Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand
Abstract
1. Introduction
2. Materials and Methods
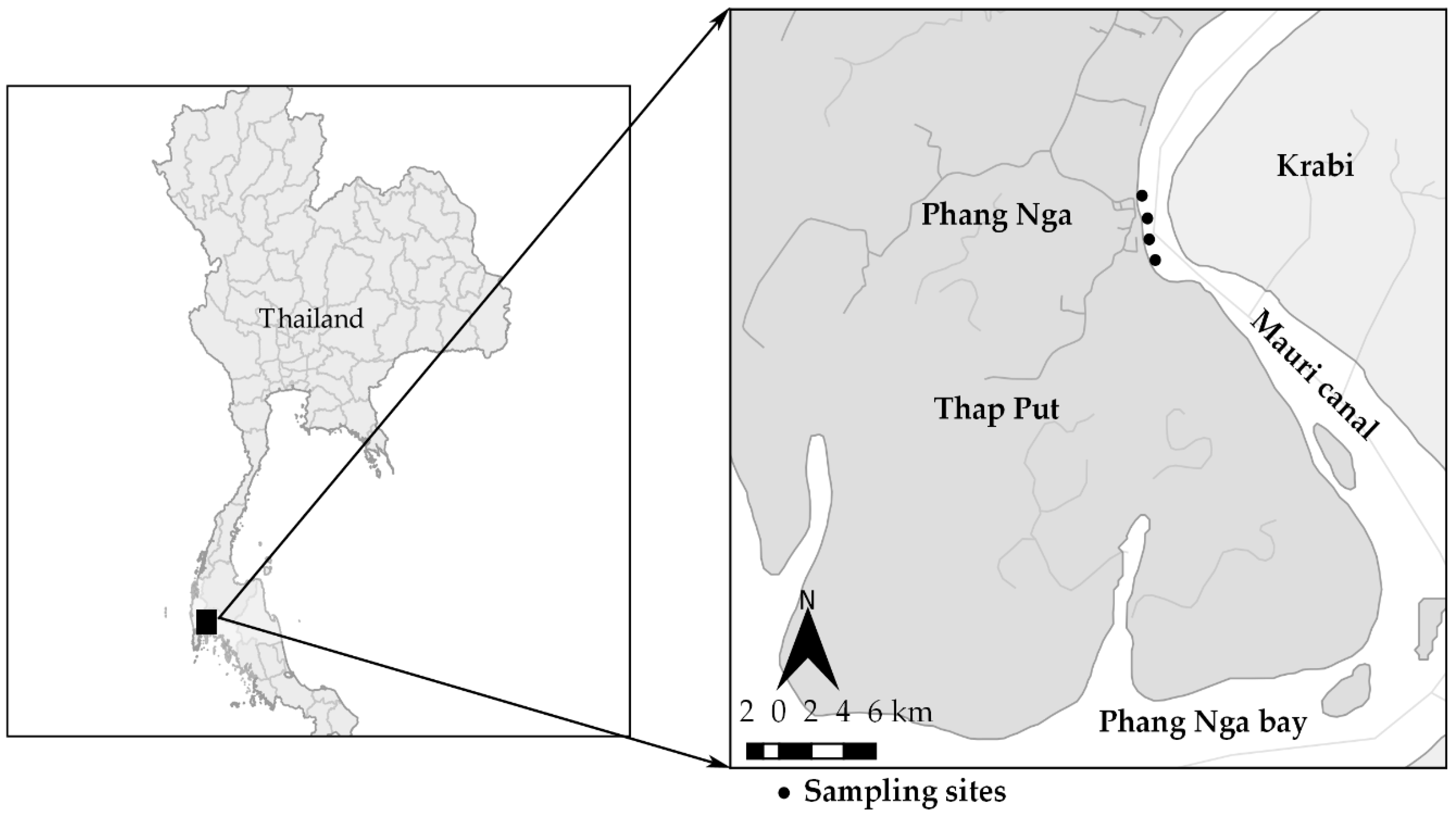
2.1. Oyster Site Selection and Oyster and Estaurine Water Sample Collection
2.2. Measurement of Environmental Factors
