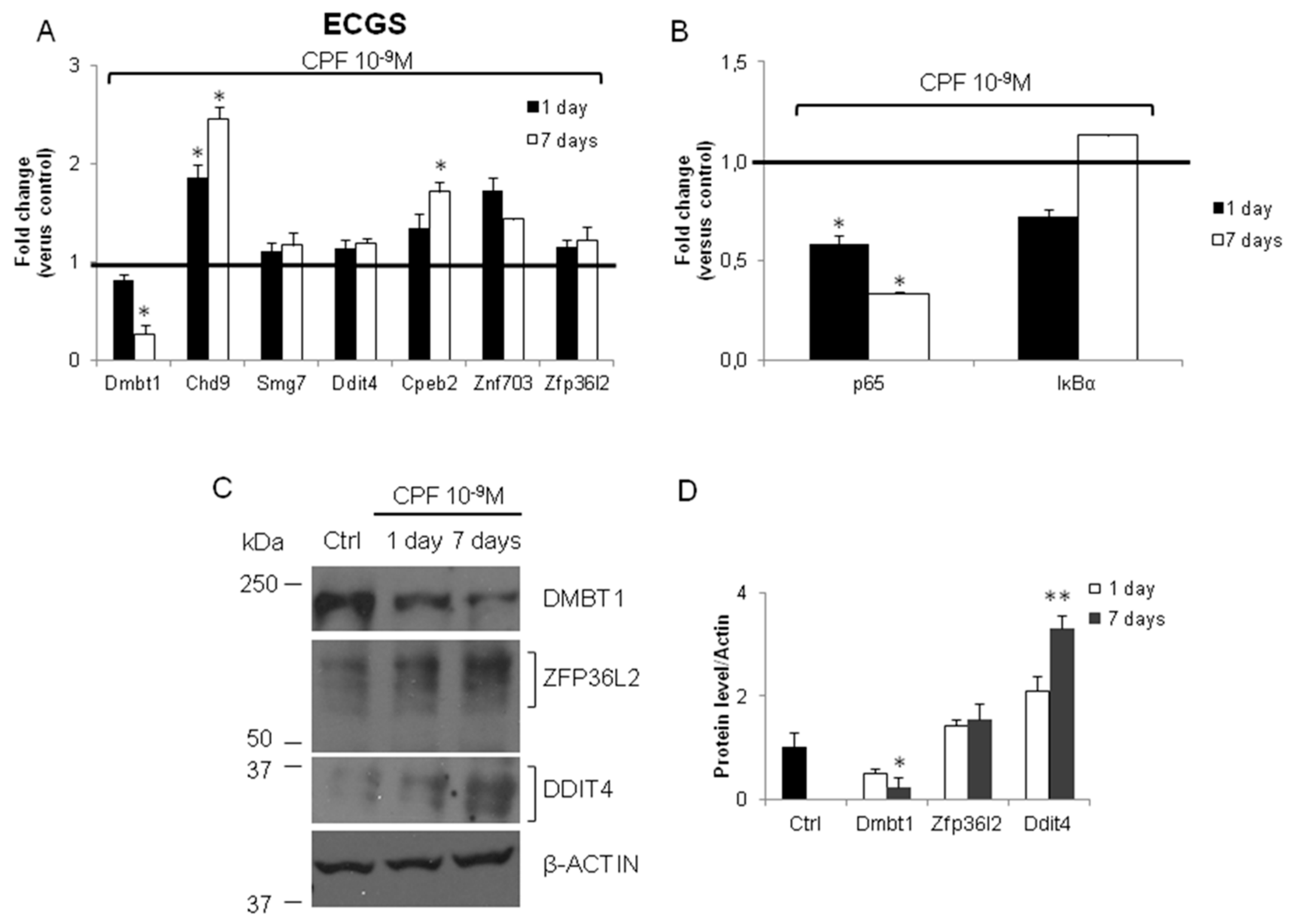
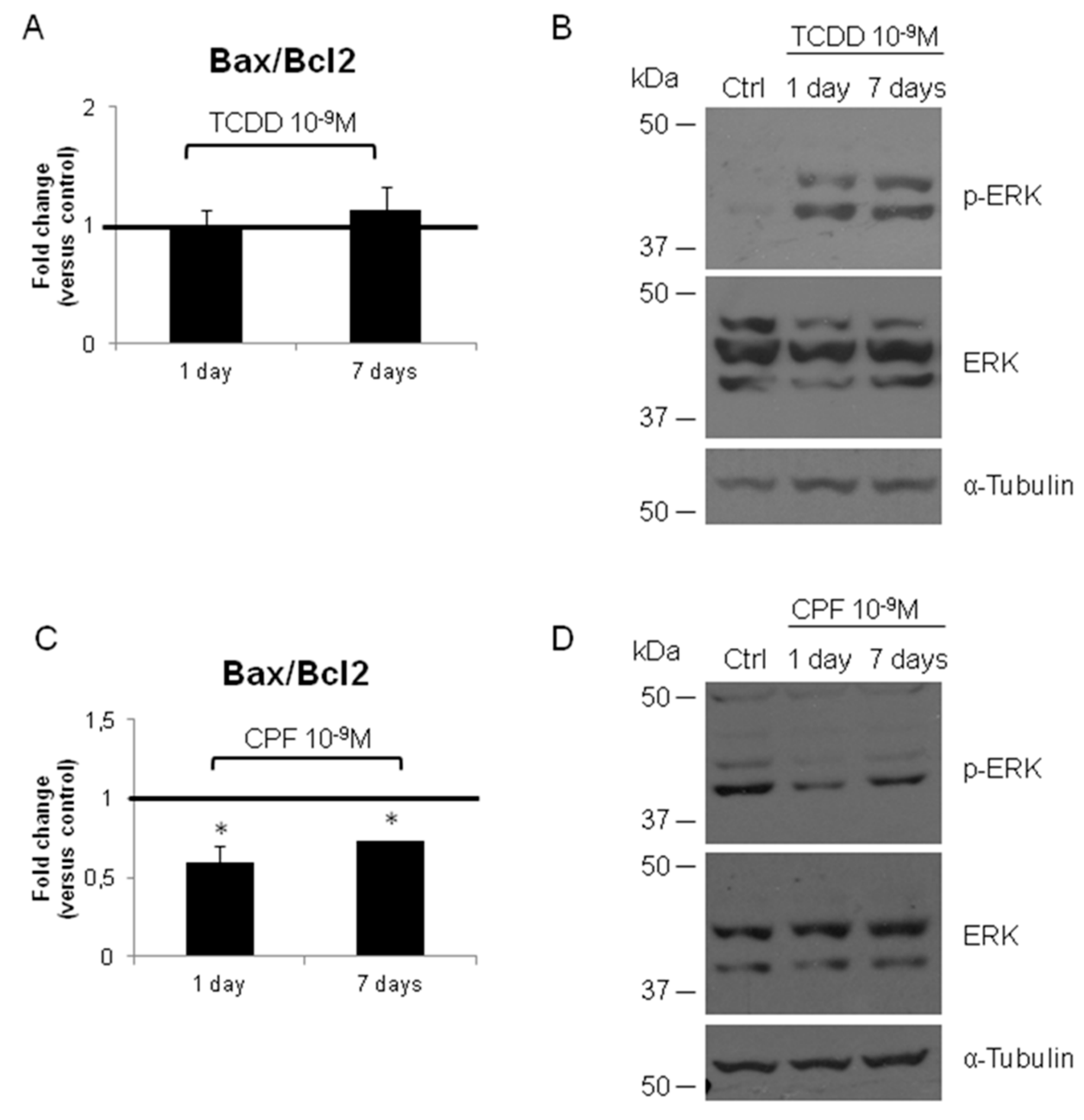
3.2. Validation of the New Gene-Network in Cellular and Animal Models of Thyroid Carcinogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutleb, A.C.; Cambier, S.; Serchi, T. Impact of Endocrine Disruptors on the Thyroid Hormone System. Horm. Res. Paediatr. 2016, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, T.R. Environmental chemicals targeting thyroid. Hormones (Athens) 2010, 9, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Moracci, G.; Altieri, I.; Rasinger, J.D.; Carroll, T.S.; Hogstrand, C.; Lundebye, A.K.; Mantovani, A. Dietary exposure of juvenile female mice to polyhalogenated seafood contaminants (HBCD, BDE-47, PCB-153, TCDD): Comparative assessment of effects in potential target tissues. Food Chem. Toxicol. 2013, 56, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Porreca, I.; Ulloa Severino, L.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; Altucci, L.; Amendola, E.; Nebbioso, A.; Mallardo, M.; De Felice, M.; et al. “Stockpile” of Slight Transcriptomic Changes Determines the Indirect Genotoxicity of Low-DoseBPA in Thyroid Cells. PLoS ONE 2016, 11, e0151618. [Google Scholar] [CrossRef] [PubMed]
- Reale, C.; Porreca, I.; Russo, F.; Marotta, M.; Roberto, L.; Russo, N.A.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Genetic background and window of exposure contribute to thyroid dysfunction promoted by low-dose exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Sci. Rep. 2018, 8, 16324. [Google Scholar] [CrossRef] [PubMed]
