Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs
Abstract
1. Introduction
2. Materials and Methods
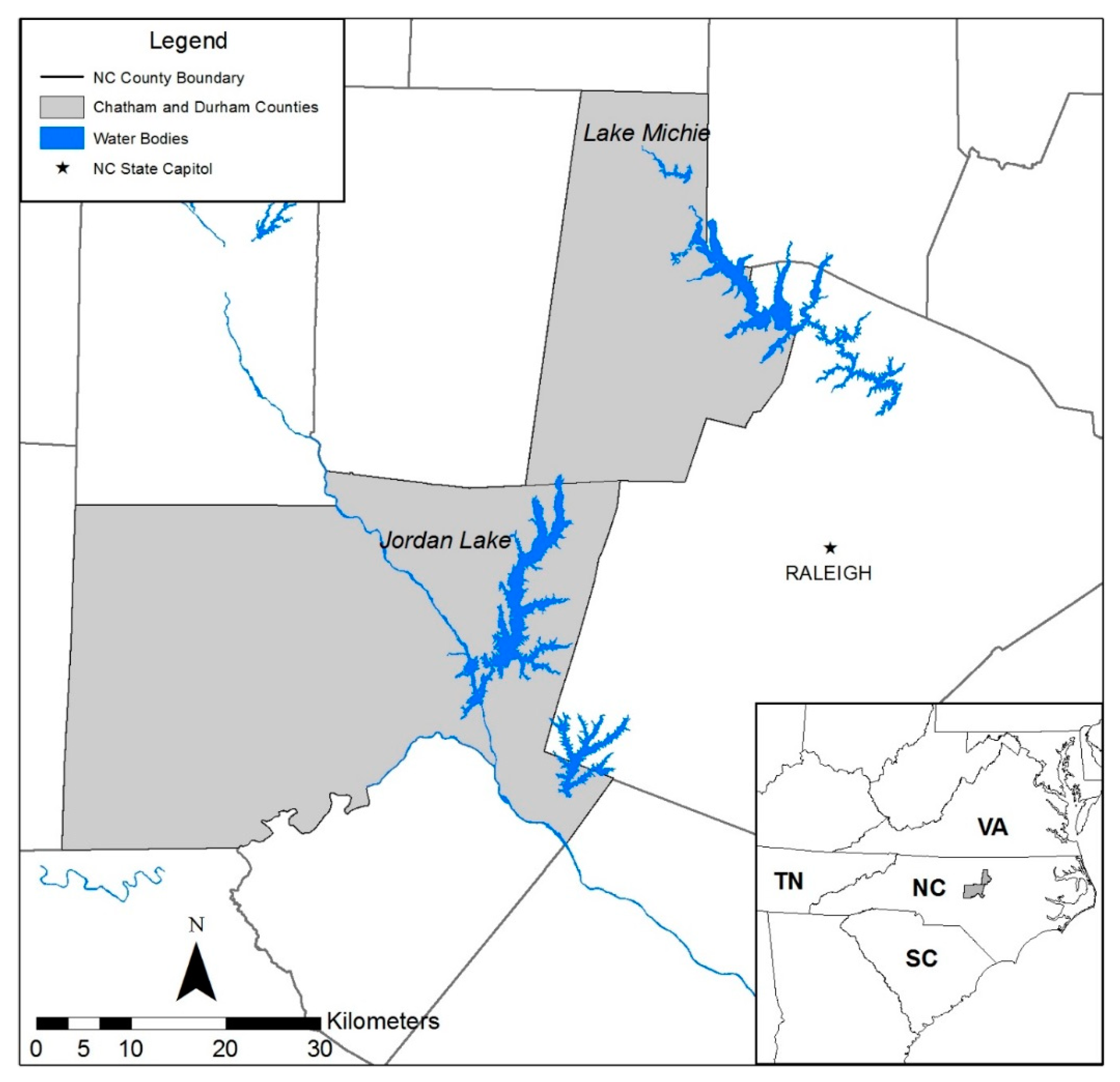
2.1. Site Selection and Sampling
2.2. Fish Tissue Analysis
2.3. Statistical Analyses
3. Results
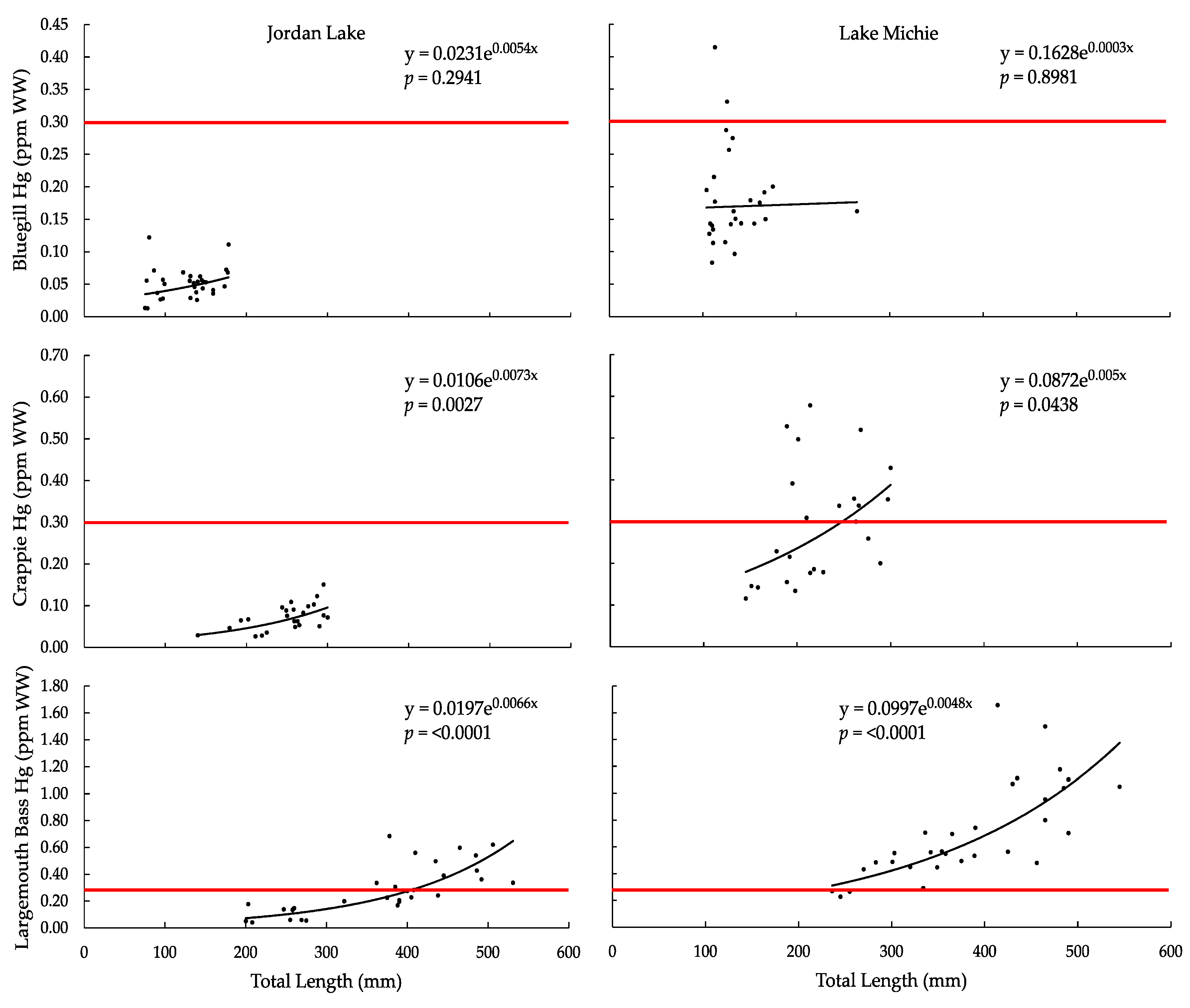
3.1. Mercury
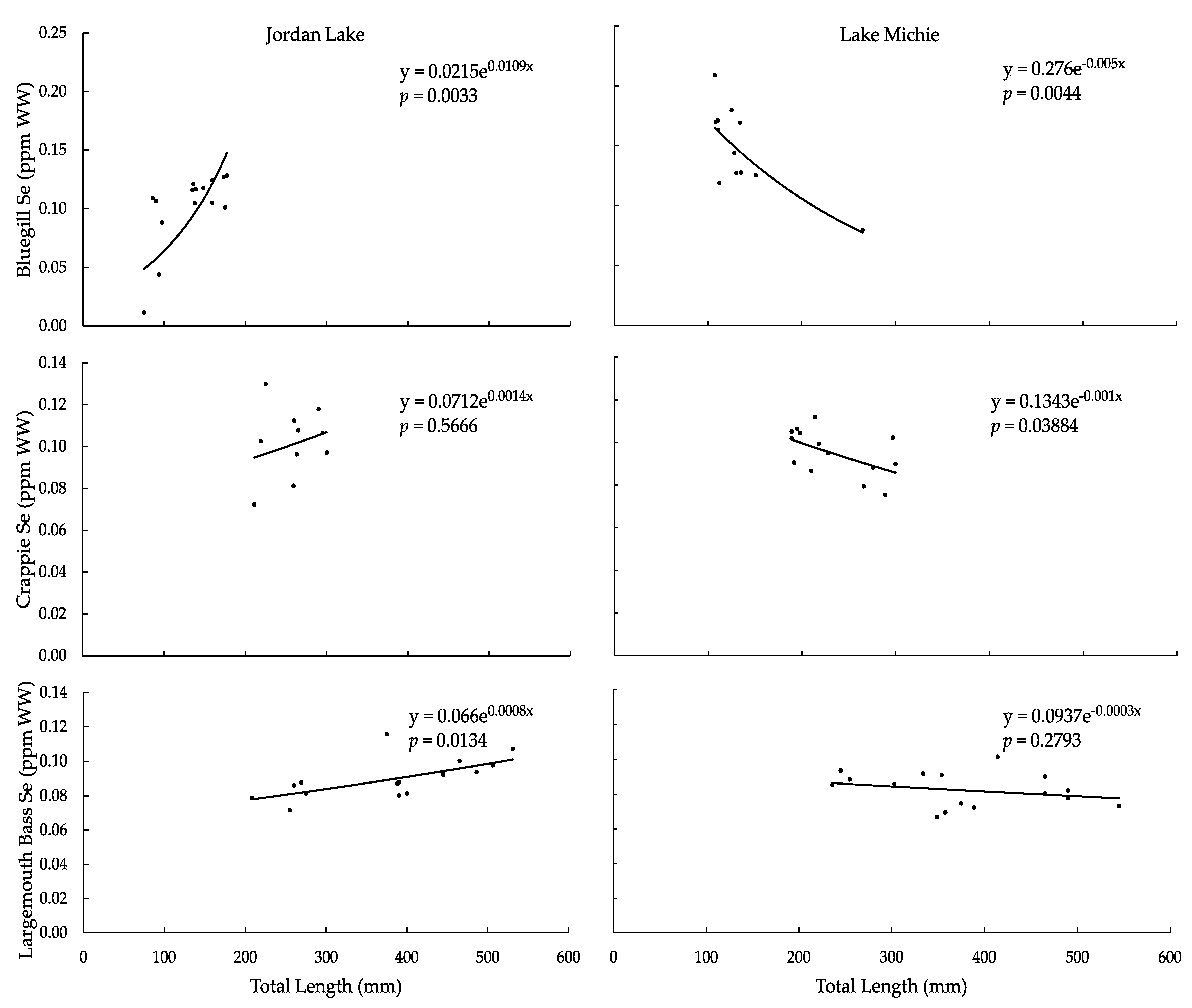
