Bias Adjustment Techniques Are Underutilized in HIV Sexual Risk Estimation: A Systematic Review
Abstract
1. Introduction
Consequences of Misclassifying Sexual Behavior in HIV Risk Estimation
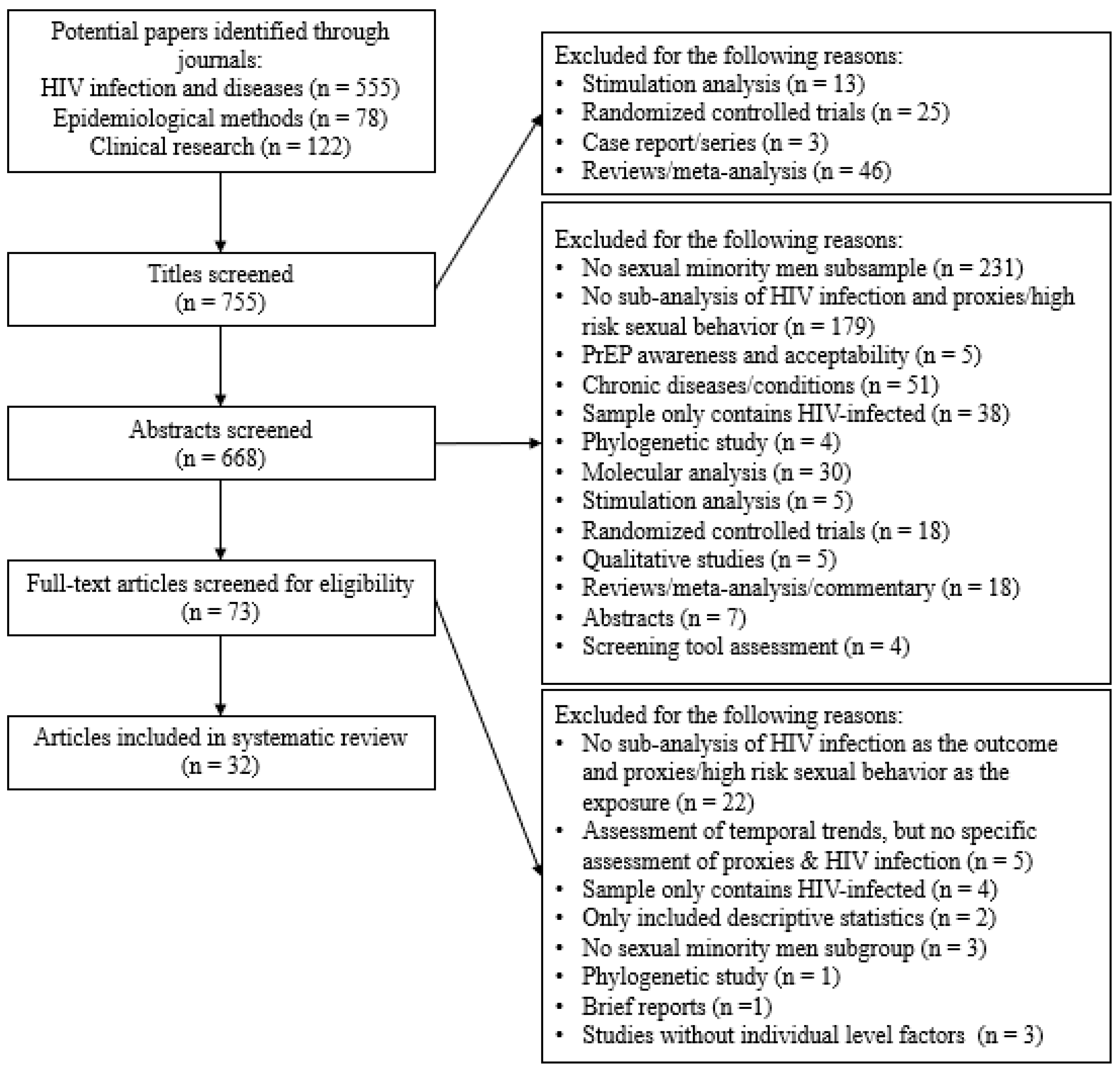
2. Methods
- HIV- and STI-focused journals: Acquired Immunodeficiency Syndrome, Journal of Acquired Immunodeficiency Syndrome, Acquired Immunodeficiency Syndrome and Behavior, Sexually Transmitted Disease, Sexually Transmitted Infections,
- Epidemiology-focused journals: American Journal of Epidemiology, Epidemiology, International Journal of Epidemiology, European Journal of Epidemiology, and Annals of Epidemiology,
- General medical journals: New England Journal of Medicine, Journal of American Medical Association, Lancet, and Annals of Internal Medicine.