- Caughlan, A.; Newhouse, K.; Namgung, U.; Xia, Z. Chlorpyrifos induces apoptosis in rat cortical neurons that is regulated by a balance between p38 and ERK/JNK MAP kinases. Toxicol. Sci. 2004, 78, 125–134. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Q.; Guo, S.; Zhang, Q.; Tang, J.; Liu, G.; Kong, D.; Li, J.; Yan, S.; Wang, R.; et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin promotes endothelial cell apoptosis through activation of EP3/p38MAPK/Bcl-2 pathway. J. Cell Mol. Med. 2017, 21, 3540–3551. [Google Scholar] [CrossRef]
- Ye, Z.Q.; Gu, D.N.; Hu, H.Y.; Zhou, Y.L.; Hu, X.Q.; Zhang, X.H. Hashimoto’s thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J. Surg. Oncol. 2013, 11, 56. [Google Scholar] [CrossRef]
- Hrafnkelsson, J.; Tulinius, H.; Kjeld, M.; Sigvaldason, H.; Jonasson, J.G. Serum thyroglobulin as a risk factor for thyroid carcinoma. Acta Oncol. 2000, 39, 973–977. [Google Scholar] [CrossRef]
- Jonklaas, J.; Nsouli-Maktabi, H.; Soldin, S.J. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid 2008, 18, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Ozdemir, D.; Dirikoc, A.; Oguz, A.; Tuzun, D.; Baser, H.; Ersoy, R.; Cakir, B. Are endogenously lower serum thyroid hormones new predictors for thyroid malignancy in addition to higher serum thyrotropin? Endocrine 2010, 37, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484. [Google Scholar] [CrossRef] [PubMed]
- Goldner, W.S.; Sandler, D.P.; Yu, F.; Hoppin, J.A.; Kamel, F.; Levan, T.D. Pesticide use and thyroid disease among women in the Agricultural Health Study. Am. J. Epidemiol. 2010, 171, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Lerro, C.; Lavoué, J.; Huang, H.; Siemiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Aschebrook-Kilfoy, B.; Ward, M.H.; Della Valle, C.T.; Friesen, M.C. Occupation and thyroid cancer. Occup. Environ. Med. 2014, 71, 366–380. [Google Scholar] [CrossRef] [Green Version]
