Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota
Abstract
1. Introduction
2. Materials and Methods
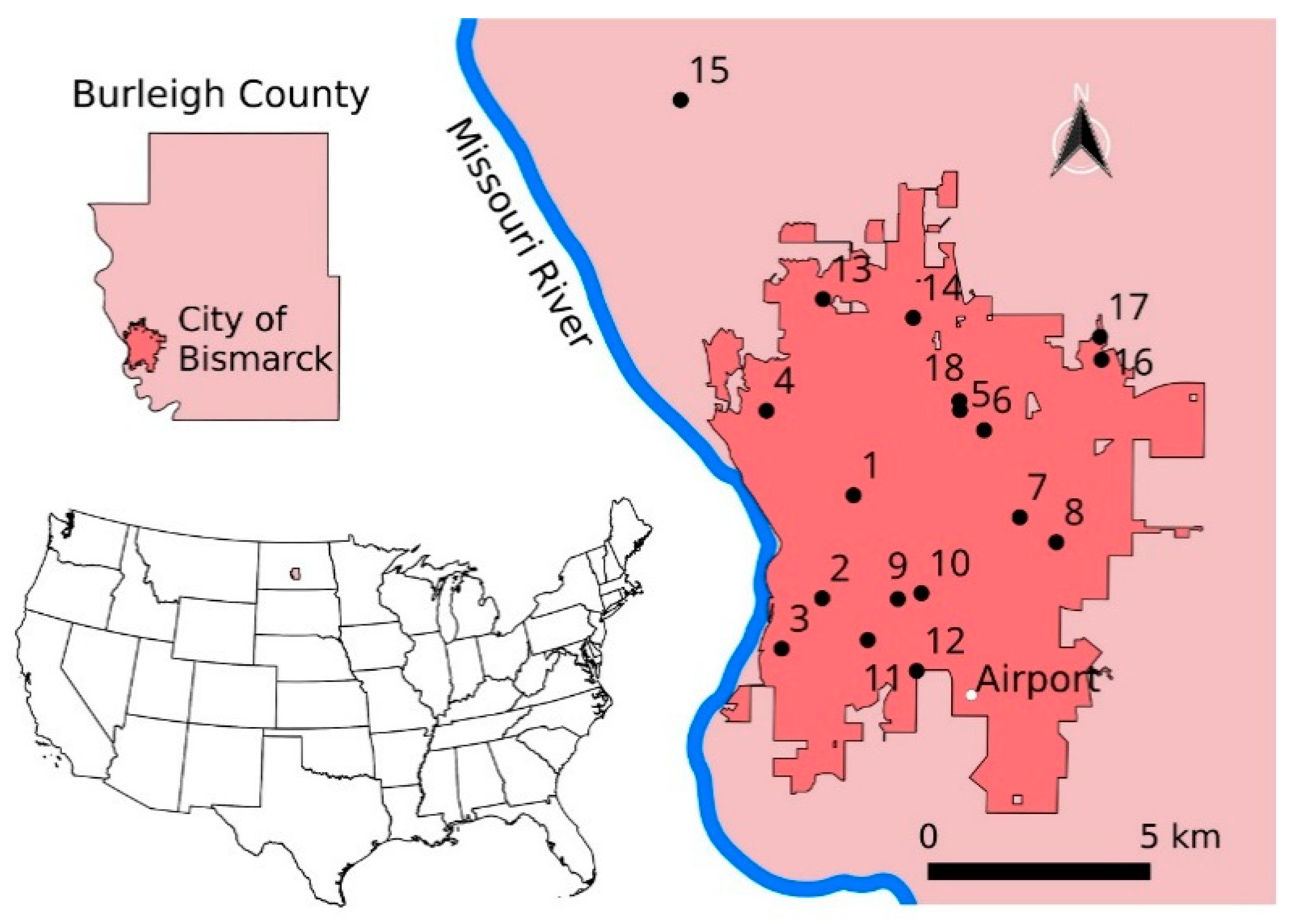
2.1. Study Area
2.2. Data on Mosquito Numbers and West Nile Virus Cases
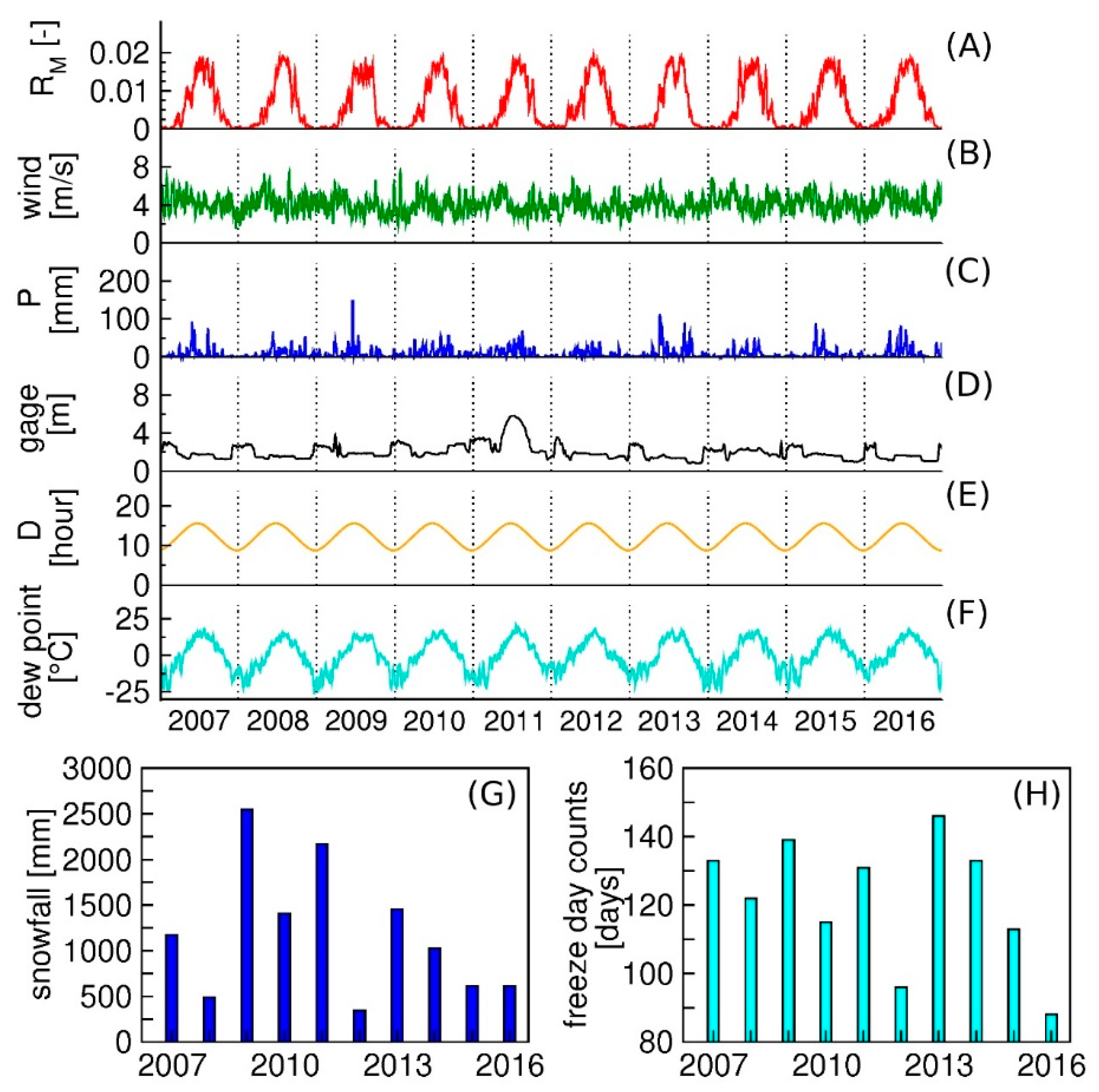