2.3. Determination of Total Coliforms (TC), Fecal Coliforms (FC) and Escherichia coli (EC)
2.4. Determination of Vibrio Parahaemolyticus
2.5. Isolation of Salmonella spp.
2.6. Isolation of Shigella spp.
2.7. Determination of Heavy Metals
2.8. Statistical Analyses
3. Results
3.1. Sampling Location and Environmental Condition
3.2. Concentrations of Total Coliforms, Fecal Coliforms, and E. coli
3.3. Concentrations of V. parahaemolyticus
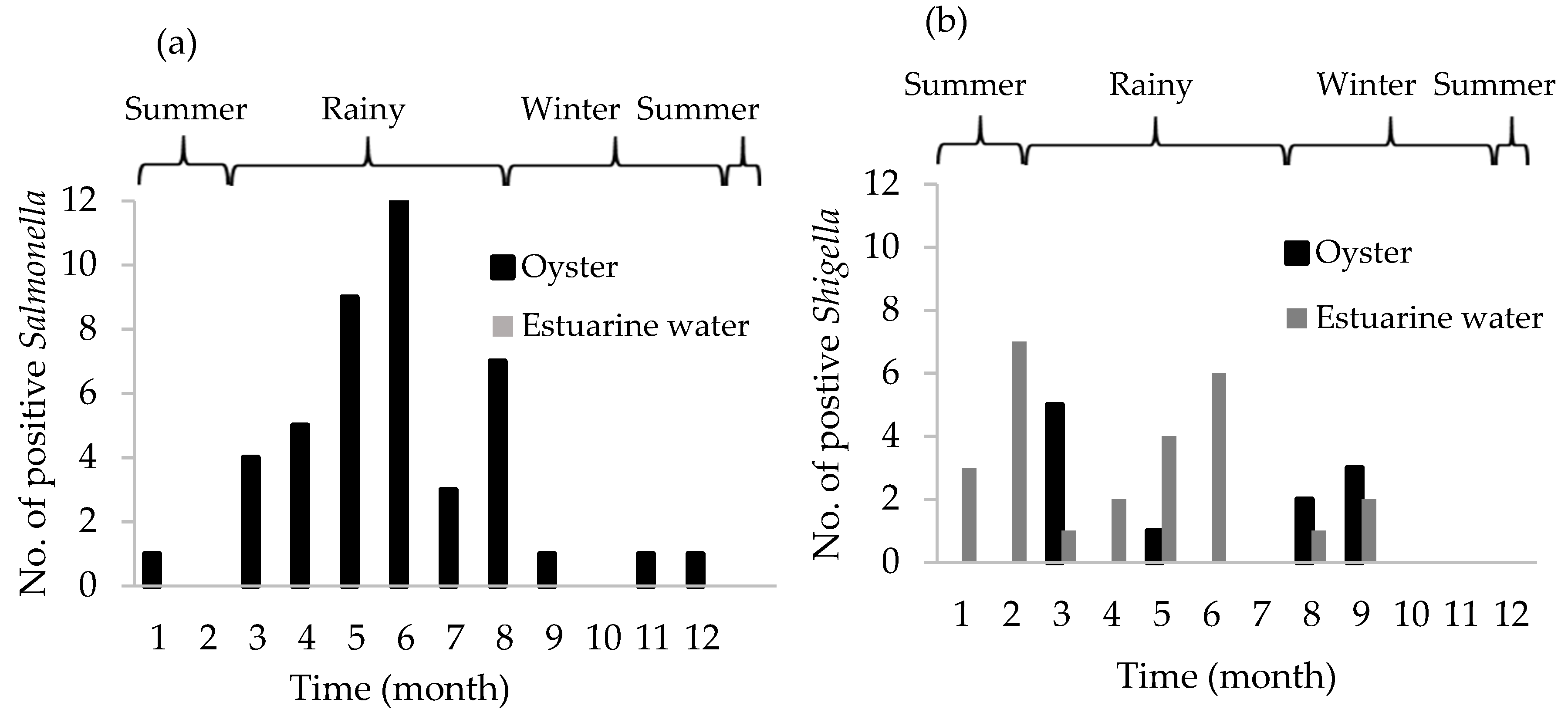
3.4. Presence of Salmonella spp. and Shigella spp.
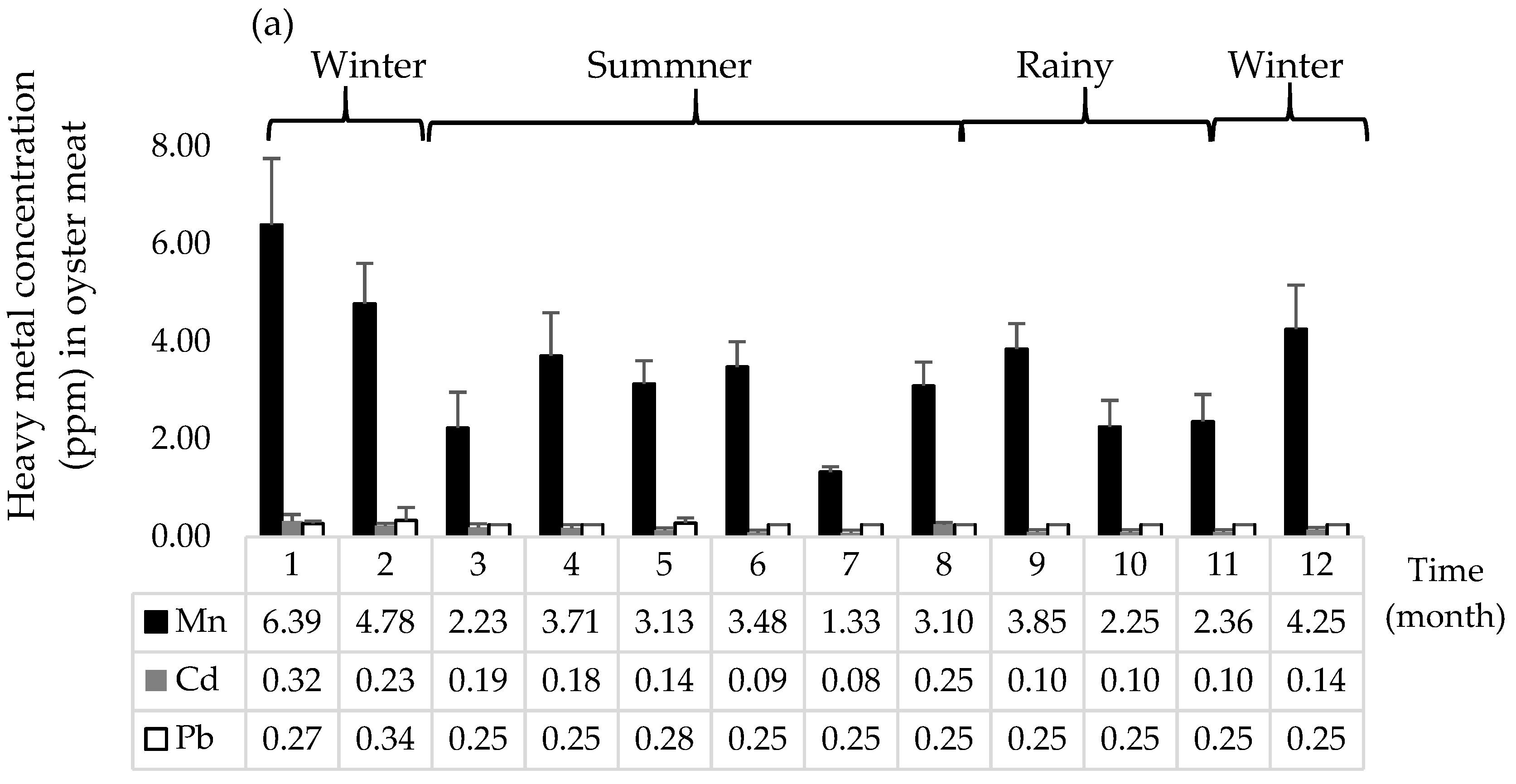
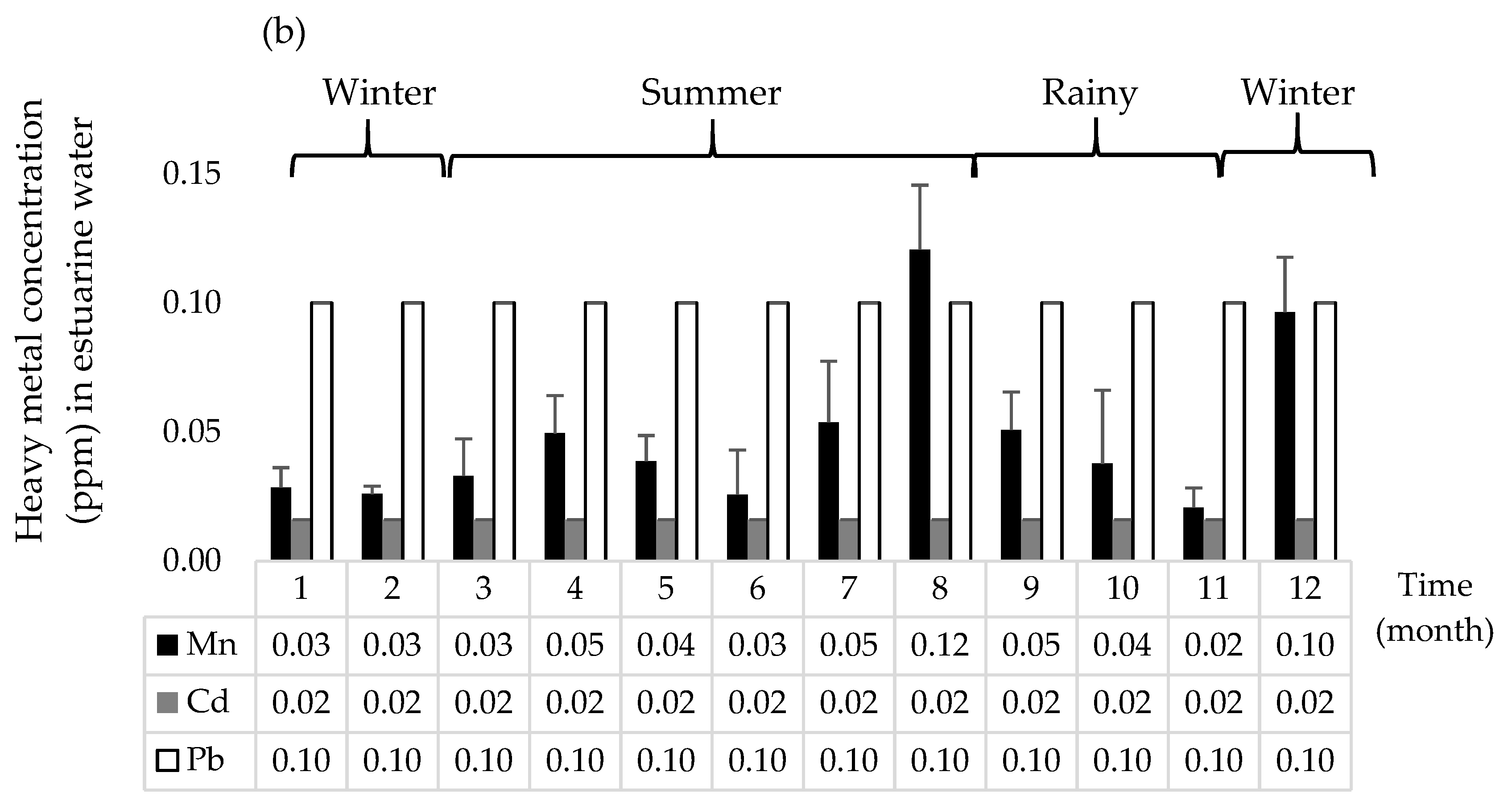
3.5. Heavy Metal Concentrations