- Goldner, W.S.; Sandler, D.P.; Yu, F.; Shostrom, V.; Hoppin, J.A.; Kamel, F.; LeVan, T.D. Hypothyroidism and pesticide use among male private pesticide applicators in the agricultural health study. J. Occup. Environ. Med. 2013, 55, 1171–1178. [Google Scholar] [CrossRef]
- Toft, G.; Flyvbjerg, A.; Bonde, J.P. Thyroid function in Danish greenhouse workers. Environ. Health 2006, 5, 32. [Google Scholar] [CrossRef]
- De Angelis, S.; Tassinari, R.; Maranghi, F.; Eusepi, A.; Di Virgilio, A.; Chiarotti, F.; Ricceri, L.; Venerosi Pesciolini, A.; Gilardi, E.; Moracci, G.; et al. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicol. Sci. 2009, 108, 311–319. [Google Scholar] [CrossRef]
- Maranghi, F.; De Angelis, S.; Tassinari, R.; Chiarotti, F.; Lorenzetti, S.; Moracci, G.; Marcoccia, D.; Gilardi, E.; Di Virgilio, A.; Eusepi, A.; et al. Reproductive toxicity and thyroid effects in Sprague Dawley rats exposed to low doses of ethylenethiourea. Food Chem. Toxicol. 2013, 59, 261–271. [Google Scholar] [CrossRef]
- Crofton, K.M. Thyroid disrupting chemicals: Mechanisms and mixtures. Int. J. Androl. 2008, 31, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Zhang, Y.F.; Xu, H.X.; Zhang, X.P. The role of BRAF in the pathogenesis of thyroid carcinoma. Front. Biosci. (Landmark Ed.) 2015, 20, 1068–1078. [Google Scholar] [PubMed]
- Shirokawa, J.M.; Elisei, R.; Knauf, J.A.; Hara, T.; Wang, J.; Saavedra, H.I.; Fagin, J.A. Conditional Apoptosis Induced by Oncogenic Ras in Thyroid Cells. Mol. Endocrinol. 2000, 14, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Porreca, I.; D’Angelo, F.; Gentilcore, D.; Carchia, E.; Amoresano, A.; Affuso, A.; Ceccarelli, M.; De Luca, P.; Esposito, L.; Guadagno, F.M.; et al. Cross-species toxicogenomic analyses and phenotypic anchoring in response to groundwater low-level pollution. BMC Genom. 