3.2. Selenium
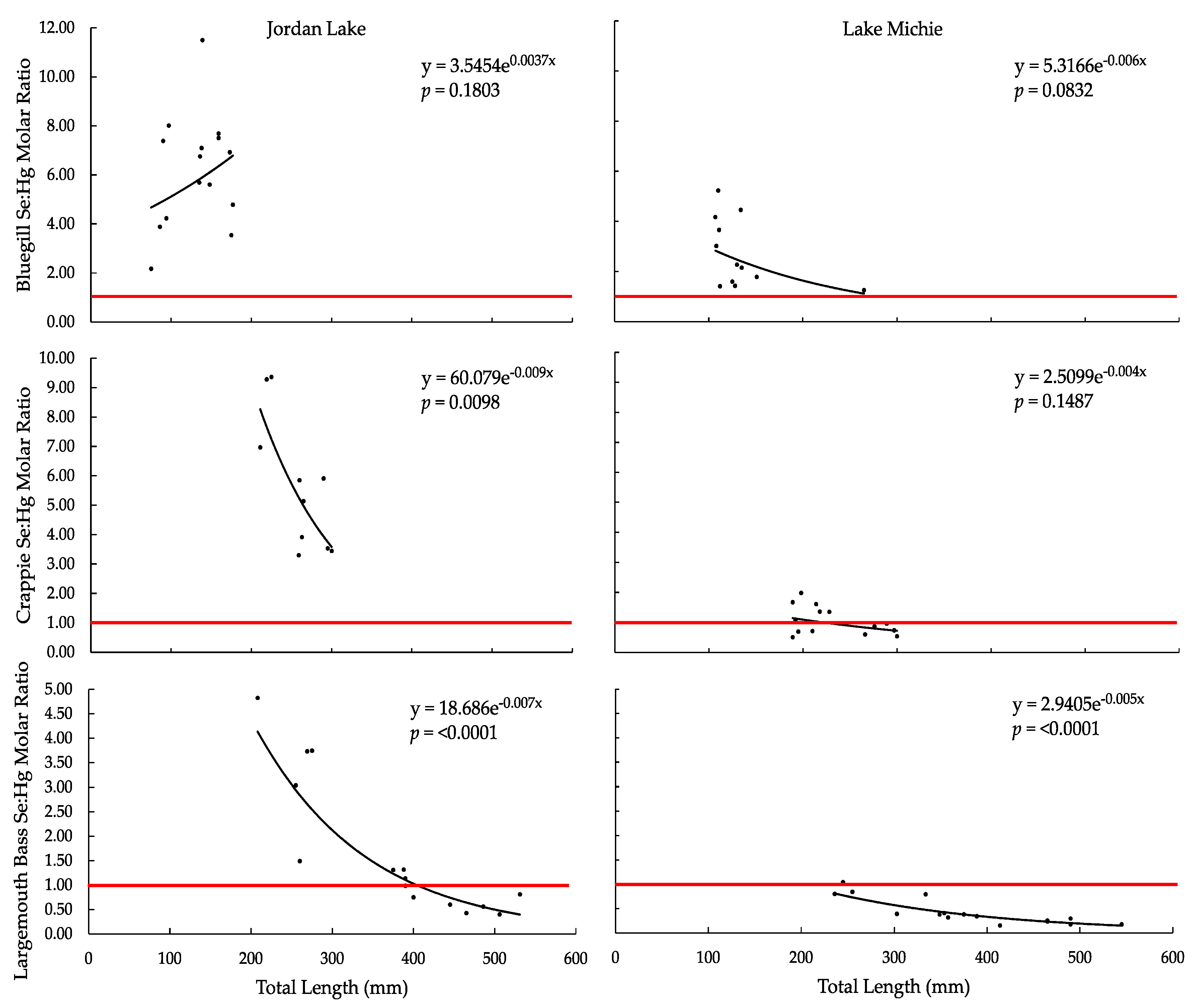
3.3. Selenium:Mercury Molar Ratio
3.4. Selenium Health Benefit Values
4. Discussion
4.1. Mercury
4.2. Selenium
4.3. Selenium:Merucry Molar Ratio
4.4. HBVSe Determination
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burger, J.; Jehl, J.R.J.; Gochfeld, M. Selenium: Mercury molar ratio in eared grebes (Podiceps nigricollis) as a possible biomarker of exposure. Ecol. Indic. 2013, 34, 60–68. [Google Scholar] [CrossRef]
- Sackett, D.K.; Aday, D.D.; Rice, J.A.; Cope, W.G.; Buchwalter, D. Does proximity to coal-fired power plants influence fish tissue mercury? Ecotoxicology 2010, 19, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- National Listing of Fish Advisories: Technical Fact Sheet. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/technical-factsheet-2011.pdf (accessed on 15 April 2018).
- Mercury and Fish Consumption Fact Sheet. Available online: http://www.env-health.org/IMG/pdf/Fish_consumption.pdf (accessed on 15 April 2018).