2.3. Climatological Factors Considered for the Mosquito Model
2.4. Transmission Factors Considered for the Model of Human Disease Cases
2.5. Statistical Analyses and Model Evaluation
3. Results
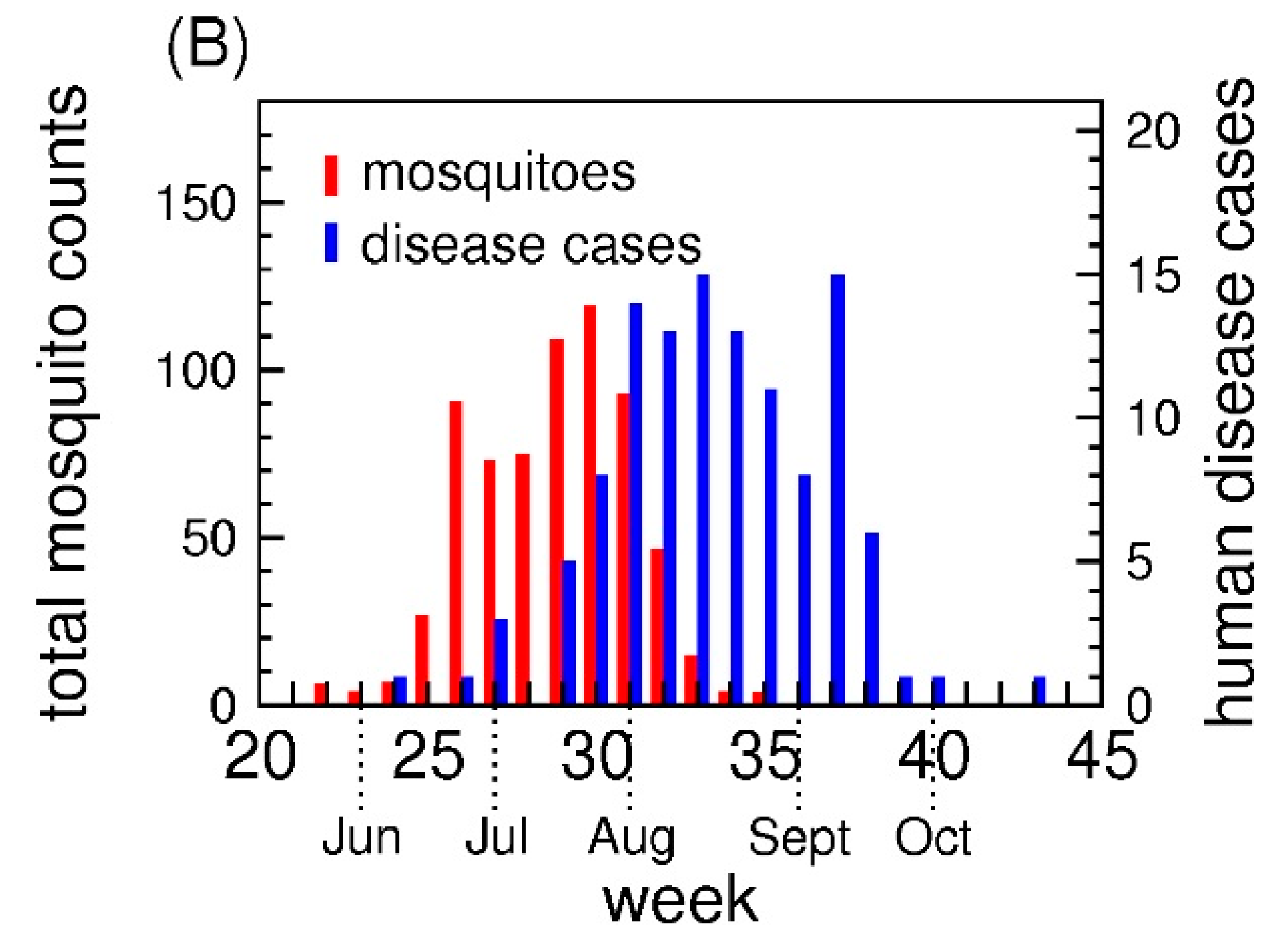
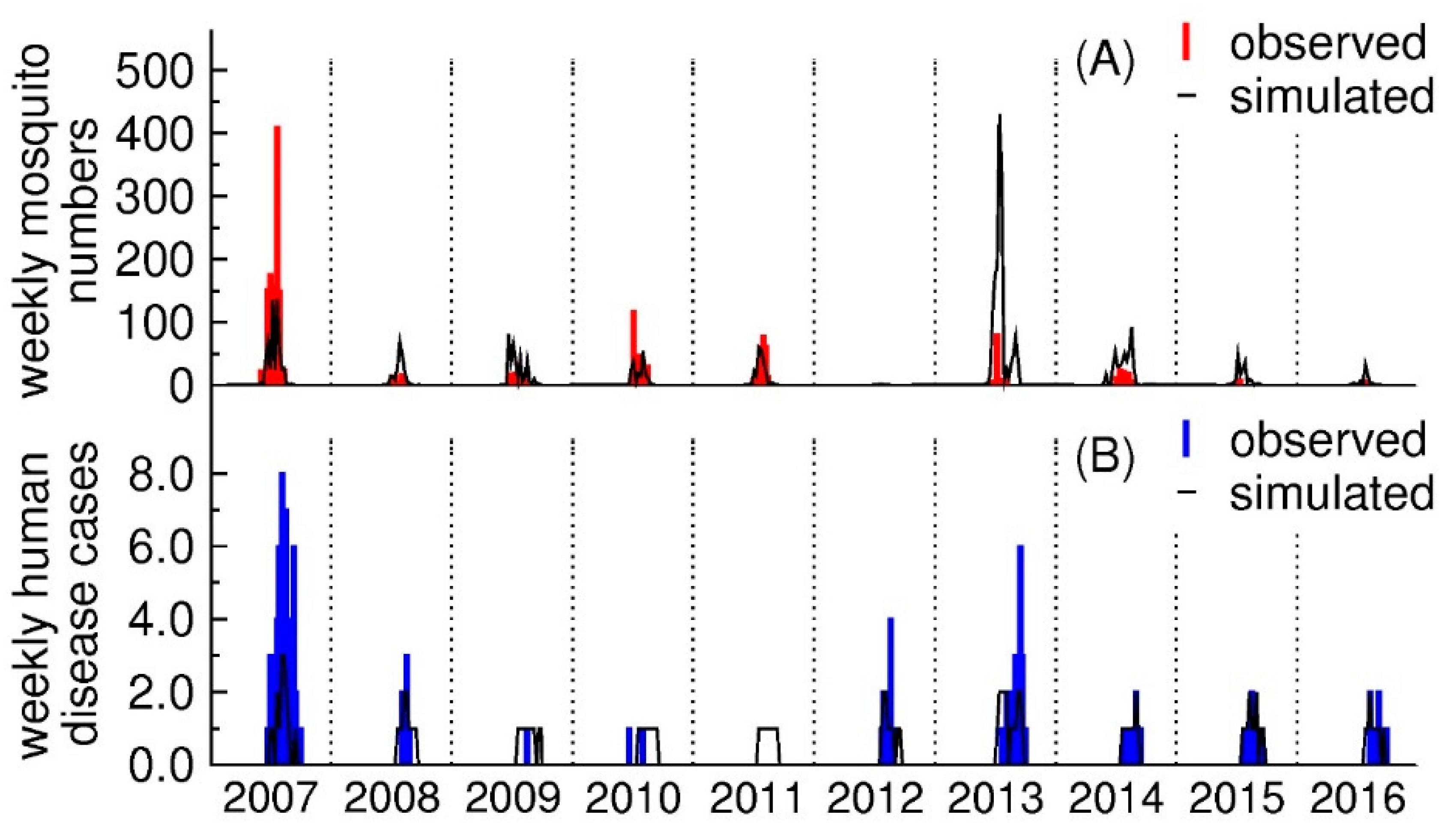