2014, 15, 1067. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, D.; Porreca, I.; Cobellis, G.; Tarallo, R.; Nassa, G.; Falco, G.; Nardone, A.; Rizzo, F.; Mallardo, M.; Ambrosino, C. Carcinogenic risk and Bisphenol A exposure: A focus of molecular aspects in endoderm derived glands. Mol. Cell. Endocrinol. 2017, 457, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, A.J.; Veerman, E.C.; Nieuw Amerongen, A.V.; Mollenhauer, J. Salivary agglutinin/glycoprotein-340/DMBT1: A single molecule with variable composition and with different functions in infection, inflammation and cancer. Biol. Chem. 2007, 388, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrogio, A.; Nagaoka, K.; Richter, J.D. Translation control of cell growth and malignancy by the CPEBs. Nat. Rev. Cancer 2013, 13, 283. [Google Scholar] [CrossRef]
- De Vita, G.; Bauer, L.; da Costa, V.M.; De Felice, M.; Baratta, M.G.; De Menna, M.; Di Lauro, R. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol. Endocrinol. 2005, 19, 76–89. [Google Scholar] [CrossRef]
- De Menna, M.; D’Amato, V.; Ferraro, A.; Fusco, A.; Di Lauro, R.; Garbi, C.; De Vita, G. Wnt4 inhibits cell motility induced by oncogenic Ras. Oncogene 2013, 32, 4110–4119. [Google Scholar] [CrossRef]
- Frezzetti, D.; De Menna, M.; Zoppoli, P.; Guerra, C.; Ferraro, A.; Bello, A.M.; De Luca, P.; Calabrese, C.; Fusco, A.; Ceccarelli, M.; et al. Upregulation of miR-21 by Ras in vivo and its role in tumor growth. Oncogene 2011, 30, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Porreca, I.; D’Angelo, F.; De Franceschi, L.; Mattè, A.; Ceccarelli, M.; Iolascon, A.; Zamò, A.; Russo, F.; Ravo, M.; Tarallo, R.; et al. Pesticide toxicogenomics across scales: In vitro transcriptome predicts mechanisms and outcomes of exposure in vivo. Sci. Rep. 2016, 6, 38131. