Elevated Indoor Volatile Organic Compound Exposure in the Niger Delta Region of Nigeria
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. VOC Monitoring
2.3. Questionnaire
2.4. Health Risk Assessment and Adverse Health Symptoms
3. Results
3.1. VOC Exposure and Risk Estimates
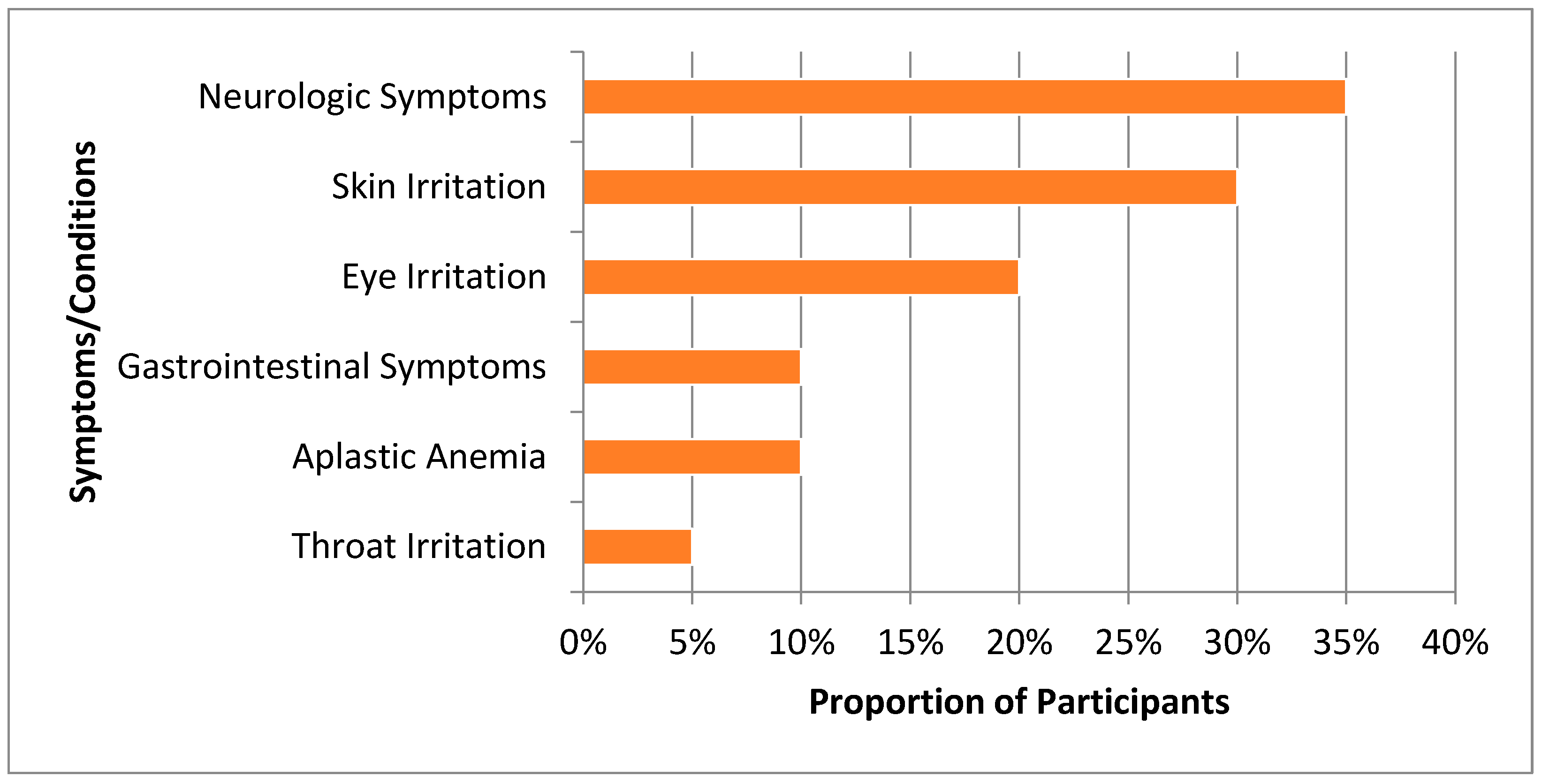
3.2. Non-Cancer Health Outcomes
4. Discussion
4.1. Indoor Air Pollution Sources for VOCs Posing Cancer and Non-Cancer Health Risks
4.2. Health Risks and Concerns
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zabbey, N.; Sam, K.; Onyebuchi, A.T. Remediation of contaminated lands in the Niger Delta, Nigeria: Prospects and challenges. Sci. Total Environ. 2017, 586, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Ana, G.R.; Sridhar, M.K.; Bamgboye, E.A. Environmental risk factors and health outcomes in selected communities of the Niger Delta area, Nigeria. Perspect. Public Health 2009, 129, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Boele, R.; Heike, F.; Wheeler, D. Shell, Nigeria and the Ogoni. A study in unsustainable development: I. The story of Shell, Nigeria and the Ogoni people-environment, economy, relationships: Conflicts and prospects for resolution. Sustain. Dev. 2001, 9, 74–86. [Google Scholar] [CrossRef]
- Nriagu, J.; Udofia, E.; Ekong, I.; Ebuk, G. Health Risks Associated with Oil Pollution in the Niger Delta, Nigeria. Int. J. Environ. Res. Public. Health 2016, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Enivironmental Assessment of Ogoniland; UNEP: Athens, Greece, 2011. [Google Scholar]
- Osuji, L.C.; Avwiri, G.O. Flared Gases and Other Pollutants Associated with Air Quality in Industrial Areas of Nigeria: An Overview. Chem. Biodivers. 2005, 2, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Umoh, V.A.; Peters, E. The relationship between lung function and indoor air pollution among rural women in the Niger Delta region of Nigeria. Lung India Off. Organ Indian Chest Soc. 2014, 31, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, B.A.; Blangiardo, M.; Briggs, D.J.; Hansell, A.L. Traffic Air Pollution and Other Risk Factors for Respiratory Illness in Schoolchildren in the Niger-Delta Region of Nigeria. Environ. Health Perspect. 2011, 119, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Kponee, K.Z.; Chiger, A.; Kakulu, I.I.; Vorhees, D.; Heiger-Bernays, W. Petroleum contaminated water and health symptoms: A cross-sectional pilot study in a rural Nigerian community. Environ. Health 2015, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Hellén, H.; Hakola, H.; Laurila, T.; Hiltunen, V.; Koskentalo, T. Aromatic hydrocarbon and methyl tert-butyl ether measurements in ambient air of Helsinki (Finland) using diffusive samplers. Sci. Total Environ. 2002, 298, 55–64. [Google Scholar] [CrossRef]