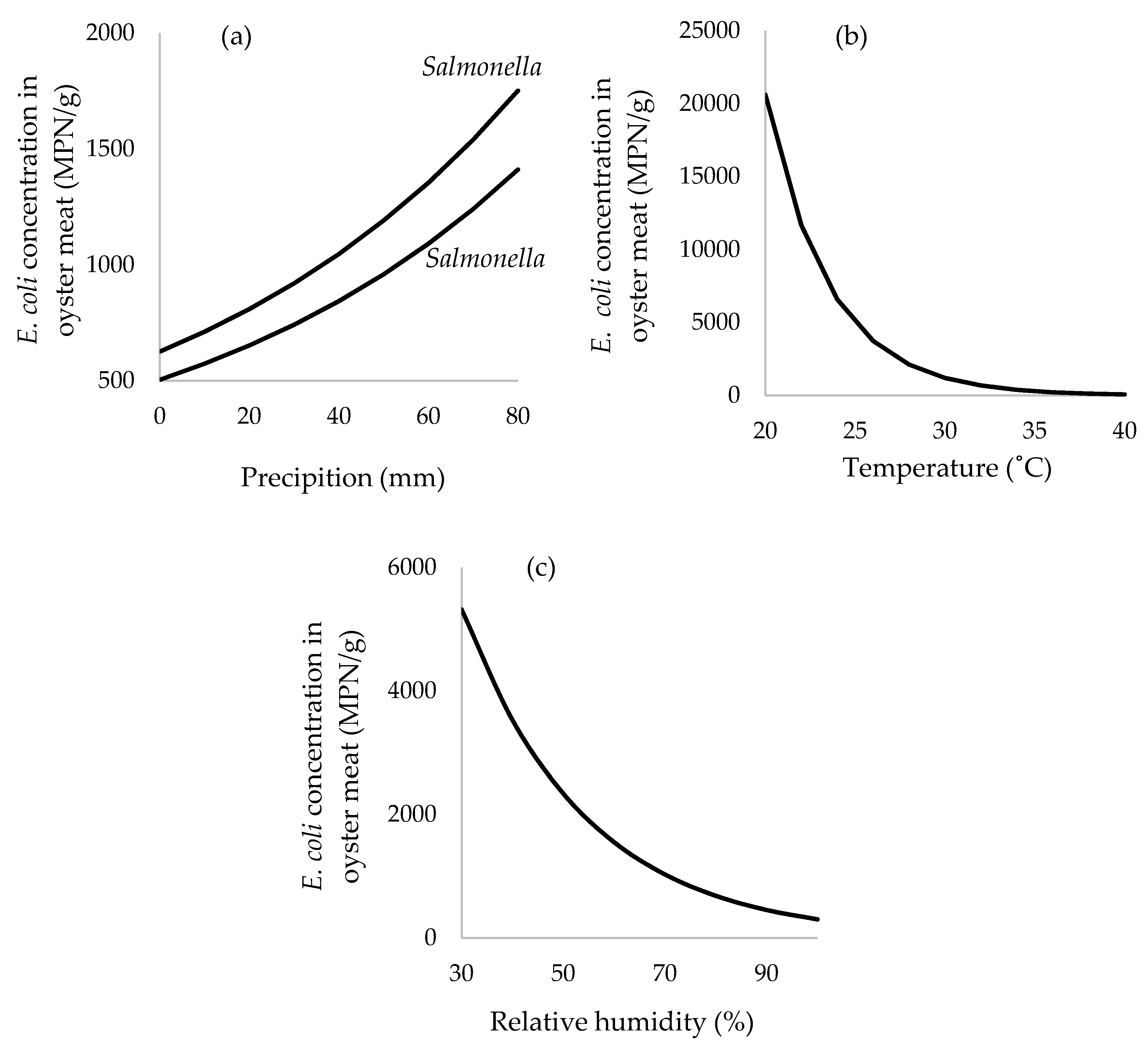
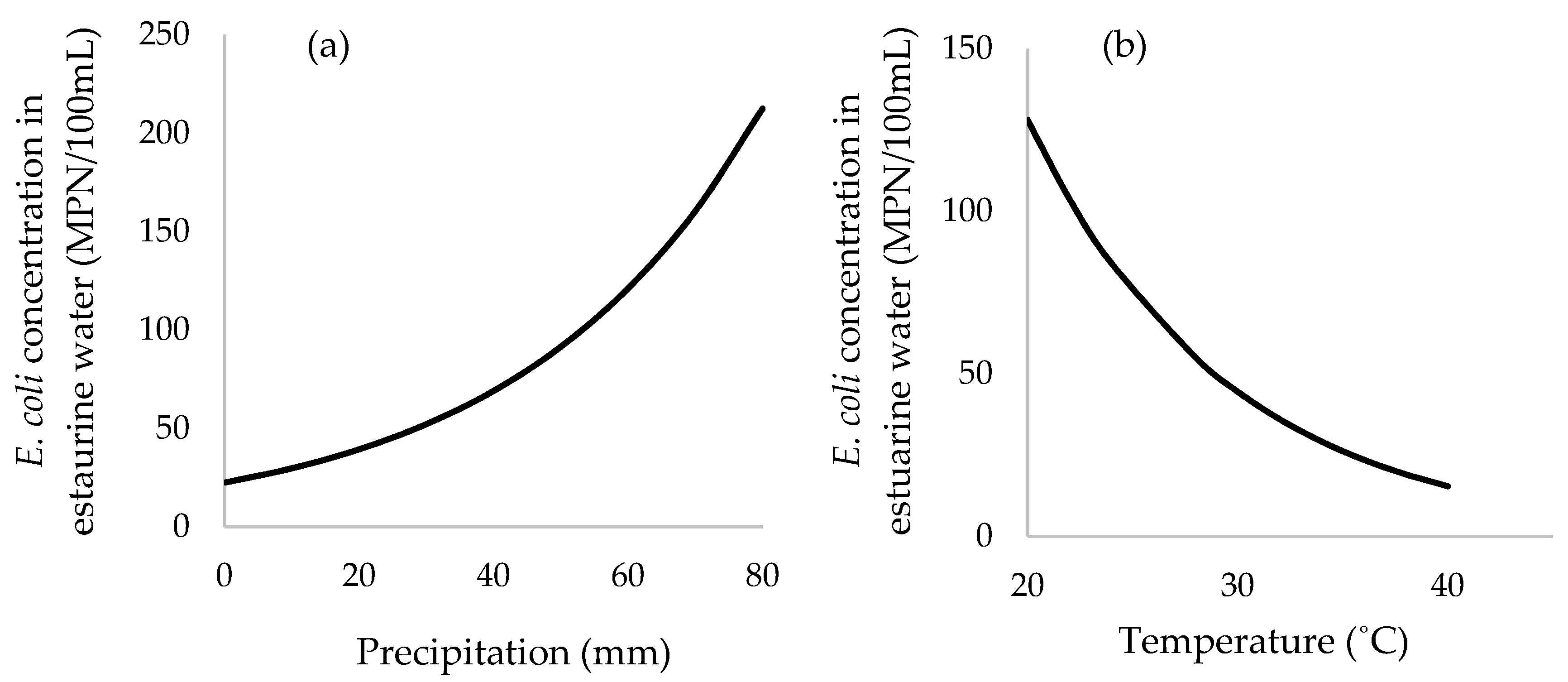
3.6. Multivariable Mixed-Effects Negative Binomial Regression Model
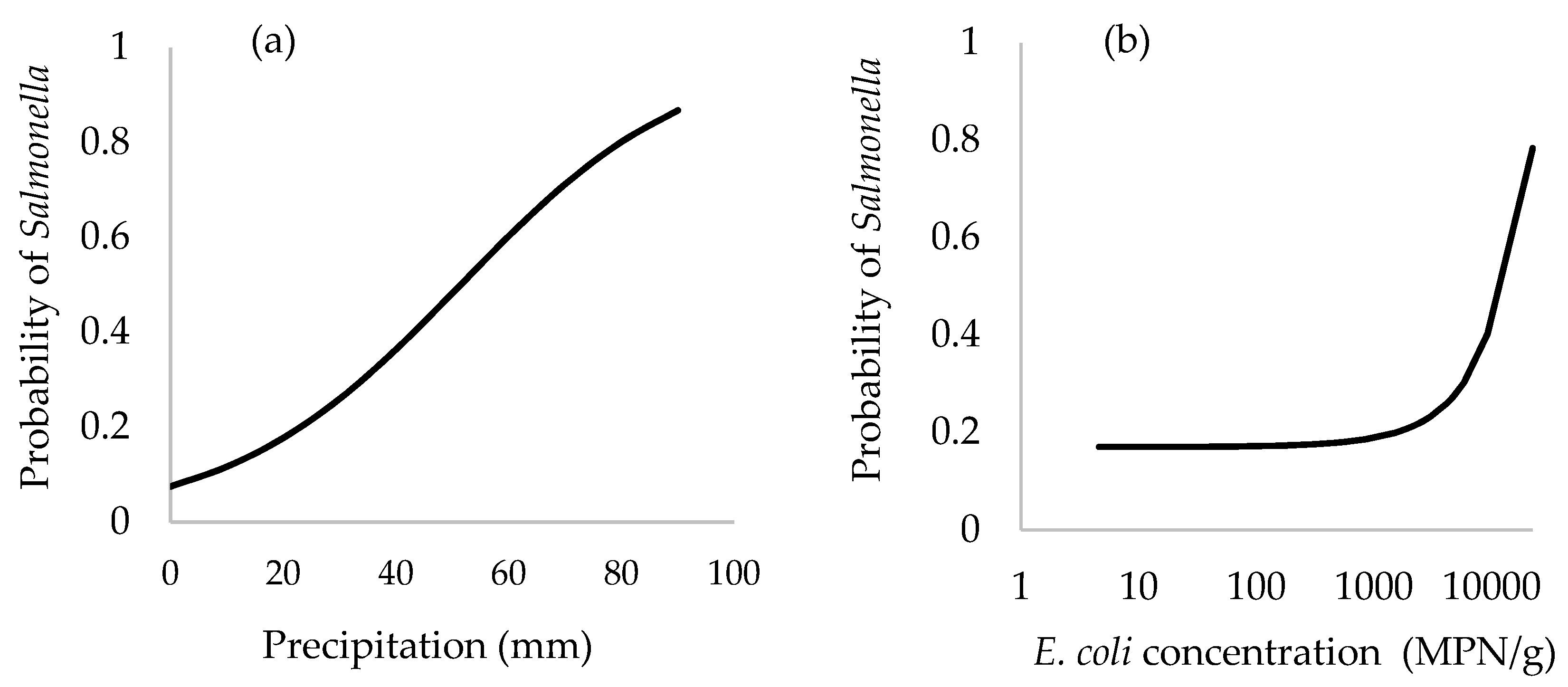
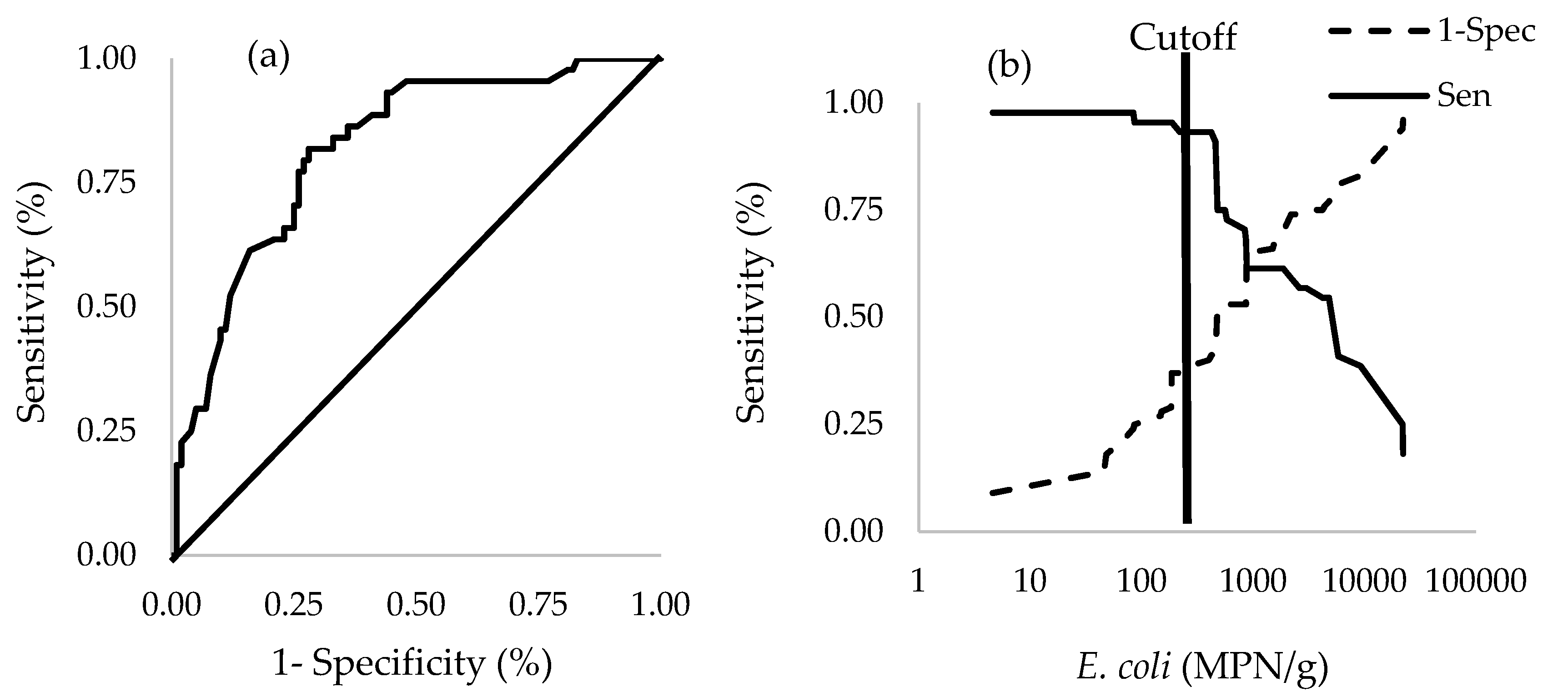
3.7. Logistic Regression and Receiver Operating Characteristic (ROC) Curve Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350, 117–129. [Google Scholar] [CrossRef]
- U.S. Food and Agricuture Organization (FAO). The State of World Fisheries and Aquaculture Opportunities and Challenges. Available online: http://www.fao.org/3/a-i3720e.pdf (accessed on 14 December 2017).