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Porreca, I.; Rizzo, F.; Ganbaatar, E.; Carchia, E.; Mallardo, M.; De Felice, M.; Ambrosino, C. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013, 304, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Namba, H.; Saenko, V.; Yamashita, S. Nuclear factor-kB in thyroid carcinogenesis and progression: A novel therapeutic target for advanced thyroid cancer. Arq. Bras. Endocrinol. Metabol. 2007, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, N.; Guyton, K.Z.; Loomis, D.; Barupal, D.K. Prioritizing Chemicals for Risk Assessment Using Chemoinformatics: Examples from the IARC Monographs on Pesticides. Environ. Health Perspect. 2016, 124, 1823. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Kaseb, A.; Li, D.; Patt, Y.Z.; Vauthey, J.N.; Thomas, M.B.; Curley, S.A.; Spitz, M.R.; Sherman, S.I.; Abdalla, E.K.; et al. Association between hypothyroidism and hepatocellular carcinoma: A case-control study in the United States. Hepatology 2009, 49, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jin, W.; Zhou, S.; Yang, J.D.; Harmsen, W.S.; Giama, N.H.; Wongjarupong, N.; Heimbach, J.K.; Watt, K.D.; Malhi, H.; et al. Hypothyroidism is associated with worse outcomes of hepatocellular carcinoma patients after liver transplantation. Cancer Med. 2018. [Google Scholar] [CrossRef]
- Mollenhauer, J.; Wiemann, S.; Scheurlen, W.; Korn, B.; Hayashi, Y.; Wilgenbus, K.K.; von Deimling, A.; Poustka, A. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3-26.1 is deleted in malignant brain tumours. Nat. Genet. 1997, 17, 32–39. [Google Scholar] [CrossRef]