Study Inclusion/Exclusion Criteria
3. Results
3.1. Assessment of High-Risk Behaviors and MSM Exposure
3.2. Self-Reported Proxy of High-Risk Sexual Behavior
3.3. Acknowledgement of Purported Sexual Behavior Misclassification
4. Discussion
4.1. Correcting Risk Estimates
4.2. Implications of Biased HIV Risk Data
4.3. Towards Improving the Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Durso, L.E.; Gates, G.J. Best practices: Collecting and analyzing data on sexual minorities. In International Handbook on the Demography of Sexuality; Baumle, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 21–42. [Google Scholar]
- Richters, J. Understanding sexual orientation: A plea for clarity. Reprod. Health Matters 1998, 6, 144–149. [Google Scholar] [CrossRef]
- Beyrer, C.; Baral, S.D.; van Griensven, F.; Goodreau, S.M.; Chariyalertsak, S.; Wirtz, A.L.; Brookmeyer, R. Global epidemiology of hiv infection in men who have sex with men. Lancet 2012, 380, 367–377. [Google Scholar] [CrossRef]
- Institute of Medicine of the National Academies. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- McFarland, W.; Caceres, C.F. HIV surveillance among men who have sex with men. AIDS 2001, 15, S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Pathela, P.; Hajat, A.; Schillinger, J.; Blank, S.; Sell, R.; Mostashari, F. Discordance between sexual behavior and self-reported sexual identity: A population-based survey of new york city men. Ann. Intern. Med. 2006, 145, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Latkin, C.; Yang, C.; Tobin, K.; Roebuck, G.; Spikes, P.; Patterson, J. Social network predictors of disclosure of msm behavior and hiv-positive serostatus among african american msm in baltimore, maryland. AIDS Behav. 2012, 16, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zea, M.C.; Reisen, C.A.; Poppen, P.J.; Echeverry, J.J.; Bianchi, F.T. Disclosure of hiv-positive status to latino gay men’s social networks. Am. J. Community Psychol. 2004, 33, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Savin-Williams, R.C. Who’s gay? Does it matter? Curr. Dir. Psychol. Sci. 2006, 15, 40–44. [Google Scholar] [CrossRef]
- Lash, T.L.; Fink, A.K.; Fox, M.P. Misclassification. In Applying Quantitative Bias Analysis to Epidemiologic Data; Springer: New York, NY, USA, 2009; pp. 79–108. [Google Scholar]
- Igartua, K.; Thombs, B.D.; Burgos, G.; Montoro, R. Concordance and discrepancy in sexual identity, attraction, and behavior among adolescents. J. Adolesc. Health 2009, 45, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Cole, S.R. Use of multiple imputation in the epidemiologic literature. Am. J. Epidemiol. 2008, 168, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Brakenhoff, T.B.; Mitroiu, M.; Keogh, R.H.; Moons, K.G.M.; Groenwold, R.H.H.; van Smeden, M. Measurement error is often neglected in medical literature: A systematic review. J. Clin. Epidemiol. 2018, 98, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ko, N.Y.; Lee, H.C.; Hung, C.C.; Tseng, F.C.; Chang, J.L.; Lee, N.Y.; Chang, C.M.; Lee, M.P.; Chen, B.J.; Wang, S.W.; et al. Trends of HIV and sexually transmitted infections, estimated hiv incidence, and risky sexual behaviors among gay bathhouse attendees in taiwan: 2004–2008. AIDS Behav. 2011, 15, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-D.; Zhao, J.; Gan, Y.-X.; Zhang, Y.; Yang, Z.-R.; Cheng, J.-Q.; Lin, S.-H.; He, M.-L.; Chen, L.; Wang, X.-R. A comparison of HIV infection and related risk factors between money boys and noncommercial men who have sex with men in shenzhen, china. Sex. Transm. Dis. 2012, 39, 942–948. [Google Scholar]