- Sackett, D.K.; Aday, D.D.; Rice, J.A.; Cope, W.G. Maternally transferred mercury in wild largemouth bass, Micropterus salmoides. Environ. Pollut. 2013, 178, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Drevnick, P.E.; Sandheinrich, M.B. Effects of dietary methylmercury on reproductive endocrinology of fathead minnows. Environ. Sci. Technol. 2003, 37, 4390–4396. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.K.; Cope, W.G.; Rice, J.A.; Aday, D.D. The influence of fish length on tissue mercury dynamics: Implications for natural resource management and human health risk. Int. J. Environ. Res. Public Health 2013, 10, 638–659. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Qiu, G. Methylmercury exposure and health effects from rice and fish consumption: A review. Int. J. Environ. Res. Public Health 2010, 7, 2666–2691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McDonald, T.; Sohn, M.; Anquandah, G.; Pettine, M.; Zboril, R. Biogeochemistry of selenium: A review. Environ. Chem. Lett. 2015, 13, 49–58. [Google Scholar] [CrossRef]
- Lemly, A.D. Symptoms and implications of selenium toxicity in fish: The Belews Lake case example. Aquat. Toxicol. 2002, 57, 39–49. [Google Scholar] [CrossRef]
- Lemly, A.D. Teratogenic effects and monetary cost of selenium poisoning of fish in Lake Sutton, North Carolina. Ecotoxicol. Environ. Saf. 2014, 104, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.E.; Bernhardt, E.S.; Dwyer, G.S.; Di Giulio, R.T. Selenium ecotoxicology in freshwater lakes receiving coal combustion residual effluents: A North Carolina example. Environ. Sci. Technol. 2017, 51, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New insights into traditional health risk assessments of mercury exposure: Implications of selenium. Environ. Sci. Technol. 2014, 48, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.S.; Chapman, P.M. Human health risks of selenium-contaminated fish: A case study for risk assessment of essential elements. Hum. Ecol. Risk Assess. Int. J. 2007, 13, 1192–1213. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Jeitner, C.; Burke, S.; Stamm, T.; Snigaroff, R.; Snigaroff, D.; Patrick, R.; Weston, J. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci. Total Environ. 2007, 384, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Fish, mercury, selenium and cardiovascular risk: Current evidence and unanswered questions. Int. J. Environ. Res. Public Health 2009, 6, 1894–1916. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000; pp. 1–365. ISBN 9780309071406. [Google Scholar]
- Borum, D.; Schoeny, R.; Manibusan, M.K.; Winchester, E.L. Water Quality Criterion for the Protection of Human Health: Methylmercury; USEPA: Washington, DC, USA, 2001; pp. 1–308.
- Foulke, J.E. Mercury in fish: Cause for concern? FDA Consum. 1994, 28, 5. [Google Scholar]
- Mercury: What Fish Are Safe to Eat? Available online: http://epi.publichealth.nc.gov/oee/mercury/safefish.html (accessed on 30 March 2018).
- Williams, L.K. (North Carolina Department of Health and Human Services, Raleigh, North Carolina, USA). NC Fish Advisory Action Levels for DDT, DDE, DDD, Dioxins, Mercury, PCBs, PBDEs, and Selenium. Personal communication, 2007. [Google Scholar]
- Ganther, H.E.; Goudie, C.; Sunde, M.L.; Kopecky, M.J.; Wagner, P.; Oh, S.; Hoekstra, W.G. Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science 1972, 175, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in commercial fish from New Jersey and Illinois: Variation within species and relevance to risk communication. Food Chem. Toxicol. 2013, 57, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M.; Burger, J.; Jeitner, C.; Donio, M.; Pittfield, T. Seasonal, locational and size variations in mercury and selenium levels in striped bass (Morone saxatilis) from New Jersey. Environ. Res. 2012, 112, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in saltwater fish from New Jersey: Individual and species variability complicate use in human health fish consumption advisories. Environ. Res. 2012, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.A.; Ralston, N.V.C.; Peck, D.V.; Sickle, J.V.; Robertson, J.D.; Spate, V.L.; Morris, J.S. How might selenium moderate the toxic effects of mercury in stream fish of the western U.S.? Environ. Sci. Technol. 2009, 43, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L. Selenium and mercury molar ratios in commercial fish from the Baltic Sea: Additional risk assessment criterion for mercury exposure. Food Control 2015, 50, 881–888. [Google Scholar] [CrossRef]