3.1. The Number of Human Disease Cases and Mosquitoes
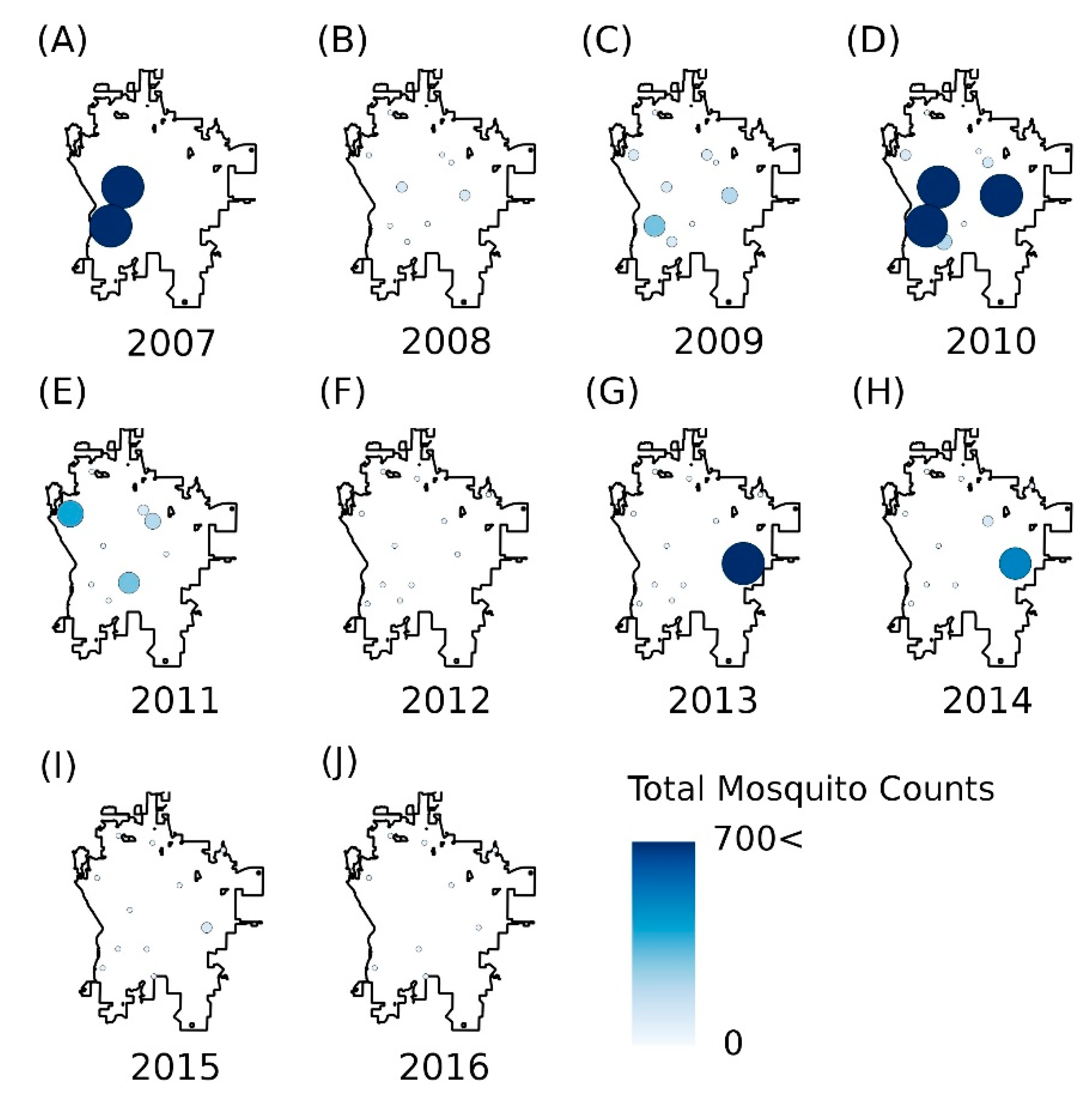
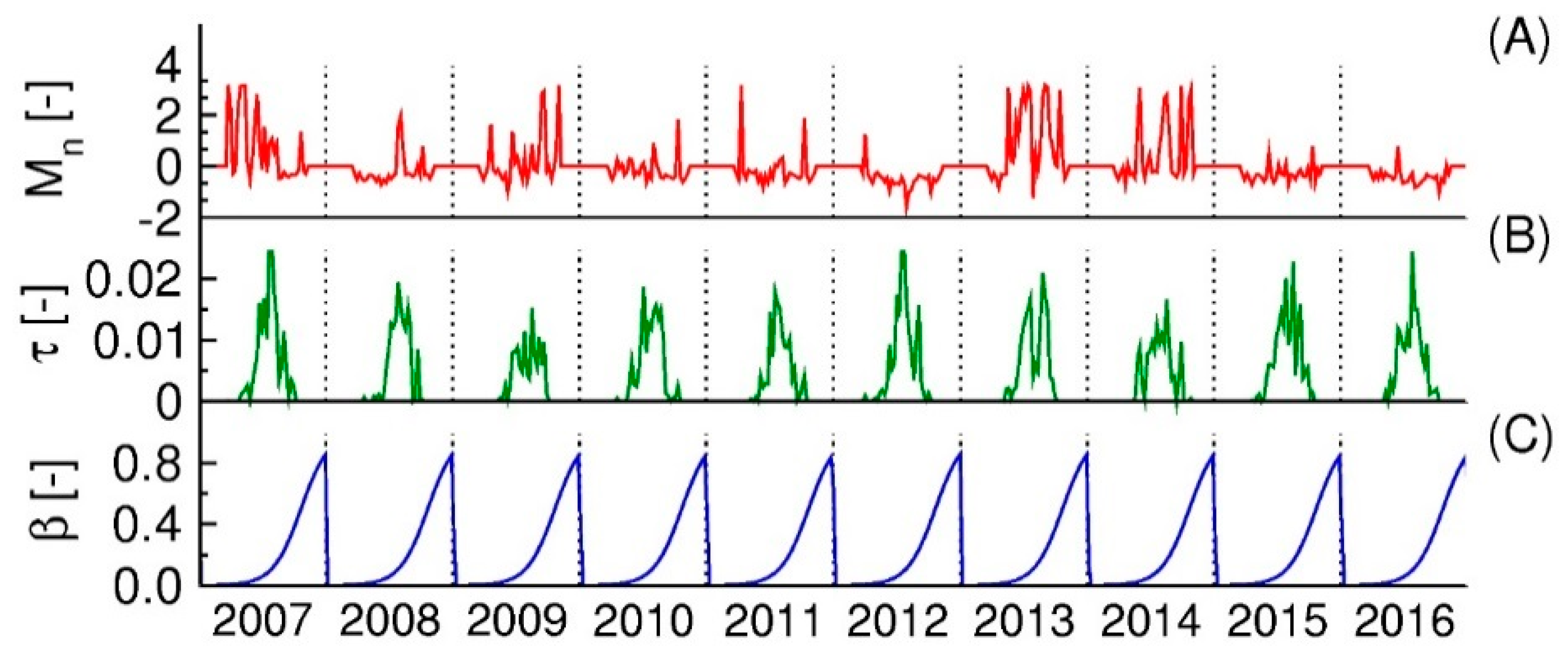
3.2. Identification of Key Factors Affecting Mosquito Abundances
3.3. Identification of Key Factors Affecting Human Disease Cases
3.4. Model Validation for Mosquito Numbers and Human Disease Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gould, E.; Solomon, T. Pathogenic Flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Current Trends in West Nile Virus Vaccine Development. Expert Rev. Vaccines 2014, 13, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. West Nile Virus Disease Cases Reported to CDC by State of Residence, 1999–2015. Available online: https://www.cdc.gov/westnile/resources/pdfs/data/2-west-nile-virus-disease-cases-reported-to-cdc-by-state_1999-2015_07072016.pdf (accessed on 19 July 2018).