- Solomon, S.S.; Mehta, S.H.; Srikrishnan, A.K.; Vasudevan, C.K.; McFall, A.M.; Balakrishnan, P.; Anand, S.; Nandagopal, P.; Ogburn, E.L.; Laeyendecker, O.; et al. High HIV prevalence and incidence among msm across 12 cities in india. AIDS 2015, 29, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yan, H.; Liu, Y.; Leng, Z.; Wang, B.; Zhao, J. Increasing prevalence of hiv and syphilis but decreasing rate of self-reported unprotected anal intercourse among men who had sex with men in harbin, China: Results of five consecutive surveys from 2006 to 2010. Int. J. Epidemiol. 2012, 41, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Lin, P.; Xu, H.; Wang, Y.; Wang, M.; He, Q.; Fan, L.; Li, Y.; Wen, F.; Liang, Y.; et al. Possible increase in hiv and syphilis prevalence among men who have sex with men in guangzhou, China: Results from a respondent-driven sampling survey. AIDS Behav. 2011, 15, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.Z.; Ruan, Y.; Liu, Y.; Milam, D.F.; Spiegel, H.M.; Yin, L.; Li, D.; Shepherd, B.E.; Shao, Y.; Vermund, S.H. Lower HIV risk among circumcised men who have sex with men in China: Interaction with anal sex role in a cross-sectional study. J. Acquir. Immune Defic. Syndr. 2016, 71, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Kunzweiler, C.P.; Bailey, R.C.; Okall, D.O.; Graham, S.M.; Mehta, S.D.; Otieno, F.O. Factors associated with prevalent HIV infection among Kenyan MSM: The Anza Mapema Study. J. Acquir. Immune Defic. Syndr. 2017, 76, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tafuma, T.A.; Merrigan, M.B.; Okui, L.A.; Lebelonyane, R.; Bolebantswe, J.; Mine, M.; Chishala, S.; Moyo, S.; Thela, T.; Rajatashuvra, A. HIV/sexually transmitted infection prevalence and sexual behavior of men who have sex with men in 3 districts of Botswana: Results from the 2012 biobehavioral survey. Sex. Transm. Infect. 2014, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, A.L.; Trapence, G.; Kamba, D.; Gama, V.; Chalera, R.; Jumbe, V.; Kumwenda, R.; Mangochi, M.; Helleringer, S.; Beyrer, C.; et al. Geographical disparities in hiv prevalence and care among men who have sex with men in Malawi: Results from a multisite cross-sectional survey. Lancet HIV 2017, 4, e260–e269. [Google Scholar] [CrossRef]
- Konda, K.A.; Lescano, A.G.; Celentano, D.D.; Hall, E.; Montano, S.M.; Kochel, T.J.; Coates, T.J.; Cáceres, C.F.; NIMH Collaborative HIVSTD Prevention Trial Group. In Peru, reporting male sex partners imparts significant risk of incident HIV/sexually transmitted infection: All men engaging in same-sex behavior need prevention services. Sex. Transm. Dis. 2013, 40, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Konda, K.A.; Leon, S.R.; Silva-Santisteban, A.; Salazar, X.; Klausner, J.D.; Coates, T.J.; Caceres, C.F. HIV and sexually transmitted infection incidence and associated risk factors among high-risk msm and male-to-female transgender women in Lima, Peru. J. Acquir. Immune Defic. Syndr. 2015, 69, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Van den Boom, W.; Konings, R.; Davidovich, U.; Sandfort, T.; Prins, M.; Stolte, I.G. Is serosorting effective in reducing the risk of HIV infection among men who have sex with men with casual sex partners? J. Acquir. Immune Defic. Syndr. 2014, 65, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Seaman, S.R.; De Angelis, D.; Presanis, A.M.; Dodds, J.P.; Johnson, A.M.; Mercey, D.; Gill, O.N.; Copas, A.J. A synthesis of convenience survey and other data to estimate undiagnosed HIV infection among men who have sex with men in England and Wales. Int. J. Epidemiol. 2011, 40, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Beymer, M.R.; Weiss, R.E.; Halkitis, P.N.; Kapadia, F.; Ompad, D.C.; Bourque, L.; Bolan, R.K. Disparities within the disparity-determining HIV risk factors among latino gay and bisexual men attending a community-based clinic in Los Angeles, CA. J. Acquir. Immune Defic. Syndr. 2016, 73, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Eaton, L.A.; Maksut, J.L.; Gamarel, K.E.; Siembida, E.J.; Driffin, D.D.; Baldwin, R. Online sex partner meeting venues as a risk factor for testing HIV positive among a community-based sample of black men who have sex with men. Sex. Transm. Dis. 2016, 43, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.D.; Burstyn, I.; Welles, S.L. Bayesian approaches to racial disparities in HIV risk estimation among men who have sex with men. Epidemiology 2017, 28, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.D.; Welles, S.L.; Burstyn, I. To be or not to be: Bayesian correction for misclassification of self-reported sexual behaviors among men who have sex with men. Epidemiology 2015, 26, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Joseph, H.A.; Marks, G.; Belcher, L.; Millett, G.A.; Stueve, A.; Bingham, T.A.; Lauby, J. Older partner selection, sexual risk behaviour and unrecognised HIV infection among black and latino men who have sex with men. Sex. Transm. Infect. 