- Martin, N.J.; Marlow, D.J.; Henderson, M.H.; Goody, B.A.; Quincey, P.G. Studies using the sorbent Carbopack X for measuring environmental benzene with Perkin-Elmer-type pumped and diffusive samplers. Atmos. Environ. 2003, 37, 871–879. [Google Scholar] [CrossRef]
- McClenny, W.A.; Jacumin, H.H., Jr.; Oliver, K.D.; Hunter Daughtrey, E., Jr.; Whitaker, D.A. Comparison of 24 h averaged VOC monitoring results for residential indoor and outdoor air using Carbopack X-filled diffusive samplers and active sampling—A pilot study. J. Environ. Monit. 2006, 8, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.T.; Wright, M.D. Diffusive Sampling of C7–C16 Hydrocarbons in Workplace Air: Uptake Rates, Wall Effects and Use in Oil Mist Measurements. Ann. Occup. Hyg. 2008, 52, 249–257. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. Standard Practice for Choosing Sorbents, Sampling Parameters, and Thermal Desorption Analytical Conditions for Monitoring Volatile Organic Chemicals in Air; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Jia, C.; Fu, X. Diffusive Uptake Rates of Volatile Organic Compounds on Standard ATD Tubes for Environmental and Workplace Applications. Environments 2017, 4, 87. [Google Scholar] [CrossRef]
- USEPA. Integrated Risk Information System (IRIS); USEPA: Washington, DC, USA, 2017. [Google Scholar]
- USEPA. Dose-Response Assessment for Assessing Health Risks Associated with Exposure to Hazardous Air Pollutants; USEPA: Washington, DC, USA, 2017. [Google Scholar]
- Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Toxicity of Naphthalene and Benzene on Tribollium castaneum Herbst. Int. J. Environ. Res. Public. Health 2017, 14, 667. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Toxicological Review of Benzene; USEPA: Washington, DC, USA, 2002. [Google Scholar]
- Charles, S.M.; Jia, C.; Batterman, S.A.; Godwin, C. VOC and Particulate Emissions from Commercial Cigarettes: Analysis of 2,5-DMF as an ETS Tracer. Environ. Sci. Technol. 2008, 42, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, S.; Adair-Rohani, H.; Wolf, J.; Bruce, N.G.; Mehta, S.; Prüss-Ustün, A.; Lahiff, M.; Rehfuess, E.A.; Mishra, V.; Smith, K.R. Solid fuel use for household cooking: Country and regional estimates for 1980–2010. Environ. Health Perspect. 