- Garay, J.; Piazuelo, M.B.; Lopez-Carrillo, L.; Leal, Y.A.; Majumdar, S.; Li, L.; Cruz-Rodriguez, N.; Serrano-Gomez, S.J.; Busso, C.S.; Schneider, B.G.; et al. Increased expression of deleted in malignant brain tumors (DMBT1) gene in precancerous gastric lesions: Findings from human and animal studies. Oncotarget 2017, 8, 47076–47089. [Google Scholar] [CrossRef]
- Salomon-Kent, R.; Marom, R.; John, S.; Dundr, M.; Schiltz, L.R.; Gutierrez, J.; Workman, J.; Benayahu, D.; Hager, G.L. New Face for Chromatin-Related Mesenchymal Modulator: N-CHD9 Localizes to Nucleoli and Interacts With Ribosomal Genes. J. Cell. Physiol. 2015, 230, 2270–2780. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hazekawa, M.; Yasukochi, A.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M. miR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Mol. Carcinog. 2018, 57, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fagman, H.; Amendola, E.; Parrillo, L.; Zoppoli, P.; Marotta, P.; Scarfò, M.; De Luca, P.; De Carvalho, D.P.; Ceccarelli, M.; De Felice, M.; et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev. Biol. 2011, 359, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Abulaiti, A.; Fikaris, A.J.; Tsygankova, O.M.; Meinkoth, J.L. Ras induces chromosome instability and abrogation of the DNA damage response. Cancer Res. 2006, 66, 10505–10512. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Srivastava, N. In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 761, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Das, D.N.; Panda, P.K.; Sinha, N.; Mukhopadhyay, S.; Naik, P.P.; Bhutia, S.K. DNA damage by 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced p53-mediated apoptosis through activation of cytochrome P450/aryl hydrocarbon receptor. Environ. Toxicol. Pharmacol. 2017, 55, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, E.; Xing, Q.; Yan, J.; Arrington, A.; Wang, C.; Tully, D.; Kowolik, C.M.; Lu, D.M.; Frankel, P.H.; et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer. Lett. 2015, 358, 170–179. [Google Scholar] [CrossRef]