2011, 87, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Oster, A.M.; Wiegand, R.E.; Sionean, C.; Miles, I.J.; Thomas, P.E.; Melendez-Morales, L.; Le, B.C.; Millett, G.A. Understanding disparities in HIV infection between black and white msm in the United States. AIDS 2011, 25, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Reisner, S.L.; Falb, K.L.; Mimiaga, M.J. Early life traumatic stressors and the mediating role of PTSD in incident HIV infection among US men, comparisons by sexual orientation and race/ethnicity: Results from the Nesarc, 2004–2005. J. Acquir. Immune Defic. Syndr. 2011, 57, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.S.; Rosenberg, E.S.; Sanchez, T.H.; Kelley, C.F.; Luisi, N.; Cooper, H.L.; Diclemente, R.J.; Wingood, G.M.; Frew, P.M.; Salazar, L.F.; et al. Explaining racial disparities in HIV incidence in black and white men who have sex with men in Atlanta, GA: A prospective observational cohort study. Ann. Epidemiol. 2015, 25, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.B.; Bowles, K.E.; Le, B.C.; Paz-Bailey, G.; Oster, A.M. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS 2013, 27, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.; Hotton, A.L.; Kuhns, L.M.; Gratzer, B.; Mustanski, B. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J. Acquir. Immune Defic. Syndr. 2016, 72, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Halkitis, P.; Kapadia, F.; Ompad, D. Incidence of HIV infection in young gay, bisexual, and other YMSM: The p18 cohort study. J. Acquir. Immune Defic. Syndr. 2015, 69, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.R.; Stein, R.; Williams, W.O.; Wang, G.; Xu, S.; Uhl, G.; Cheng, Q.; Rasberry, C.N. HIV testing in nonhealthcare facilities among adolescent MSM. AIDS 2017, 31 (Suppl. 3), S261–S265. [Google Scholar] [CrossRef]
- Sanders, E.J.; Okuku, H.S.; Smith, A.D.; Mwangome, M.; Wahome, E.; Fegan, G.; Peshu, N.; van der Elst, E.M.; Price, M.A.; McClelland, R.S.; et al. High HIV-1 incidence, correlates of HIV-1 acquisition, and high viral loads following seroconversion among MSM. AIDS 2013, 27, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Crosby, R.; Mena, L.; Yarber, W.L.; Graham, C.A.; Sanders, S.A.; Milhausen, R.R. Condom use errors and problems: A comparative study of HIV-positive versus HIV-negative young black men who have sex with men. Sex. Transm. Dis. 2015, 42, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Davey, D.J.; Beymer, M.; Roberts, C.P.; Bolan, R.K.; Klausner, J.D. Differences in risk behavior and demographic factors between men who have sex with men with acute and nonacute human immunodeficiency virus infection in a community-based testing program in Los Angeles. J. Acquir. Immune Defic. Syndr. 2017, 74, e97–e103. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.; Welles, S.L. Associations of sexual identity or same-sex behaviors with history of childhood sexual abuse and HIV/STI risk in the United States. J. Acquir. Immune Defic. Syndr. 2012, 59, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, C.M.; Dombrowski, J.C.; Kerani, R.P.; Katz, D.A.; Barbee, L.A.; Golden, M.R. Changes in condomless sex and serosorting among men who have sex with men after HIV diagnosis. J. Acquir. Immune Defic. Syndr. 2016, 73, 475–481. [Google Scholar] [CrossRef] [PubMed]
- German, D.; Brady, K.; Kuo, I.; Opoku, J.; Flynn, C.; Patrick, R.; Park, J.N.; Adams, J.; Carroll, M.; Simmons, R.; et al. Characteristics of black men who have sex with men in Baltimore, Philadelphia, and Washington, D.C.: Geographic diversity in socio-demographics and HIV transmission risk. J. Acquir. Immune Defic. Syndr. 2017, 75, S296–S308. [Google Scholar] [CrossRef] [PubMed]