- Liu, H.; Weng, Q.; Gaines, D. Spatio-Temporal analysis of the relationship between WNV dissemination and environmental variables in Indianapolis, USA. Int. J. Health Geogr. 2008, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local Impact of Temperature and Precipitation on West Nile Virus Infection in Culex Species Mosquitoes in Northeast Illinois, USA. Parasites Vectors 2010, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.; Henebry, G.M.; Kimball, J.S.; VanRoekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite Microwave Remote Sensing for Environmental Modeling of Mosquito Population Dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.M.; Buseman, C.M.; Joyner, S.N.; Hughes, S.M.; Fomby, T.B.; Luby, J.P.; Haley, R.W. The 2012 West Nile Encephalitis Epidemic in Dallas, Texas. JAMA 2013, 310, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Pape, W.J. Predicting Human West Nile Virus Infections with Mosquito Surveillance Data. Am. J. Epidemiol. 2013, 178, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Komar, N.; Nasci, R.S.; Montgomery, S.P.; O’Leary, D.R.; Campbell, G.L. Epidemiology and Transmission Dynamics of West Nile Virus Disease. Emerg. Infect. Dis. 2005, 11, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Barker, C.M.; Moore, C.G.; Pape, W.J.; Eisen, L. Seasonal Patterns for Entomological Measures of Risk for Exposure to Culex Vectors and West Nile Virus in Relation to Human Disease Cases in Northeastern Colorado. J. Med. Entomol. 2009, 46, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Colborn, J.M.; Smith, K.A.; Townsend, J.; Damian, D.; Nasci, R.S.; Mutebi, J. West Nile Virus Outbreak in Phoenix, Arizona—2010: Entomological Observations and Epidemiological Correlations. J. Am. Mosq. Control Assoc. 2013, 29, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.V.; Turner, K.W.; Hunter, F.F. Geospatial Analysis and Seasonal Distribution of West Nile Virus Vectors (Diptera: Culicidae) in Southern Ontario, Canada. Int. J. Environ. Res. Public Health 2018, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Hantel, M.; Chvala-Mannsberger, S.; Bakonyi, T.; Weissenböck, H.; Nowotny, N. Explaining Usutu Virus Dynamics in Austria: Model Development and Calibration. Prev. Vet. Med. 2008, 85, 166–186. [Google Scholar] [CrossRef] [PubMed]
- Little, E.; Campbell, S.R.; Shaman, J. Development and Validation of a Climate-Based Ensemble Prediction Model for West Nile Virus Infection Rates in Culex Mosquitoes, Suffolk County, New York. Parasites Vectors 2016, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Hadler, J.L.; Patel, D.; Nasci, R.S.; Petersen, L.R.; Hughes, J.M.; Bradley, K.; Etkind, P.; Kan, L.; Engel, J. Assessment of Arbovirus Surveillance 13 Years After Introduction of West Nile Virus, United States. Emerg. Infect. Dis. 2015, 21, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Rudolfs, W. Relation between Temperature, Humidity and Activity of House Mosquitoes. J. N. Y. Entomol. Soc. 1925, 33, 163–169. [Google Scholar]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.M.; Edwards, E.; Hettler, D.L.; Davis, B.S.; Bowen, R.A.; Bunning, M.L. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile Virus Epidemics in North America are Driven by Shifts in Mosquito Feeding Behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Andreadis, T.G.; Armstrong, P.M.; Bueno, R., Jr.; Dennett, J.A.; Real, S.V.; Sargent, C.; Bala, A.; Randle, Y.; Guzman, H.; et al. Host Feeding Pattern of Culex Quinquefasciatus (Diptera: Culicidae) and its Role in Transmission of West Nile Virus in Harris County, Texas. Am. J. Trop. Med. Hyg. 2007, 77, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Aggarwal, D.; Apperson, C.S.; Katholi, C.R.; Gordon, E.; Hassan, H.K.; Anderson, M.; Charnetzky, D.; McMillen, L.; Unnasch, E.A. Host Choice and West Nile Virus Infection Rates in Blood-Fed Mosquitoes, Including Members of the Culex Pipiens Complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007, 7, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Miller, S.N.; Schmidtmann, E.T. A GIS Tool to Estimate West Nile Virus Risk Based on a Degree-Day Model. Environ. Monit. Assess. 2007, 129, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, Viral Genetics, and the Transmission of West Nile Virus by Culex Pipiens Mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host Selection by Culex Pipiens Mosquitoes and West Nile Virus Amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.S.; Mead, D.G.; Hamer, G.L.; Brosi, B.J.; Hedeen, D.L.; Hedeen, M.W.; McMillan, J.R.; Bisanzio, D.; Kitron, U.D. Supersuppression: Reservoir Competency and Timing of Mosquito Host Shifts Combine to Reduce Spillover of West Nile Virus. Am. J. Trop. Med. Hyg. 2016, 95, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Stilianakis, N.I.; Syrris, V.; Petroliagkis, T.; Pärt, P.; Gewehr, S.; Kalaitzopoulou, S.; Mourelatos, S.; Baka, A.; Pervanidou, D.; Vontas, J. Identification of Climatic Factors Affecting the Epidemiology of Human West Nile Virus Infections in Northern Greece. PLoS ONE 2016, 11, e0161510. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Mickelson, N.J.; Vaughan, J.A. West Nile Virus in Host-Seeking Mosquitoes within a Residential Neighborhood in Grand Forks, North Dakota. Vector Borne Zoonotic Dis. 2005, 5, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.; Kramer, L.D.; Campbell, S.R.; Oscar, A.E.; Dobson, A.P.; Daszak, P. West Nile Virus Risk Assessment and the Bridge Vector Paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Cartographic Boundary Shapefiles—States. Available online: https://www.census.gov/geo/maps-data/data/cbf/cbf_state.html (accessed on 29 September 2017).