- Bougeard, M.; Le Saux, J.C.; Pérenne, N.; Baffaut, C.; Robin, M.; Pommepuy, M. Modeling of Escherichia coli fluxes on a catchment and the impact on coastal water and shellfish quality. J. Am. Water Resour. Assoc. 2011, 47, 350–366. [Google Scholar] [CrossRef]
- Hood, M.; Ness, G.; Rodrick, G. Isolation of Vibrio cholerae serotype O1 from the eastern oyster, Crassostrea virginica. Appl. Environ. Microbiol. 1981, 41, 559–560. [Google Scholar] [PubMed]
- Odeyemi, O.A. Incidence and prevalence of Vibrio parahaemolyticus in seafood: A systematic review and meta-analysis. SpringerPlus 2016, 5, 464. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, D.; Leoni, F.; Rocchegiani, E.; Santarelli, S.; Canonico, C.; Masini, L.; Ditrani, V.; Carraturo, A. First clinical report of pandemic Vibrio parahaemolyticus O3:K6 infection in Italy. J. Clin. Microbiol. 2008, 46, 2144–2145. [Google Scholar] [CrossRef] [PubMed]
- Brands, D.A.; Inman, A.E.; Gerba, C.P.; Maré, C.J.; Billington, S.J.; Saif, L.A.; Levine, J.F.; Joens, L.A. Prevalence of Salmonella spp. in Oysters in the United States. Appl. Environ. Microbiol. 2005, 71, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Reeve, G.; Martin, D.L.; Pappas, J.; Thompson, R.E.; Greene, K.D. An outbreak of shigellosis associated with the consumption of raw oysters. N. Engl. J. Med. 1989, 321, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Terajima, J.; Tamura, K.; Hirose, K.; Izumiya, H.; Miyahara, M.; Konuma, H.; Watanabe, H. A Multi-Prefectural Outbreak of Shigella sonnei Infections Associated with Eating Oysters in Japan. Microbiol. Immunol. 2004, 48, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, G.M.; Nordstrom, J.L.; Vickery, M.C.; Bowen, M.D.; Meyer, R.F.; DePaola, A. Detection of pathogenic Vibrio parahaemolyticus in oyster enrichments by real time PCR. J. Microbiol. Methods 2003, 53, 149–155. [Google Scholar] [CrossRef]
- Garcia, K.; Torres, R.; Uribe, P.; Hernandez, C.; Rioseco, M.L.; Romero, J.; Espejo, R.T. Dynamics of clinical and environmental Vibrio parahaemolyticus strains during seafood-related summer diarrhea outbreaks in southern Chile. Appl. Environ. Microbiol. 2009, 75, 7482–7487. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Wang, X.; Ji, R. Foodborne disease outbreaks in China from 1992 to 2001 national foodborne disease surveillance system. Wei Sheng Yan Jiu 2004, 33, 725–727. [Google Scholar] [PubMed]
- Wong, H.C.; Liu, S.H.; Wang, T.K.; Lee, C.L.; Chiou, C.S.; Liu, D.P.; Nishibuchi, M.; Lee, B.K. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 2000, 66, 3981–3986. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Q.; Cheung, R.Y.; Wong, M.H. Heavy metals in oysters, mussels and clams collected from coastal sites along the Pearl River Delta, South China. J. Environ. Sci. 2003, 15, 9–24. [Google Scholar]
- Sarong, M.A.; Jihan, C.; Muchlisin, Z.A.; Fadli, N.; Sugianto, S. Cadmium, lead and zinc contamination on the oyster Crassostrea gigas muscle harvested from the estuary of Lamnyong River, Banda Aceh City, Indonesia. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. (AACL Bioflux) 2015, 8, 1–6. [Google Scholar]
- Guilarte, T.R. Manganese and Parkinson’s Disease: A Critical Review and New Findings. Environ. Health Perspect. 2010, 118, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Langlois, D.; Olley, J. Heavy metals in Tasmanian Shellfish 1—Monitoring heavy metal contamination in the Derwent estuary: Use of oysters and mussels. J. Appl. Toxicol. 1982, 2, 99–109. [Google Scholar] [CrossRef]
- Huggett, R.J.; Bender, M.E.; Slone, H.D. Utilizing metal concentration relationships in the eastern oyster (Crassostrea virginica) to detect heavy metal pollution. Water Res. 1973, 7, 451–460. [Google Scholar] [CrossRef]
- Liang, L.; He, B.; Jiang, G.; Chen, D.; Yao, Z. Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Sci. Total Environ. 2004, 324, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.A.; Ness, G.E.; Blake, N.J. Relationship among fecal coliforms, Escherichia coli, and Salmonella spp. in shellfish. Appl. Environ. Microbiol. 1983, 45, 122–126. [Google Scholar] [PubMed]
- Feng, P.; Weagant, S.D.; Grant, M.A.; Burkhardt, W.; Shellfish, M.; Water, B. BAM: Enumeration of Escherichia coli and the Coliform Bacteria. Bacteriological Analytical Manual (BAM), 2002. Available online: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm064948.htm (accessed on 18 January 2017).