- Dailey Garnes, N.J.M.; Moore, Z.S.; Cadwell, B.L.; Fleischauer, A.T.; Leone, P. Previously undiagnosed HIV infections identified through cluster investigation, North Carolina, 2002–2007. AIDS Behav. 2015, 19, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.S.; Rosenberg, E.S. Commentary: Breaking bias: Improved methods for analyzing HIV/AIDS data. Epidemiology 2015, 26, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.L.; Fox, M.P.; MacLehose, R.F.; Maldonado, G.; McCandless, L.C.; Greenland, S. Good practices for quantitative bias analysis. Int. J. Epidemiol. 2014, 43, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sternberg, M.R.; Markowitz, L.E. Men who have sex with men in the United States: Demographic and behavioral characteristics and prevalence of HIV and HSV-2 infection: Results from national health and nutrition examination survey 2001–2006. Sex. Transm. Dis. 2010, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kates, J.; Ranji, U.; Beamesderfer, A.; Salganicoff, A.; Dawson, L. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender Individuals in the U.S.; Henry, J., Ed.; Kaiser Family Foundation: Menlo Park, CA, USA, 2014. [Google Scholar]
- Bird, J.D.; Voisin, D.R. “You’re an open target to be abused”: A qualitative study of stigma and HIV self-disclosure among black men who have sex with men. Am. J. Public Health 2013, 103, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Welles, S.L.; Corbin, T.J.; Rich, J.A.; Reed, E.; Raj, A. Intimate partner violence among men having sex with men, women, or both: Early-life sexual and physical abuse as antecedents. J. Community Health 2011, 36, 477–485. [Google Scholar] [CrossRef] [PubMed]
| Reference | Data Source | Study Description | Population-Based vs. Community-Based Survey | Survey Administration | Proxy Variables | Behavioral Variables | Treatment of Misclassification | ||
|---|---|---|---|---|---|---|---|---|---|
| Sexual Identify | Partner Gender | Condomless Anal Intercourse | Position | ||||||
| Cai et al., 2012 [16] | Cross-sectional survey administered in Shenzhen, China, 2008 | Cross-sectional analysis assessing factors associated with HIV prevalence among male sex workers | Community-based Survey | Self-administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Ko et al., 2011 [15] | Community-based cross-sectional survey, Taiwan, 2004–2008 | Cross-sectional analysis of factors associated with HIV/STI | Community-based Survey | Self-administered questionnaire | X | Acknowledgement, but no adjustment | |||
| Joseph et al., 2011 [32] | Brothers y Hermanos Study, 2005–2006 | Cross-sectional analysis of risk for unrecognized HIV infection among Black and Latino MSM | Community-based Survey | Self-administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Oster et al., 2011 [33] | National HIV Behavioral Surveillance Survey (NHBS), 2008 | Cross-sectional CI analysis assessing disparities in HIV infection between racial groups | Population-based Survey | Interviewer administered computer-based questionnaire | X | None | |||
| Reisner et al., 2011 [34] | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), 2004–2005 | Cross-sectional analysis assessing the association between HIV infection and early life stressors among MSM using a national survey | Population-based Survey | Interviewer administered computer-based questionnaire | X | Acknowledgement, but no adjustment | |||
| Walker et al., 2011 [27] | Gay men sexual health survey (GMSHS), British National Survey of Sexual Attitudes and Lifestyles (NATSAL), genitourinary medicine (GUM) clinic | Cross-sectional analysis estimating undiagnosed HIV infection among MSM | Population- and Community-based Survey | Self-administered questionnaire; self-administered computer-based questionnaire; disease reporting system | X | Acknowledgement, but no adjustment | |||
| Zhong et al., 2011 [19] | Cross-sectional survey, Guangzhou, China, 2008 | Cross-sectional analysis assessing prevalence of HIV among MSM | Population-based Survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Sweet et al., 2012 [43] | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), 2004–2005 | Cross-sectional analysis assessing the association between HIV risk and CSA | Population-based Survey | Interviewer administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Wang et al., 2012 [18] | Community based survey, Harbin, China, 2006–2010 | Cross-section analysis of HIV prevalence/syphilis and the context of lower rates of condomless anal sex | Community-based Survey | Interviewer administered questionnaire | X | Acknowledgement, but no adjustment | |||