- Looi, L.J.; Aris, A.Z.; Haris, H.; Yusoff, F.M.; Hashim, Z. The levels of mercury, methylmercury and selenium and the selenium health benefit value in grey-eel catfish (Plotosus canius) and giant mudskipper (Periophthalmodon schlosseri) from the Strait of Malacca. Chemosphere 2016, 152, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.; Silbergeld, E.K.; Basu, N.; Bustamante, P.; Diaz-Barriga, F.; Hopkins, W.A.; Kidd, K.A.; Nyland, J.F. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 2018, 47, 170–197. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.; Ralston, C.; Raymond, L. Selenium health benefit values: Updated criteria for mercury risk assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cusack, L.K.; Eagles-Smith, C.; Harding, A.K.; Kile, M.; Stone, D. Selenium: Mercury molar ratios in freshwater fish in the Columbia River Basin: Potential applications for specific fish consumption advisories. Biol. Trace Elem. Res. 2017, 178, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Reash, R.J.; Brown, L.; Merritt, K. Mercury and other trace elements in Ohio River fish collected near coal-fired power plants: Interspecific patterns and consideration of consumption risks. Integr. Environ. Assess. Manag. 2015, 11, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, M.J.; Olson, J.T.; Holub, B.J.; Ripley, M.P. Mercury, polychlorinated biphenyls, selenium, and fatty acids in tribal fish harvests of the Upper Great Lakes. Risk Anal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ai, Z.; Zheng, S.; Gu, B.; Li, Y. Effects of dryout and inflow water quality on mercury methylation in a constructed wetland. Water Air Soil Pollut. 2014, 225, 1929. [Google Scholar] [CrossRef]
- Tremblay, A.; Lucotte, M.; Schetagne, R.; Therien, N. (Eds.) Mercury in the Biogeochemical Cycle: Natural Environments and Hydroelectric Reservoirs of Northern Quebec (Canada); Springer: New York, NY, USA, 1999; pp. 1–334. ISBN 354065755X. [Google Scholar]
- Willacker, J.J.; Eagles-Smith, C.A.; Lutz, M.A.; Tate, M.T.; Lepak, J.M.; Ackerman, J.T. Reservoirs and water management influences fish mercury concentrations in the western United States and Canada. Sci. Total Environ. 2016, 568, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, J.W.; Jagoe, C.H.; Bryan, A.L., Jr.; Brant, H.A.; Burger, J. Effects of trophic status and wetland morphology, hydroperiod, and water chemistry on mercury concentrations in fish. Can. J. Fish. Aquat. Sci. 2000, 57, 171–180. [Google Scholar] [CrossRef]
- Jordan Lake Water Supply Allocation: Background Information. Available online: https://deq.nc.gov/about/divisions/water-resources/planning/basin-planning/map-page/cape-fear-river-basin-landing/jordan-lake-water-supply-allocation/jordan-lake-water-supply-allocation-background-info (accessed on 15 April 2018).
- NC Department of Environmental Quality. Division of Water Resources Round 4 Jordan Lake Allocations: Final Decision. Available online: https://files.nc.gov/ncdeq/Water%20Resources/files/permits/jordanlake/EMC_Final_Decision_Round_4_Jordan_Lake_Allocation_2017-04-04.pdf (accessed on 23 July 2018).
- Water Management, Water Supply and Treatment Division. Available online: https://web.archive.org/web/20071016212933/http://durhamnc.gov/departments/wm/water_supply.cfm (accessed on 15 April 2018).
- Lake Levels: Current Conditions, Source Water (Reservoir) Levels. Available online: https://durhamnc.gov/1225/Lake-Levels (accessed on 15 April 2018).
- Weaver, J.C. Bathymetry of Lake Michie, Durham County, North Carolina; Water Resources Investigations Report 1990–1992 (No. 93-4031); U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 1993.
- Water Supply Status: Current Data, Days of Supply. Available online: https://durhamnc.gov/1214/Current-Data (accessed on 15 April 2018).
- Durham Parks and Recreation, Lake Michie Boathouse. Available online: http://durhamnc.gov/facilities/facility/details/lake-michie-recreation-area-118 (accessed on 15 April 2018).
- Hall, B.; Bodaly, R.; Fudge, R.; Rudd, J.; Rosenberg, D. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut. 1997, 100, 13–24. [Google Scholar]
- What to Catch: Common Sportfish Species. Available online: http://ncwildlife.org/Fishing/What-to-Catch (accessed on 15 April 2018).
- Werner, E.E.; Hall, D.J. Ontogenetic habitat shifts in bluegill: The foraging rate-predation risk trade-off. Ecology 1988, 69, 1352–1366. [Google Scholar] [CrossRef]
- Pine, W.E.; Allen, M.S. Differential growth and survival of weekly age-0 black crappie cohorts in a Florida lake. Trans. Am. Fish. Soc. 2001, 130, 80–91. [Google Scholar] [CrossRef]
- Olson, M.H. Ontogenetic niche shifts in largemouth bass: Variability and consequences for first-year growth. Ecology 1996, 77, 179–190. [Google Scholar] [CrossRef]
- Inland Fishing Regulations and Information. Available online: http://ncwildlife.org/Portals/0/Regs/Documents/Fishing-Regulations.pdf (accessed on 15 April 2018).
- US EPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. In Fish Sampling and Analysis, 3rd ed.; US EPA: Washington, DC, USA, 2000; Volume 1, pp. 1–485. [Google Scholar]
- Bloom, N.S. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry: US EPA Method 7473. Available online: https://www.epa.gov/sites/production/files/2015-07/documents/epa-7473.pdf (accessed on 15 April 2018).
- Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf (accessed on 15 April 2018).