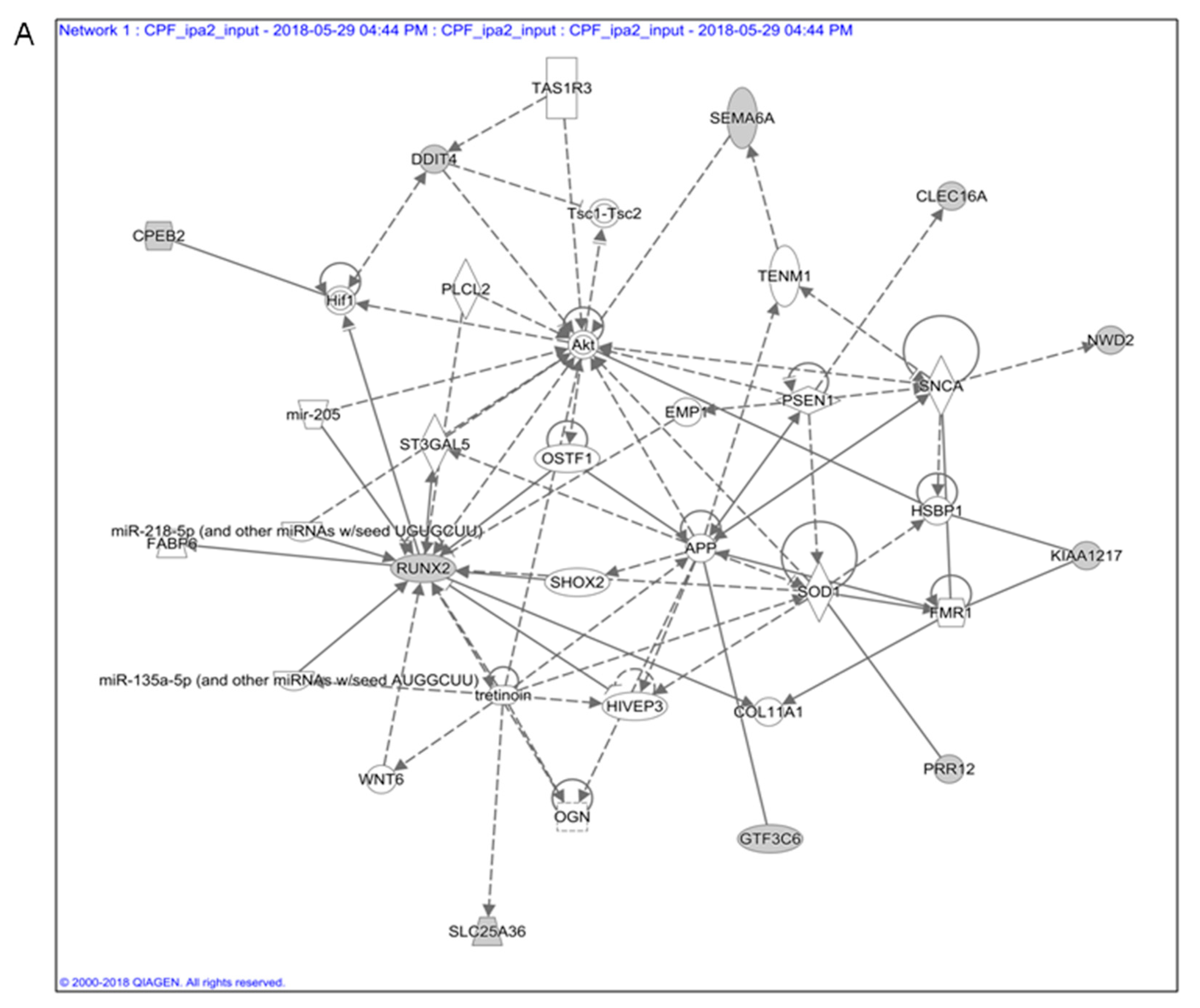
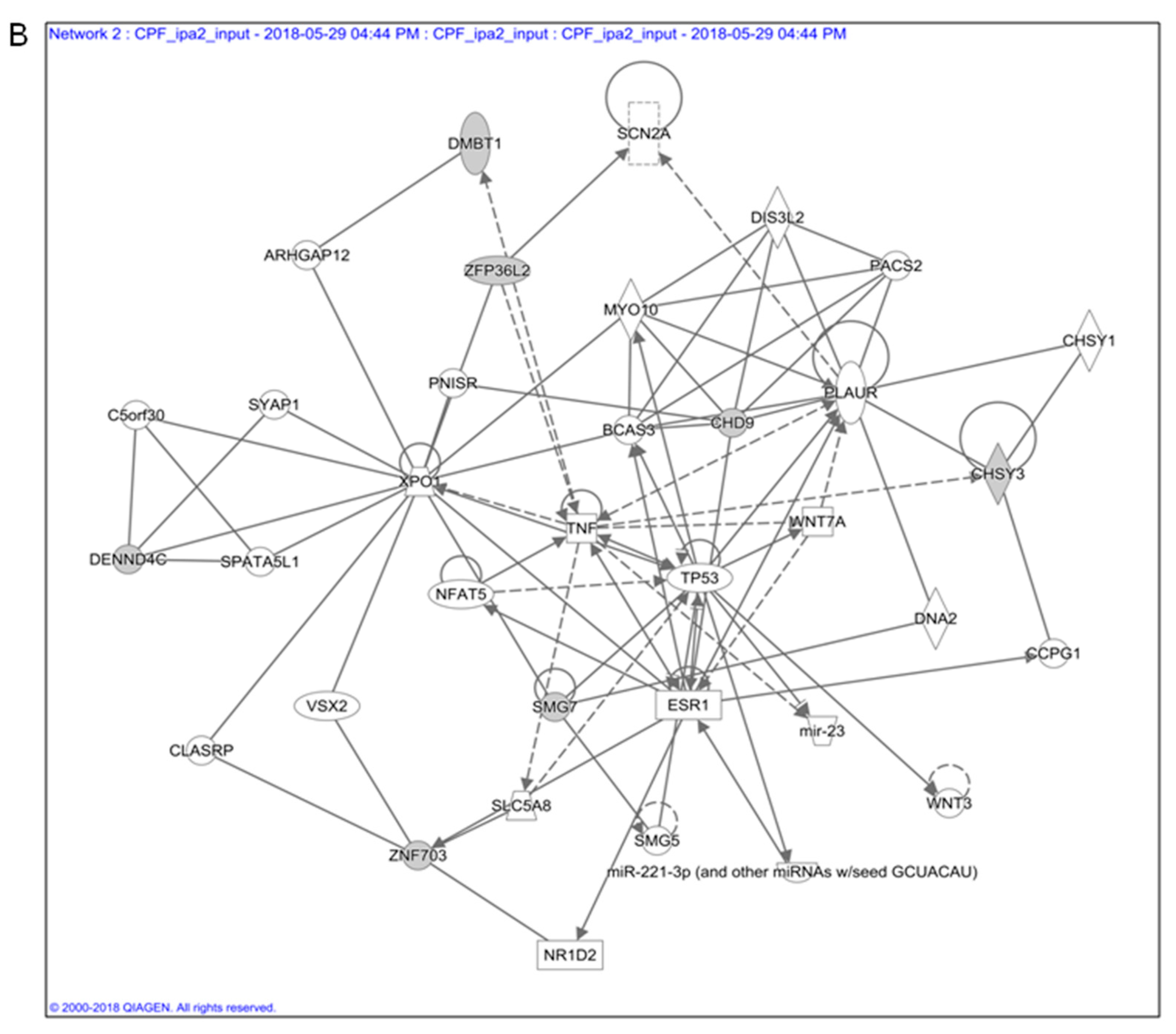
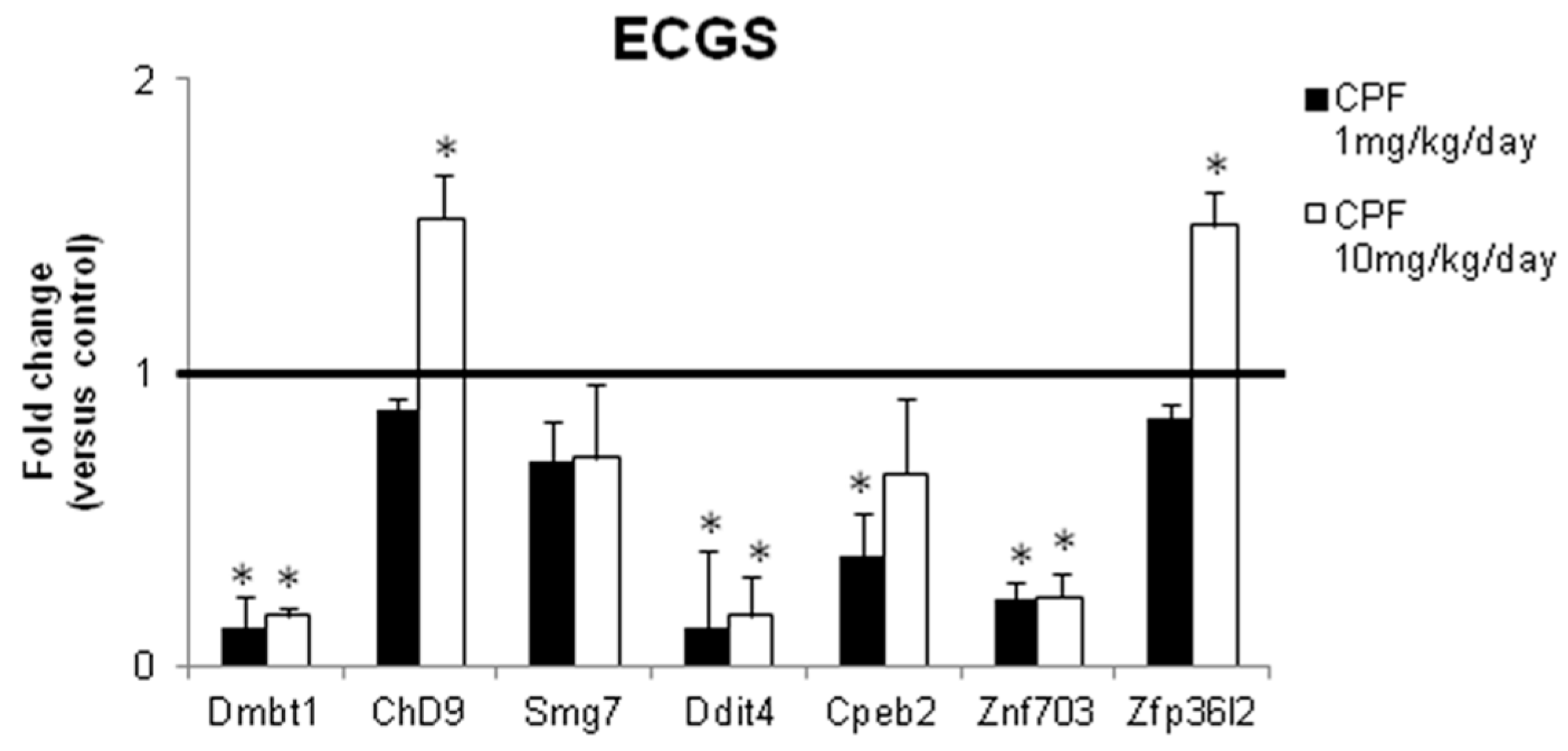
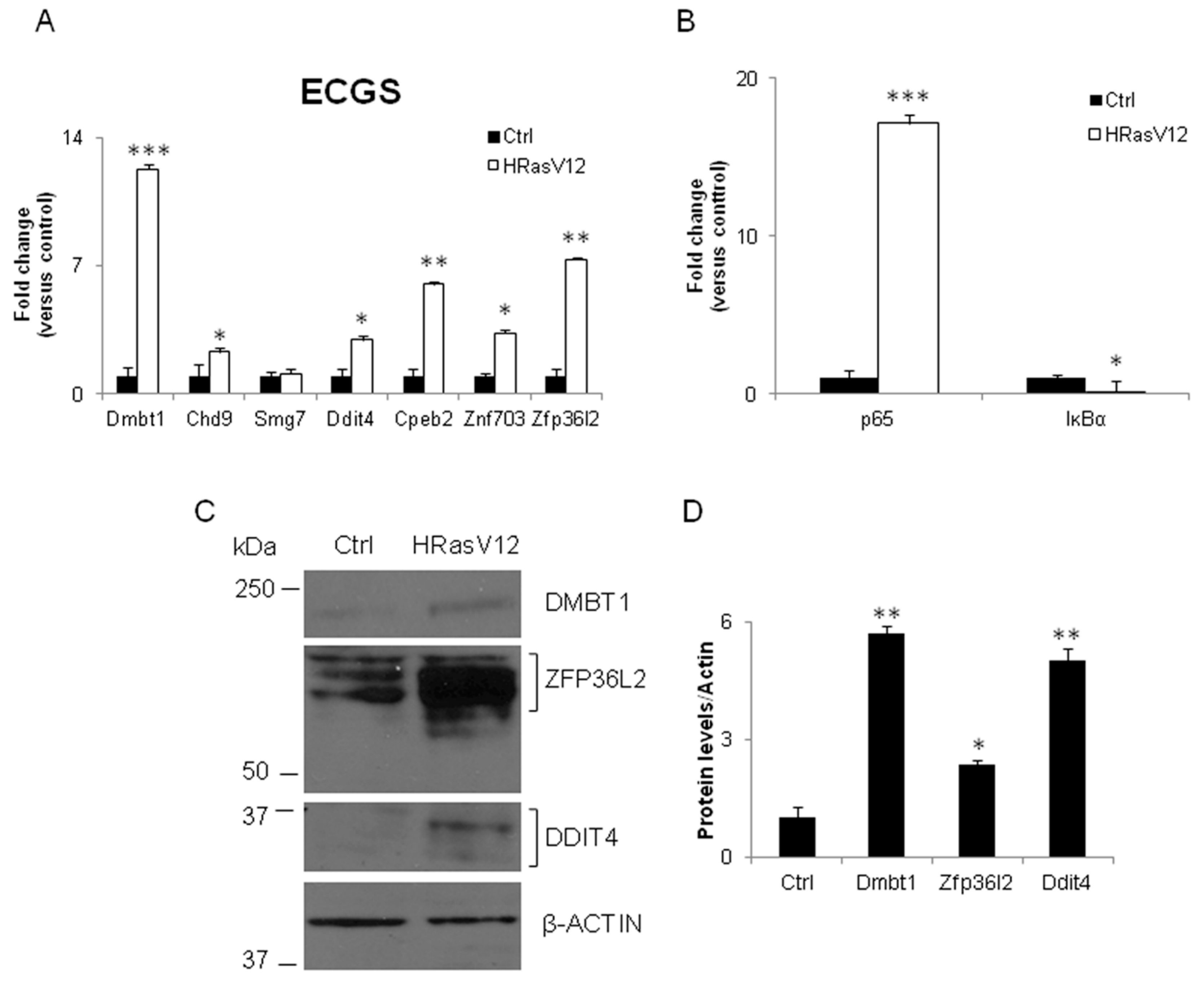
| Categories | Diseases or Functions Annotation | Corrected p-Value | Genes |
|---|---|---|---|
| Cell Cycle | Entry into G2 phase | 2.86 × 10−2 | EGR1, RUNX2 |
| Cellular Growth and Proliferation, Embryonic Development | Formation of embryonic cells | 2.86 × 10−2 | GNAO1, RUNX2 |
| Gene Expression | Transcription of DNA | 2.86 × 10−2 | BEX1, EGR1, FZD5, GTF3C6, HEXIM2, HMGA1, RUNX2, TARBP1, ZFP36L2, ZNF628, ZNF703 |
| Cancer, Organismal Injury and Abnormalities | Epithelial cancer | 2.86 × 10−2 | BEX1, C9orf172, CDKL4, CHD9, CHSY3, CLEC16A, CPEB2, CTNNAL1, DDIT4, DENND4C, DMBT1, EGR1, FZD5, GNAO1, GTF3C6, HEXIM2, HMGA1, INPP5J, KIAA1217, NWD2, PRR12, RUNX2, SEMA6A, SLC25A36, SMG7, SUSD6, TARBP1, WDR76, ZFP36L2, ZNF628, ZNF703 |
| Cellular Development, Hematological System Development and Function, Hematopoiesis | Erythropoiesis of cells | 2.86 × 10−2 | EGR1, HMGA1 |
| Cancer, Organismal Injury and Abnormalities, Reproductive System Disease | Mammary tumor | 2.86 × 10−2 | BEX1, CPEB2, CTNNAL1, DDIT4, EGR1, GNAO1, INPP5J, RUNX2, SUSD6, ZFP36L2, ZNF703 |
| Cell-mediated Immune Response, Cellular Development, Cellular Function and Maintenance, Hematological System Development and Function, Hematopoiesis, Lymphoid Tissue Structure and Development | Differentiation of T-lymphocytes | 2.86 × 10−2 | EGR1, FZD5, HMGA1, RUNX2, ZFP36L2 |