| Balaji et al., 2013 [36] | National HIV Behavioral Surveillance Survey (NHBS), 2008 | Cross-sectional analysis assessing factors associated with HIV incidence and prevalence | Population-based Survey | Interviewer administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Konda et al., 2013 [24] | National Institute of Mental Health (NIMH) Collaborative HIV/STD Prevention Trial Group, 2002–2007 | Longitudinal analysis of factors associated with HIV/STI | Community-based survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Sanders et al., 2013 [40] | Prospective cohort study assessing sexual behaviors and HIV/STI acquisition | Longitudinal analysis assessing factors for HIV infection | Community-based Survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Tafuma et al., 2014 [22] | Ministry of Health and Family Health International 360 Survey, Botswana, 2012 | Cross-sectional survey analysis assessing factors associated with HIV/STI | Population-based Survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| van den Boom et al., 2014 [26] | Amsterdam Cohort Study (ACS), 2007–2011 | Cross-sectional survey analysis assessing factors associated with HIV | Population-based Survey | Self-administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Castillo et al., 2015 [25] | Longitudinal study assessing sexual behaviors and HIV/STI acquisition at baseline, 9- and 18-month follow-up | Discrete time proportional hazard models assessing factors associated with incidence of HIV/STI | Community-based Survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Crosby et al., 2015 [41] | Convenience sample recruited from National Institute of Health—RCT for safer sex intervention, 2012–2014 | Cross-sectional analysis assessing differences between HIV-positive and negative MSM in regard to condom use | Community-based Survey | Self-administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Dailey Garnes et al., 2015 [46] | Sexually Transmitted Disease Management Information System (STD-MIS) | Cross-sectional analysis assessing odds of identifying new HIV infection among social contacts using surveillance data | Neither | Disease reporting system | X | Acknowledgement, but no adjustment | |||
| Halkitis et al., 2015 [38] | Prospective cohort study assessing factors associated with HIV infection | Survival analysis assessing factors associated with HIV incidence among young MSM (18–19 years) | Community-based Survey | Interviewer administered questionnaire | X | X | None | ||
| Qian et al., 2015 [20] | Cross-sectional survey, Beijing, China, 2010–2011 | Cross-sectional analysis assessing risk factors associated with HIV prevalence | Community-based Survey | Self-administered questionnaire | X | Acknowledgement, but no adjustment | |||
| Solomon et al., 2015 [17] | Multicenter cross-sectional survey, 12 cities | Cross-sectional analysis assessing prevalence, incidence and factors associated with HIV infection | Population-based Survey | Interviewer administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Sullivan et al., 2015 [35] | Prospective cohort study, Atlanta, GA, 2010–2014 | Survival analysis assessing racial disparities in HIV infection | Community-based Survey | Self-administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Beymer et al., 2016 [28] | Longitudinal study assessing HIV risk factors using a community-based survey, Los Angeles, CA, 2009–2014 | Survival analysis assessing factors associated with HIV infection | Community-based Survey | Interviewer administered questionnaire | X | X | None | ||
| Eaton et al., 2016 [29] | Cross-sectional survey of Black MSM | Cross-sectional analysis assessing factors associated with testing HIV positive | Community-based Survey | Self-administered computer-based questionnaire | X | Acknowledgement, but no adjustment | |||