2013, 121, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Batterman, S.A.; Relyea, G.E. Variability of indoor and outdoor VOC measurements: An analysis using variance components. Environ. Pollut. 2012, 169, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.M. The Central Nervous System and Exposure to Toluene: A Risk Characterization. Environ. Res. 1997, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kraut, A.; Lilis, R.; Marcus, M.; Valciukas, J.A.; Wolff, M.S.; Landrigan, P.J. Neurotoxic effects of solvent exposure on sewage treatment workers. Arch. Environ. Health 1988, 43, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Wickliffe, J.; Overton, E.; Frickel, S.; Howard, J.; Wilson, M.; Simon, B.; Echsner, S.; Nguyen, D.; Gauthe, D.; Blake, D. Evaluation of Polycyclic Aromatic Hydrocarbons Using Analytical Methods, Toxicology, and Risk Assessment Research: Seafood Safety after a Petroleum Spill as an Example. Environ. Health Perspect. 2014, 122, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Na, J.U.; Sim, M.S.; Jo, I.J.; Song, H.G. The duration of acute health problems in people involved with the cleanup operation of the Hebei Spirit oil spill. Mar. Pollut. Bull. 2012, 64, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- San Sebastián, M.; Armstrong, B.; Córdoba, J.A.; Stephens, C. Exposures and cancer incidence near oil fields in the Amazon basin of Ecuador. Occup. Environ. Med. 2001, 58, 517–522. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Toxicological Review of Naphthalene. In Integrated Risk Information System (IRIS); USEPA: Washington, DC, USA, 1998. [Google Scholar]
- Sudakin, D.L.; Stone, D.L.; Power, L. Naphthalene Mothballs: Emerging and Recurring Issues and their Relevance to Environmental Health. Curr. Top. Toxicol. 2011, 7, 13–19. [Google Scholar] [PubMed]
- Lindén, O.; Pålsson, J. Oil Contamination in Ogoniland, Niger Delta. Ambio 2013, 42, 685–701. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for 1,4-Dichlorobenzene (Update); Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- U.S. Department of Health and Human Services. Toxicological Profile for Dichlorobenzenes; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2006.
- U.S. Department of Health and Human Services. Toxicological Profile for Ethylbenzene; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2010.
- ATSDR. Toxicological Profile for Carbon Tetrachloride; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2005.