- Kaysner, C.A.; DePaola, A. Bacteriological Analytical Manual (BAM): Vibrio. 2004. Available online: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm070830.htm (accessed on 18 January 2017).
- Andrews, W.H.; Wang, H.; Jacobson, A.; Hammack, T. BAM: Salmonella. 2007. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm (accessed on 18 January 2017).
- Grimont, P.A.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; WHO Collaborating Centre for Reference and Research on Salmonella: Paris, France, 2007. [Google Scholar]
- Andrews, W.H.; Jacobson, A. BAM: Shigella. Available online: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm070789.htm (accessed on 18 January 2017).
- Association of Official Analytical Chemists (AOAC). Official Methos of Analysis; AOAC: Washington, DC, USA, 1975. [Google Scholar]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar] [PubMed]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Forcelini, H.C.D.-L.; Kolm, H.E.; Absher, T.M. Escherichia coli in the surface waters and in oysters of two cultivations of Guaratuba Bay-Paraná-Brazil. Braz. Arch. Biol. Technol. 2013, 56, 319–326. [Google Scholar] [CrossRef]
- Silva, A.I.; Vieira, R.H.; Menezes, F.G.; Fonteles-Filho, A.A.; Torres, R.C.; Sant’Anna, E.S. Bacteria of fecal origin in mangrove oysters (Crassostrea rhizophorae) in the Cocó River estuary, Ceará State, Brazil. Braz. J. Microbiol. 2004, 35, 126–130. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 854/2004 of the European Parliament and of the council of 29 April 2004 laying down specific rules for the organisation of official controls on products of animal origin intended for human consumption. Off. J. Eur. Union 2004, L226, 83. [Google Scholar]
- Codex Alimentarius. Standard for Live and Raw Bivalve Molluscs CODEX STAN 292-2008 in Codex Alimentarius; Adopted in 2008, Amendments 2013, Revised 2014; Codex Alimentarius, International Food Standards: Rome, Italy, 2014; pp. 1–9. [Google Scholar]
- U.S. Food and Drug Administration (FDA). National Shellfish Sanitation Program (NSSP), Guide for the Control of Molluscan Shellfish. Available online: https://www.fda.gov/food/guidanceregulation/federalstatefoodprograms/ucm2006754.htm (accessed on 18 February 2017).
- Chinnadurai, S.; Mohamed, K.S.; Sharma, J.; Venkatesan, V.; Kripa, V. Assessment of bio-accumulation of bacteria in oysters from shellfish growing waters in Ashtamudi Lake (Kerala, India): A RAMSAR wetland. Reg. Stud. Mar. Sci. 2016, 7, 118–122. [Google Scholar] [CrossRef]
- Derolez, V.; Soudant, D.; Fiandrino, A.; Cesmat, L.; Serais, O. Impact of weather conditions on Escherichia coli accumulation in oysters of the Thau lagoon (the Mediterranean, France). J. Appl. Microbiol. 2013, 114, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Atwill, E.R.; Pereira, M.; Bond, R. Spatial and Temporal Dynamics of Fecal Coliform and Associated with Suspended Solids and Water within Five Northern California Estuaries. J. Environ. Qual. 2013, 42, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in fish and seafood. J. Food. Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.J.; Martin, G.; Hernandez, S.; Cheng, Y.; Gerner-Smidt, P.; Hise, K.B.; D’Angelo, M.T.; Cole, D.; Sanchez, S.; Madden, M. Diversity and persistence of Salmonella enterica strains in rural landscapes in the Southeastern United States. PLoS ONE 2015, 10, e0128937. [Google Scholar] [CrossRef] [PubMed]
- Baudart, J.; Lemarchand, K.; Brisabois, A.; Lebaron, P. Diversity of Salmonella strains isolated from the aquatic environment as determined by serotyping and amplification of the ribosomal DNA spacer regions. Appl. Environ. Microbiol. 2000, 66, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Chandran, A.; Hatha, A.M. Survival of Escherichia coli in a tropical estuary. South Pac. J. Nat. Appl. Sci. 2003, 21, 41–46. [Google Scholar]
- Chigbu, P.; Gordon, S.; Tchounwou, P.B. The seasonality of fecal coliform bacteria pollution and its influence on closures of shellfish harvesting areas in Mississippi Sound. Int. J. Environ. Res. Public Health 2005, 2, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Šolić, M.; Krstulović, N. Separate and combined effects of solar radiation, temperature, salinity, and pH on the survival of faecal coliforms in seawater. Mar. Pollut. Bull. 1992, 24, 411–416. [Google Scholar] [CrossRef]