| Garofalo et al., 2016 [37] | Crew 45—Longitudinal study assessing HIV risk in Chicago for 2 years | Survival analysis assessing factors associated with HIV incidence among young MSM (16–20 years) | Community-based Survey | Self-administered computer-based questionnaire | X | Acknowledgement, but no adjustment | |||
| Khosropour et al., 2016 [44] | Data from STD clinic in Seattle & King County, 2001–2013 | Retrospective case-control study assessing factors associated with seroconversion and difference in sexual behavior after seroconversion | Population-based Survey | Disease reporting system | X | Acknowledgement, but no adjustment | |||
| Davey et al., 2017 [42] | Retrospective study of HIV testing data from LA LGBT Center, 2011–2015 | Cross-sectional analysis of MSM assessing factors associated with acute HIV infection | Community-based Survey | Interviewer administered questionnaire | X | X | Acknowledgement, but no adjustment | ||
| German et al., 2017 [45] | National HIV Behavioral Surveillance Survey (NHBS), 2008 | Cross-sectional analysis of factors associated with HIV transmission risk | Population-based Survey | Interviewer administered computer-based questionnaire | X | X | Acknowledgement, but no adjustment | ||
| Goldstein et al., 2015 [31] | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-2) & Black and African American Men’s Health Study (BAAMHS) | Bayesian analysis for the odds of self-reported HIV infection when adjusted for misclassification of same-sex behavior | Population- and Community-based Survey | Interviewer administered computer-based questionnaire; self-administered computer-based questionnaire | X | X | X | X | Acknowledgement and Bayesian adjustment |
| Goldstein et al., 2017 [30] | National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-2) & Black and African American Men’s Health Study (BAAMHS) | Bayesian analysis to adjust for residual confounding and correct misclassification of MSM status to help explain racial disparity in HIV infection | Population- and Community-based Survey | Interviewer administered computer-based questionnaire; self-administered computer-based questionnaire | X | X | X | X | Acknowledgement and Bayesian adjustment |
| Kunzweiler et al., 2017 [21] | The Anza Mapema Study, 2015–2016 | Cross-sectional analysis of risk reduction behaviors associated with HIV prevalence among HIV-positive and out of care (vs. HIV negative) and newly diagnosed HIV positive and out of care | Community-based Survey | Self-administered computer-based questionnaire | X | Acknowledgement, but no adjustment | |||
| Marano et al., 2017 [39] | NHM&E—National HIV Prevention Program Monitoring and Evaluation system, 2015 | Cross-sectional analysis describing linkage to care among new HIV diagnoses and assessing factors associated with HIV incidence | Population-based Survey | Disease reporting system | X | None | |||
| Wirtz et al., 2017 [23] | Multi-center cross-sectional survey, 2010–2014 | Cross-sectional analysis assessing regional disparities in HIV prevalence and care | Population-based Survey | Interviewer administered questionnaire | X | Acknowledgement, but no adjustment | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.K.; Goldstein, N.D.; Welles, S.L. Bias Adjustment Techniques Are Underutilized in HIV Sexual Risk Estimation: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 1696. https://doi.org/10.3390/ijerph15081696
Tran NK, Goldstein ND, Welles SL. Bias Adjustment Techniques Are Underutilized in HIV Sexual Risk Estimation: A Systematic Review. International Journal of Environmental Research and Public Health. 2018; 15(8):1696. https://doi.org/10.3390/ijerph15081696
Chicago/Turabian StyleTran, Nguyen K., Neal D. Goldstein, and Seth L. Welles. 2018. "Bias Adjustment Techniques Are Underutilized in HIV Sexual Risk Estimation: A Systematic Review" International Journal of Environmental Research and Public Health 15, no. 8: 1696. https://doi.org/10.3390/ijerph15081696
APA StyleTran, N. K., Goldstein, N. D., & Welles, S. L. (2018). Bias Adjustment Techniques Are Underutilized in HIV Sexual Risk Estimation: A Systematic Review. International Journal of Environmental Research and Public Health, 15(8), 1696. https://doi.org/10.3390/ijerph15081696