- North Dakota GIS Hub Data Portal. North Dakota GIS Data Downloader. Available online: http://www.nd.gov/gis/apps/Download/ (accessed on 21 September 2016).
- State Water Commission. Generalized Rivers, Streams, and Shorelines. Available online: http://www.nd.gov/gis/apps/Download/?clipping=Full&coord=ND83-SF&format=SHAPE&layers=NDHUB.WATER2000K_LINE (accessed on 21 September 2016).
- State Water Commission. Counties. Available online: http://www.nd.gov/gis/apps/Download/?clipping=Full&coord=ND83-SF&format=SHAPE&layers=NDHUB.COUNTIES (accessed on 13 September 2017).
- North Dakota State Water Commission. State Border. Available online: http://www.nd.gov/gis/apps/Download/?clipping=Full&coord=ND83-SF&format=SHAPE&layers=NDHUB.STATE (accessed on 21 September 2016).
- Department of Transportation. Incorporated City Boundaries. Available online: http://www.nd.gov/gis/apps/Download/?clipping=Full&coord=ND83-SF&format=SHAPE&layers=NDHUB.CITY_POLY (accessed on 18 September 2017).
- Sloan, C.E. Ground-Water Hydrology of Prairie Potholes in North Dakota; US Government Printing Office: Washington, DC, USA, 1972.
- NOAA National Centers for Environmental Information. Climate at a Glance: U.S. Time Series. Available online: https://www.ncdc.noaa.gov/cag/ (accessed on 3 February 2017).
- Acuff, V.R. Trap Biases Influencing Mosquito Collecting. Mosq. News 1976, 36, 173–196. [Google Scholar]
- Sattler, A. (Bismarck-Burleigh Public Health, Bismarck, ND, USA). Personal Communication, 2016.
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 5 June 2018).
- Chen, C.; Epp, T.; Jenkins, E.; Waldner, C.; Curry, P.S.; Soos, C. Modeling Monthly Variation of Culex Tarsalis (Diptera: Culicidae) Abundance and West Nile Virus Infection Rate in the Canadian Prairies. Int. J. Environ. Res. Public Health 2013, 10, 3033–3051. [Google Scholar] [CrossRef] [PubMed]
- Lebl, K.; Brugger, K.; Rubel, F. Predicting Culex Pipiens/Restuans Population Dynamics by Interval Lagged Weather Data. Parasites Vectors 2013, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Rosà, R.; Marini, G.; Bolzoni, L.; Neteler, M.; Metz, M.; Delucchi, L.; Chadwick, E.A.; Balbo, L.; Mosca, A.; Giacobini, M. Early Warning of West Nile Virus Mosquito Vector: Climate and Land use Models Successfully Explain Phenology and Abundance of Culex Pipiens Mosquitoes in North-Western Italy. Parasites Vectors 2014, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Armbruster, P.A. Mosquito Diapause. Annu. Rev. Entomol. 2014, 59, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Cummins, B.; Cortez, R.; Foppa, I.M.; Walbeck, J.; Hyman, J.M. A Spatial Model of Mosquito Host-Seeking Behavior. PLoS Comput. Biol. 2012, 8, e1002500. [Google Scholar] [CrossRef] [PubMed]
- NOAA National Centers for Environmental Information. Data Tools: Find a Station. Available online: https://www.ncdc.noaa.gov/cdo-web/datatools/findstation (accessed on 3 February 2016).
- Gardner, A.M.; Hamer, G.L.; Hines, A.M.; Newman, C.M.; Walker, E.D.; Ruiz, M.O. Weather Variability Affects Abundance of Larval Culex (Diptera: Culicidae) in Storm Water Catch Basins in Suburban Chicago. J. Med. Entomol. 2012, 49, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, W.C.; Rykiel, E.J.; Stahl, R.S.; Wu, H.; Schoolfield, R.M. A Model Comparison for Daylength as a Function of Latitude and Day of Year. Ecol. Model. 1995, 80, 87–95. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University. PRISM Climate Data. Available online: http://www.prism.oregonstate.edu/ (accessed on 31 August 2017).