- Campos, C.J.; Cachola, R.A. Faecal coliforms in bivalve harvesting areas of the Alvor lagoon (Southern Portugal): Influence of seasonal variability and urban development. Environ. Monit. Assess. 2007, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.L.; Fallowfield, H.J.; Cromar, N.J. Use of microcosms to determine persistence of Escherichia coli in recreational coastal water and sediment and validation with in situ measurements. J. Appl. Microbiol. 2004, 96, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Faruque, A.S.; Terao, T.; Yunus, M.; Streatfield, K.; Yamamoto, T.; Moji, K. The Indian Ocean dipole and cholera incidence in Bangladesh: A time-series analysis. Environ. Health Perspect. 2011, 119, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Najiah, M.; Nadirah, M.; Lee, K.L.; Lee, S.W.; Wendy, W.; Ruhil, H.H.; Nurul, F.A. Bacteria flora and heavy metals in cultivated oysters Crassostrea iredalei of Setiu Wetland, East Coast Peninsular Malaysia. Vet. Res. Commun. 2008, 32, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Delshab, H.; Farshchi, P.; Mohammadi, M.; Moattar, F. Assessment of heavy metals contamination and its effects on oyster (Saccostrea cucullata) biometry parameters in the Asaluyeh port coasts, Persian Gulf, Iran. Int. J. Environ. Stud. 2017, 74, 1031–1043. [Google Scholar] [CrossRef]
- Reimer, P.S. Environmental Effects of Manganese and Proposed Guidelines to Protect Freshwater Life in British Columbia; Department of Chemical & Bio-Resource Engineering, University of British Columbia: Vancouver, BC, Canada, 1999. [Google Scholar]
- Pinsino, A.; Matranga, V.; Roccheri, M.C. Manganese: A new emerging contaminant in the environment. In Environmental Contamination; InTech Publishing: Chicago, IL, USA, 2012; pp. 17–36. [Google Scholar]
| Sample | Bacterial Concentration 1 | |||
|---|---|---|---|---|
| TC | FC | EC | VP | |
| Oyster meat (MPN/g oyster) | 9.31 × 103 (9.95 × 103) | 6.43 × 103 (8.82 × 103) | 4.58 × 103 (7.07 × 103) | 8.50 × 107 (3.78 × 106) |
| Estuarine water (MPN/100 mL) | 4.74 × 102 (1.37 × 103) | 4.18 × 102 (1.33 × 103) | 2.17 × 102 (7.06 × 102) | 3.78 × 105 (4.70 × 104) |
| Serovars | n (%) |
|---|---|
| Augustenborg | 2 (4.55) |
| Austin | 1 (2.27) |
| Braenderup | 2 (4.55) |
| Colorado | 2 (4.55) |
| Eastbourne | 3 (6.82) |
| Enteritidis | 1 (2.27) |
| Friedenau | 2 (4.55) |
| II | 1 (2.27) |
| IIIa | 1 (2.27) |
| Kentucky | 4 (9.09) |
| Larochelle | 2 (4.55) |
| Limete | 1 (2.27) |
| Lomita | 1 (2.27) |
| Ndolo | 3 (6.82) |
| Nola | 1 (2.27) |
| Paratyphi B | 10 (22.73) |
| Sao | 2 (4.55) |
| Seremban | 5 (11.36) |
| Total | 44 (00.0) |
| Factors | Coefficient | 95% CI 1 | p-Value 1 |
|---|---|---|---|
| Intercept | 18.07 | 9.47 to 26.67 | <0.0001 |
| Concentration of FC 2 | 1.49 × 10−4 | 1.28 × 10−4 to 1.70 × 10−2 | <0.0001 |
| Average precipitation prior 7 days (mm) | 1.28 × 10−2 | 4.75 × 10−3 to 2.09 × 10−4 | 0.002 |
| Average temperature (°C) | −0.28 | −0.50 to −0.065 | 0.011 |
| Relative humidity (%) | −0.041 | 0.065 to −0.017 | 0.001 |
| Salmonella present in sample | |||
| No | 0.0 | - | |
| Yes | 0.22 | 0.068 to 0.36 | 0.004 |
| Factors | Coefficient | 95% CI 1 | p-Value 1 |
|---|---|---|---|
| Intercept | 6.40 | 3.30 to 9.49 | <0.0001 |
| Concentration of FC 2 | 8.75 × 10−4 | 5.89 × 10−4 to 1.16 × 10−3 | <0.0001 |
| Average precipitation prior 7 days (mm) | 0.028 | 0.023 to 0.033 | <0.0001 |
| Average temperature (°C) | −0.11 | −0.20 to −0.01 | 0.030 |
| Factors | Coefficient | 95% CI 1 | p-Value 1 |
|---|---|---|---|
| Intercept | −2.52 | −2.78 to −2.26 | <0.0001 |
| Concentration of EC 2 | 2.21 × 10−4 | 1.30 × 10−4 to 3.13 × 10−4 | <0.0001 |
| Average precipitation prior 7 days (mm) | 0.049 | 0.031 to 0.068 | <0.0001 |
| Concentration of EC × precipitation | 4.8 × 10−6 | −6.12 × 10−6 to −3.48 × 10−6 | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeamsripong, S.; Chuanchuen, R.; Atwill, E.R. Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand. Int. J. Environ. Res. Public Health 2018, 15, 1970. https://doi.org/10.3390/ijerph15091970
Jeamsripong S, Chuanchuen R, Atwill ER. Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand. International Journal of Environmental Research and Public Health. 2018; 15(9):1970. https://doi.org/10.3390/ijerph15091970
Chicago/Turabian StyleJeamsripong, Saharuetai, Rungtip Chuanchuen, and Edward R. Atwill. 2018. "Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand" International Journal of Environmental Research and Public Health 15, no. 9: 1970. https://doi.org/10.3390/ijerph15091970
APA StyleJeamsripong, S., Chuanchuen, R., & Atwill, E. R. (2018). Assessment of Bacterial Accumulation and Environmental Factors in Sentinel Oysters and Estuarine Water Quality from the Phang Nga Estuary Area in Thailand. International Journal of Environmental Research and Public Health, 15(9), 1970. https://doi.org/10.3390/ijerph15091970