- SAS Institute Inc. JMP Pro. 13.0.0; SAS Institute Inc.: Cary, North Carolina, USA, 2016. [Google Scholar]
- Dittman, B. (North Carolina Department of Health and Human Services, Raleigh, North Carolina, USA). Re: Risk-based screening for fish consumption advisories in NC. Personal communication, 2018. [Google Scholar]
- US EPA. Aquatic Life Ambient Water Quality Criterion for Selenium—Freshwater 2016; EPA 822-R-16-006; US EPA Office of Water: Washington, DC, USA, 2016; pp. 1–807.
- Cope, W.G.; Wiener, J.G.; Rada, R.G. Mercury accumulation in yellow perch in Wisconsin seepage lakes: Relation to lake characteristics. Environ. Toxicol. Chem. 1990, 9, 931–940. [Google Scholar] [CrossRef]
- Fish Consumption Advisories: Current Advisories for, NC. Available online:. Available online: http://epi.publichealth.nc.gov/oee/fish/advisories.html#H (accessed on 16 April 2018).
- Statewide Mercury Advisory. Available online: https://appliedecology.cals.ncsu.edu/fish-consumption/mercury-advisory/ (accessed on 18 May 2018).
- Sackett, D.K.; Aday, D.D.; Rice, J.A.; Cope, W.G. A statewide assessment of mercury dynamics in North Carolina water bodies and fish. Trans. Am. Fish. Soc. 2009, 138, 1328–1341. [Google Scholar] [CrossRef]
- Sackett, D.K.; Aday, D.D.; Rice, J.A.; Cope, W.G. Validation of a predictive model for fish tissue mercury concentrations. Trans. Am. Fish. Soc. 2013, 142, 380–387. [Google Scholar] [CrossRef]
- Lake and Reservoir Assessments Neuse River Basin. Available online: https://files.nc.gov/ncdeq/Water%20Quality/Environmental%20Sciences/Reports/NEUSE%20%20RIVER%20BASIN%20ALL%202015.pdf (accessed on 1 May 2018).
- Study for the Ongoing Assessment of Water Quality in Jordan Lake 2016 Results. Available online: https://files.nc.gov/ncdeq/Water%20Quality/Environmental%20Sciences/ISU/FINAL_Jordan%20Report%202016.pdf (accessed on 1 May 2018).
- Wang, X.; Wang, W. Selenium induces the demethylation of mercury in marine fish. Environ. Pollut. 2017, 231, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Aaseth, J.; Ajsuvakova, O.P.; Nikonorov, A.A.; Skalny, A.V.; Skalnaya, M.G.; Tinkov, A.A. Molecular interaction between mercury and selenium in neurotoxicity. Coord. Chem. Rev. 2017, 332, 30–37. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Ralston, C.R.; Blackwell, J.L.I.; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 2008, 29, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Fjordside, S.; Hansen, M.G.; Petrova, M.B. Dietary selenium reduces retention of methyl mercury in freshwater fish. Environ. Sci. Technol. 2011, 45, 9793–9798. [Google Scholar] [CrossRef] [PubMed]
| Contaminant | Concentration (ppm WW) | Fish Consumption Recommendations by Population Subgroup | |
|---|---|---|---|
| Women 15–44 Years Old (Childbearing Age) and Children < 15 Years Old | General Public (Males ≥ 15 and Females > 44 Years Old) | ||
| Hg | <0.4 | 2 meals/week | 4 meals/week |
| 0.4–1.0 | do not eat | 1 meal/week | |
| >1.0–3.0 | do not eat | 1 meal/month | |
| >3.0 | do not eat | do not eat | |
| Se | <10 | no advisory | no advisory |
| 10–20 | 1 meal/week | 1 meal/week | |
| >20–50 | 1 meal/month | 1 meal/month | |
| >50 | do not eat | do not eat | |
| Species | Small | Medium | Large |
|---|---|---|---|
| Bluegill | <115 mm | 115–150 mm | >150 mm |
| Crappie | <203 mm | 203–279 mm | >279 mm |