| Cellular Development, Hematological System Development and Function, Hematopoiesis, Lymphoid Tissue Structure and Development | Development of lymphocytes | EGR1, Fus, FZD5, HMGA1, RUNX2, ZFP36L2 | |
| Endocrine System Development and Function, Lipid Metabolism, Molecular Transport, Small Molecule Biochemistry | Secretion of aldosterone | EGR1, HMGA1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reale, C.; Russo, F.; Credendino, S.C.; Cuomo, D.; De Vita, G.; Mallardo, M.; Pennino, F.; Porreca, I.; Triassi, M.; De Felice, M.; et al. A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis. Int. J. Environ. Res. Public Health 2019, 16, 122. https://doi.org/10.3390/ijerph16010122
Reale C, Russo F, Credendino SC, Cuomo D, De Vita G, Mallardo M, Pennino F, Porreca I, Triassi M, De Felice M, et al. A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis. International Journal of Environmental Research and Public Health. 2019; 16(1):122. https://doi.org/10.3390/ijerph16010122
Chicago/Turabian StyleReale, Carla, Filomena Russo, Sara Carmela Credendino, Danila Cuomo, Gabriella De Vita, Massimo Mallardo, Francesca Pennino, Immacolata Porreca, Maria Triassi, Mario De Felice, and et al. 2019. "A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis" International Journal of Environmental Research and Public Health 16, no. 1: 122. https://doi.org/10.3390/ijerph16010122
APA StyleReale, C., Russo, F., Credendino, S. C., Cuomo, D., De Vita, G., Mallardo, M., Pennino, F., Porreca, I., Triassi, M., De Felice, M., & Ambrosino, C. (2019). A Toxicogenomic Approach Reveals a Novel Gene Regulatory Network Active in In Vitro and In Vivo Models of Thyroid Carcinogenesis. International Journal of Environmental Research and Public Health, 16(1), 122. https://doi.org/10.3390/ijerph16010122