- Jia, C.; Yu, X.; Masiak, W. Blood/Air distribution of volatile organic compounds (VOCs) in a nationally representative sample. Sci. Total Environ. 2012, 419, 225–232. [Google Scholar] [CrossRef] [PubMed]
| VOC (µg/m3) | CAS Number | Detection Frequency (%) | Mean Indoor Concentrations | Median Indoor Concentrations | Standard Deviation | Min | Max |
|---|---|---|---|---|---|---|---|
| Benzene | 71-43-2 | 100 | 25.7 | 16.1 | 23.2 | 9.1 | 105.4 |
| Carbon tetrachloride | 56-23-5 | 100 | 1.0 | 1.0 | 0.2 | 0.6 | 1.5 |
| d-Limonene | 5989-27-5 | 100 | 1.8 | 1.4 | 1.2 | 0.2 | 4.2 |
| Dimethylfuran, 2,5- | 625-86-5 | 50 | 0.1 | 0.3 × 101 | 0.2 | 0.0 | 0.7 |
| Dioxane, 1,4- | 123-91-1 | 95 | 0.2 | 0.1 | 0.1 | 0.0 | 0.4 |
| Ethylbenzene | 100-41-4 | 100 | 5.2 | 2.5 | 10.4 | 1.0 | 48.2 |
| Isopropylbenzene | 98-82-8 | 100 | 0.4 | 0.2 | 0.6 | 0.1 | 3.0 |
| m,p-Xylene a | 179601-23-1 | 100 | 10.7 | 6.2 | 15.7 | 2.4 | 74.2 |
| Naphthalene | 91-20-3 | 100 | 7.6 | 2.2 | 13.8 | 0.7 | 60.0 |
| o-Xylene | 95-47-6 | 100 | 4.2 | 2.4 | 6.5 | 0.9 | 30.5 |
| p-Dichlorobenzene | 106-46-7 | 100 | 5.1 | 0.4 | 12.1 | 0.2 | 42.0 |
| p-Isopropyltoluene | 99-87-6 | 100 | 0.2 | 0.1 | 0.1 | 0.1 | 0.4 |
| Propylbenzene | 103-65-1 | 100 | 0.8 | 0.4 | 1.3 | 0.2 | 5.9 |
| sec-Butylbenzene | 135-98-8 | 100 | 0.2 | 0.1 | 0.4 | 0.0 | 1.8 |
| Styrene | 100-42-5 | 100 | 1.8 | 0.9 | 4.0 | 0.4 | 18.7 |
| Trimethylbenzene, 1,2,3- | 526-73-8 | 100 | 1.7 | 1.1 | 1.6 | 0.5 | 6.8 |
| Trimethylbenzene, 1,2,4- | 95-63-6 | 100 | 4.0 | 2.7 | 4.1 | 0.9 | 18.2 |
| Trimethylbenzene, 1,3,5- | 108-67-8 | 100 | 1.6 | 0.9 | 1.7 | 0.4 | 7.9 |
| Toluene | 108-88-3 | 100 | 21.9 | 12.5 | 34.7 | 6.2 | 163.4 |
| Xylenes (mixed) b | 1330-20-7 | 100 | 14.8 | 8.8 | 22.2 | 3.3 | 104.7 |
| VOC | Reference Concentration [16] (μg/m3) | Hazard Quotient | IUR [17] (1/106) (μg/m3)−1 | Cancer Risk | % Total Cancer Risk | Predicted Organ System(s) Impacted [16] |
|---|---|---|---|---|---|---|
| Benzene | 3 × 101 | 9 × 10−1 | 8 × 10−6 | 2 × 10−4 | 38 | Immune |
| Carbon tetrachloride | 1 × 102 | 1 × 10−2 | 6 × 10−6 | 6 × 10−6 | 1.1 | Hepatic |
| Dioxane, 1,4- | 3 × 101 | 7 × 10−3 | 5 ×10−6 | 1 × 10−6 | 0.1 | Nervous, Respiratory |
| Ethylbenzene | 1 × 103 | 5 × 10−3 | 3 × 10−6 | 1 × 10−5 | 2.5 | Developmental |
| Naphthalene | 3 × 100 | 3 × 100 | 3 × 10−5 | 3 × 10−4 | 48 | Nervous, Respiratory |
| p-Dichlorobenzene | 8 × 102 | 6 × 10−3 | 1 × 10−5 | 6 × 10−5 | 10 | Hepatic |
| Styrene | 1 × 103 | 2 × 10−3 | - | - | - | Nervous |
| Toluene | 5 × 103 | 4 × 10−3 | - | - | - | Nervous |
| Trimethylbenzene, 1,2,4- | 6 × 101 | 7 × 10−2 | - | - | - | Nervous |