- Reisen, W.K.; Milby, M.M.; Reeves, W.C.; Meyer, R.P.; Bock, M.E. Population Ecology of Culex Tarsalis (Diptera: Culicidae) in a Foothill Environment of Kern County, California: Temporal Changes in Female Relative Abundance, Reproductive Status, and Survivorship. Ann. Entomol. Soc. Am. 1983, 76, 800–808. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of Temperature on the Transmission of West Nile Virus by Culex Tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A 1972, 135, 370–384. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1983; pp. 15–34. [Google Scholar]
- Madsen, H.; Thyregod, P. Introduction to General and Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–8. ISBN 9781420091557. [Google Scholar]
- Vuong, Q.H. Likelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica 1989, 57, 307–333. [Google Scholar] [CrossRef]
- Peng, J.; Lyu, T.; Shi, J.; Nagaraja, H.N.; Xiang, H. Models for Injury Count Data in the US National Health Interview Survey. J. Sci. Res. Rep. 2014, 3, 2286–2302. [Google Scholar]
- Hilbe, J.M. Modeling Count Data; Cambridge University Press: New York, NY, USA, 2011; pp. 1–161. [Google Scholar]
- Reisen, W.K. Effect of Temperature on Culex tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J. Med. Entomol. 1995, 32, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Shaman, J.; Harding, K.; Campbell, S.R. Meteorological and Hydrological Influences on the Spatial and We Temporal Prevalence of West Nile Virus in Culex Mosquitoes, Suffolk County, New York. J. Med. Entomol. 2011, 48, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Ott, L. An Introduction to Statistical Methods and Data Analysis; Duxbury Press: Boston, MA, USA, 1977; pp. 219–222. [Google Scholar]
- Team, R. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Centers for Disease Control and Prevention. Rapid Assessment of Vector-Borne Diseases during the Midwest flood—United States, 1993. Mor. Mortal. Wkly. Rep. 1994, 43, 481–483. [Google Scholar]
- Petersen, L.R.; Marfin, A.A. West Nile Virus: A Primer for the Clinician. Ann. Intern. Med. 2002, 137, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Paull, S.H.; Horton, D.E.; Ashfaq, M.; Rastogi, D.; Kramer, L.D.; Diffenbaugh, N.S.; Kilpatrick, A.M. Drought and Immunity Determine the Intensity of West Nile Virus Epidemics and Climate Change Impacts. Proc. Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Quick Facts Burleigh County, North Dakota. Available online: https://www.census.gov/quickfacts/fact/table/burleighcountynorthdakota/PST045217 (accessed on 23 July 2018).
- United States Census Bureau. Quick Facts Bismarck City, North Dakota. Available online: https://www.census.gov/quickfacts/fact/table/bismarckcitynorthdakota/PST045217 (accessed on 23 July 2018).

| Variables 1 | Coefficients | Std. Error | p-Value |
|---|---|---|---|
| intercept | −2.712 × 101 | 8.696 | 1.817 × 10−3 |
| dew point lag 0 | 3.980 × 10−1 | 1.025 × 10−1 | 1.041 × 10−4 |
| day length lag 2 | 1.075 | 5.534 × 10−1 | 5.203 × 10−2 |
| freeze days | 5.534 × 10−2 | 1.567 × 10−2 | 4.126 × 10−4 |
| gage height lag 0 | −3.808 × 10−1 | 1.326 × 10−1 | 4.068 × 10−3 |
| gage height lag 1 year | −7.062 × 10−1 | 2.258 × 10−1 | 1.765 × 10−3 |
| reproduction rate of mosquito lag 0 | 2.084 × 102 | 9.733 × 101 | 3.225 × 10−2 |
| Variables 1 | Log Part 2 | Logit Part 3 | ||||
|---|---|---|---|---|---|---|
| Coefficients | Std. Error | p-Value | Coefficients | Std. Error | p-Value | |
| intercept | −5.732 × 10−1 | 7.103 × 10−1 | 4.196 × 10−1 | 6.312 | 2.453 | 1.009 × 10−2 |
| transmission rate lag 2 | 6.889 × 101 | 3.078 × 101 | 2.522 × 10−2 | 3.124 × 101 | 2.001 × 102 | 8.759 × 10−1 |
| feeding pattern lag 2 | −2.297 | 2.988 | 4.422 × 10−1 | −1.110 × 10−2 | 7.440 × 101 | 1.356 × 10−1 |
| mosquito lag 2 | 1.727 × 10−1 | 1.524 × 10−1 | 2.570 × 10−1 | −8.143 × 10−1 | 6.195 × 10−1 | 1.887 × 10−1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, H.; Wu, J.; Ibaraki, M.; Schwartz, F.W. Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota. Int. J. Environ. Res. Public Health 2018, 15, 1928. https://doi.org/10.3390/ijerph15091928
Mori H, Wu J, Ibaraki M, Schwartz FW. Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota. International Journal of Environmental Research and Public Health. 2018; 15(9):1928. https://doi.org/10.3390/ijerph15091928
Chicago/Turabian StyleMori, Hiroko, Joshua Wu, Motomu Ibaraki, and Franklin W. Schwartz. 2018. "Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota" International Journal of Environmental Research and Public Health 15, no. 9: 1928. https://doi.org/10.3390/ijerph15091928
APA StyleMori, H., Wu, J., Ibaraki, M., & Schwartz, F. W. (2018). Key Factors Influencing the Incidence of West Nile Virus in Burleigh County, North Dakota. International Journal of Environmental Research and Public Health, 15(9), 1928. https://doi.org/10.3390/ijerph15091928