| Largemouth bass | <355 mm | 355–432 mm | >432 mm |
| Species | Bluegill | Crappie | Largemouth Bass | |||
|---|---|---|---|---|---|---|
| Lake | Jordan | Michie | Jordan | Michie | Jordan | Michie |
| Hg (ppm, wet weight) | ||||||
| N | 30 | 27 | 24 | 24 | 31 | 31 |
| Mean ± SE | 0.05 ± <0.01 | 0.18 ± 0.01 | 0.07 ± 0.01 | 0.29 ± 0.03 | 0.28 ± 0.03 | 0.71 ± 0.06 |
| Range | 0.01–0.12 | 0.08–0.41 | 0.03–0.15 | 0.12–0.58 | 0.04–0.68 | 0.23–1.66 |
| Mean TL | 128 | 135 | 249 | 223 | 365 | 382 |
| (mm, and range) | (75–178) | (104–265) | (140–300) | (145–300) | (200–531) | (236–545) |
| ANOVA Hg vs. TL, F-Ratio | 1.1432 | 0.0167 | 11.3894 | 4.5751 | 41.3917 | 41.3247 |
| ANOVA Hg vs. TL, p-Value | 0.2941 | 0.8981 | 0.0027 | 0.0438 | <0.0001 | <0.0001 |
| Se (ppm, wet weight) | ||||||
| N | 15 | 12 | 10 | 14 | 15 | 16 |
| Mean ± SE | 0.10 ± 0.01 | 0.15 ± 0.01 | 0.10 ± 0.01 | 0.10 ± <0.01 | 0.09 ± <0.01 | 0.08 ± <0.01 |
| Range | 0.01–0.13 | 0.08–0.21 | 0.07–0.13 | 0.08–0.11 | 0.07–0.12 | 0.07–0.10 |
| Mean TL | 132 | 135 | 259 | 233 | 376 | 379 |
| (mm, and range) | (75–177) | (107–265) | (211–300) | (189–300) | (208–531) | (236–545) |
| ANOVA Se vs. TL, F-Ratio | 12.8870 | 13.3951 | 0.3572 | 5.3774 | 8.1846 | 1.2667 |
| ANOVA Se vs. TL, p-Value | 0.0033 | 0.0044 | 0.5666 | 0.0388 | 0.0134 | 0.2793 |
| Se:Hg Molar Ratio | ||||||
| N | 15 | 12 | 10 | 14 | 15 | 16 |
| Mean ± SE | 6.18 ± 0.59 | 2.70 ± 0.40 | 5.67 ± 0.72 | 1.04 ± 0.13 | 1.67 ± 0.37 | 0.44 ± 0.07 |
| Range | 2.17–11.50 | 1.25–5.23 | 3.30–9.36 | 0.51–1.98 | 0.40–4.82 | 0.16–1.04 |
| Mean TL | 132 | 135 | 259 | 233 | 376 | 379 |
| (mm, and range) | (75–177) | (107–265) | (211–300) | (189–300) | (208–531) | (236–545) |
| ANOVA Se:Hg vs. TL, F-Ratio | 2.0049 | 3.7044 | 11.3559 | 2.3819 | 68.9019 | 48.4018 |
| ANOVA Se:Hg vs. TL, p-Value | 0.1803 | 0.0832 | 0.0098 | 0.1487 | <0.0001 | <0.0001 |
| Lake | Species | N | HBVSe Mean ± SE | HBVSe Range |
|---|---|---|---|---|
| Jordan | ||||
| Bluegill | 15 | 1.24 ± 0.11 | 0.11–1.58 | |
| Crappie | 10 | 1.24 ± 0.07 | 0.90–1.63 | |
| Largemouth Bass | 15 | −0.85 ± 0.62 | −6.50–1.03 | |
| Michie | ||||
| Bluegill | 12 | 1.46 ± 0.18 | 0.36–2.49 | |
| Crappie | 14 | −0.62 ± 0.40 | −3.88–0.99 | |
| Largemouth Bass | 16 | −12.03 ± 3.53 | −51.90–0.10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, T.K.B.; LePrevost, C.E.; Kwak, T.J.; Cope, W.G. Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs. Int. J. Environ. Res. Public Health 2018, 15, 1864. https://doi.org/10.3390/ijerph15091864
Johnson TKB, LePrevost CE, Kwak TJ, Cope WG. Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs. International Journal of Environmental Research and Public Health. 2018; 15(9):1864. https://doi.org/10.3390/ijerph15091864
Chicago/Turabian StyleJohnson, Tara K. B., Catherine E. LePrevost, Thomas J. Kwak, and W. Gregory Cope. 2018. "Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs" International Journal of Environmental Research and Public Health 15, no. 9: 1864. https://doi.org/10.3390/ijerph15091864
APA StyleJohnson, T. K. B., LePrevost, C. E., Kwak, T. J., & Cope, W. G. (2018). Selenium, Mercury, and Their Molar Ratio in Sportfish from Drinking Water Reservoirs. International Journal of Environmental Research and Public Health, 15(9), 1864. https://doi.org/10.3390/ijerph15091864