| Xylenes (mixed) | 1 × 102 | 1 × 10−1 | - | - | - | Nervous |
| VOC | Neurologic Symptoms OR (95% CI) | Skin Irritation OR (95% CI) | Eye Irritation OR (95% CI) | Throat Irritation OR (95% CI) | Aplastic Anemia OR (95% CI) | Gastrointestinal Symptoms OR (95% CI) |
|---|---|---|---|---|---|---|
| Benzene | 0.9 (0.9, 1.0) | 0.9 (0.9, 1.0) | 1.0 (1.0, 1.1) | 1.0 (0.9, 1.1) | 1.0 (1.0,1.1) | 1.1 (1.0, 1.2) |
| Carbon tetrachloride | - | - | - | - | - | - |
| Dioxane, 1,4- | - | - | - | - | - | - |
| Ethylbenzene | 0.9 (0.7, 1.3) | 0.8 (0.4, 1.6) | 1.1 (0.9, 1.3) | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.5) | 1.1 (0.9, 1.4) |
| Naphthalene | 1.0 (0.9, 1.1) | 0.8 (0.4, 1.4) | 0.9 (0.8, 1.1) | 0.9 (0.6, 1.5) | 0.9 (0.6, 1.4) | 0.9 (0.6, 1.4) |
| p-Dichlorobenzene | 0.9 (0.7, 1.2) | - | - | 1.0 (0.8, 1.2) | - | - |
| Styrene | 0.6 (0.1, 4.7) | 0.5 (0.1, 6.0) | 1.4 (0.6, 3.1) | 0.9 (0.3, 3.1) | 1.5 (0.5, 4.7) | 1.4 (0.7, 2.9) |
| Toluene | 1.0 (0.9, 1.1) | 0.9 (0.8, 1.1) | 1.1 (1.0, 1.1) | 1.1 (1.0, 1.1) | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.1) |
| Trimethylbenzene, 1,2,4- | 1.0 (0.7, 1.2) | 0.9 (0.6, 1.3) | 1.2 (0.9, 1.6) | 1.0 (0.7, 1.6) | 1.3 (1.0, 1.8) | 1.3 (1.0, 1.8) |
| Xylenes (mixed) | 1.0 (0.9, 1.1) | 1.0 (0.8, 1.1) | 1.0 (1.0, 1.1) | 1.0 (0.9, 1,1) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1,1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kponee, K.Z.; Nwanaji-Enwerem, J.C.; Fu, X.; Kakulu, I.I.; Weisskopf, M.G.; Jia, C. Elevated Indoor Volatile Organic Compound Exposure in the Niger Delta Region of Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 1939. https://doi.org/10.3390/ijerph15091939
Kponee KZ, Nwanaji-Enwerem JC, Fu X, Kakulu II, Weisskopf MG, Jia C. Elevated Indoor Volatile Organic Compound Exposure in the Niger Delta Region of Nigeria. International Journal of Environmental Research and Public Health. 2018; 15(9):1939. https://doi.org/10.3390/ijerph15091939
Chicago/Turabian StyleKponee, Kalé Z., Jamaji C. Nwanaji-Enwerem, Xianqiang Fu, Iyenemi I. Kakulu, Marc G. Weisskopf, and Chunrong Jia. 2018. "Elevated Indoor Volatile Organic Compound Exposure in the Niger Delta Region of Nigeria" International Journal of Environmental Research and Public Health 15, no. 9: 1939. https://doi.org/10.3390/ijerph15091939
APA StyleKponee, K. Z., Nwanaji-Enwerem, J. C., Fu, X., Kakulu, I. I., Weisskopf, M. G., & Jia, C. (2018). Elevated Indoor Volatile Organic Compound Exposure in the Niger Delta Region of Nigeria. International Journal of Environmental Research and Public Health, 15(9), 1939. https://doi.org/10.3390/ijerph15091939






