A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
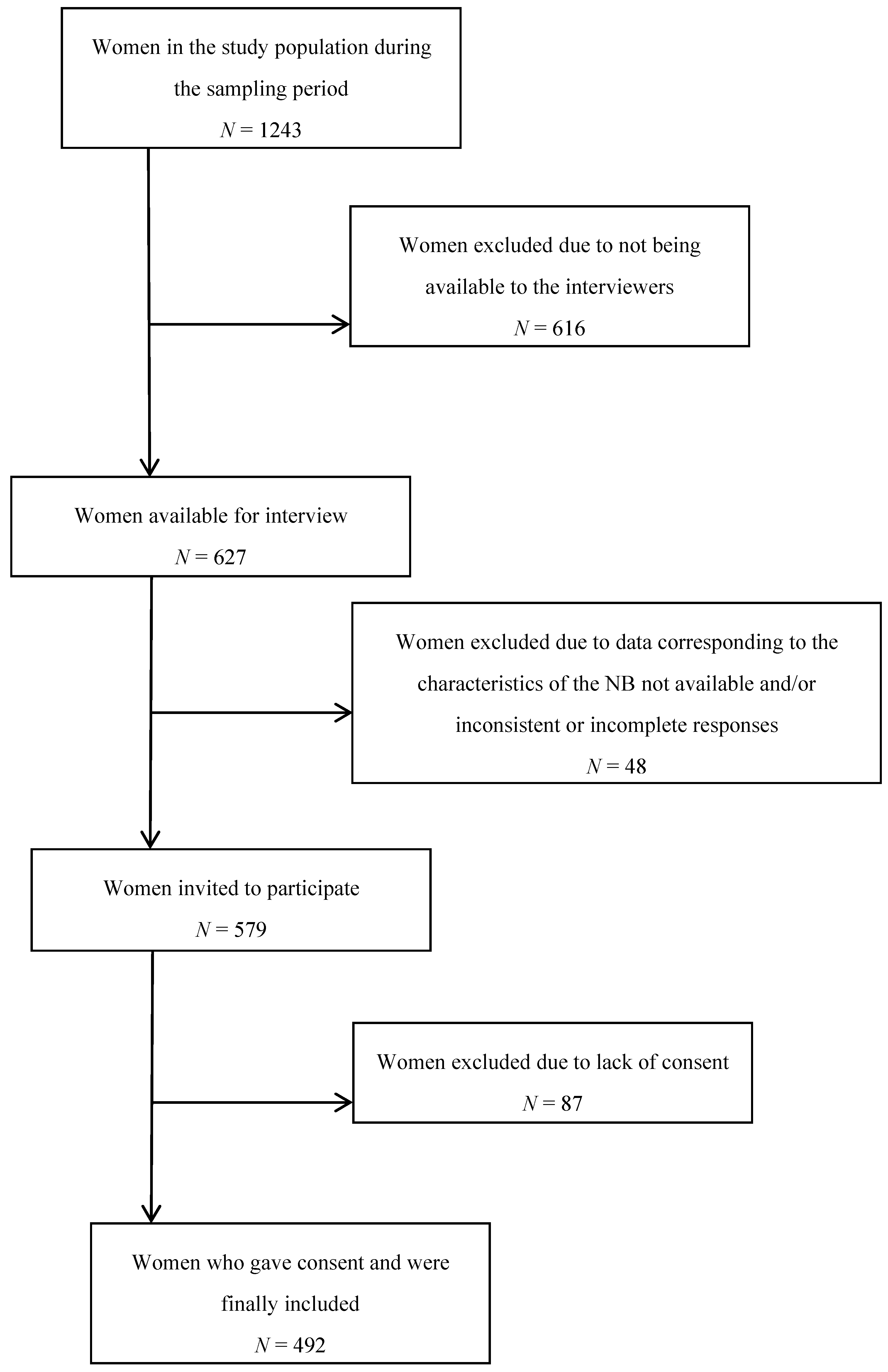
2.2. Study Population
2.3. Collected Data
2.3.1. Nutrition Assessment
2.3.2. Parental Data
2.3.3. Newborn Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Weaknesses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Michels, K.B. Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Steegers-Theunissen, R.P.; Vujkovic, M.; den Breeijen, H.; Russcher, H.; Lindemans, J.; Mackenbach, J.; Hofman, A.; Lesaffre, E.E.; Jaddoe, V.V.; et al. The Mediterranean Diet and Fetal Size Parameters: The Generation R Study. Br. J. Nutr. 2012, 108, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal Nutrition and Fetal Development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Arcas, M.; Rivas, A.; Monteagudo, C.; Granada, A.; Cerrillo, I.; Olea-Serrano, F. Proposal of a Mediterranean Diet Index for Pregnant Women. Br. J. Nutr. 2009, 102, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary Intakes of Women during Pregnancy in Low- and Middle-Income Countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.B.; Freitas Vilela, A.A.; de Oliveira, A.S.; Cabral, M.; de Souza, R.A.; Kac, G.; Sichieri, R. Sociodemographic Characteristics Determine Dietary Pattern Adherence during Pregnancy. Public Health Nutr. 2016, 19, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Brito, N.; Doreste-Alonso, J.; Nissensohn, M.; Henriquez, P.; Hermoso, M.; Berti, C.; Serra Majem, L. Methodological Aspects of the Study of Dietary Patterns during Pregnancy and Maternal and Infant Health Outcomes. A Systematic Review. Matern. Child. Nutr. 2010, 6 (Suppl. 2), 100–111. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.; Guldner, L.; Costet, N.; Kadhel, P.; Rouget, F.; Monfort, C.; Thome, J.P.; Multigner, L.; Cordier, S. Effect of a Mediterranean Diet during Pregnancy on Fetal Growth and Preterm Delivery: Results from a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr. Perinat. Epidemiol. 2014, 28, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean Diet and Nutritional Adequacy: A Review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.B.; Osterdal, M.L.; Knudsen, V.K.; Haugen, M.; Meltzer, H.M.; Bakketeig, L.; Olsen, S.F. Association between a Mediterranean-Type Diet and Risk of Preterm Birth among Danish Women: A Prospective Cohort Study. Acta Obstet. Gynecol. Scand. 2008, 87, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a Cholesterol-Lowering Diet on Maternal, Cord, and Neonatal Lipids, and Pregnancy Outcome: A Randomized Clinical Trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Penzias, A.; Missmer, S.A.; Chavarro, J.E. Prepregnancy Dietary Patterns and Risk of Pregnancy Loss. Am. J. Clin. Nutr. 2014, 100, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Di Cintio, E.; Parazzini, F.; Chatenoud, L.; Surace, M.; Benzi, G.; Zanconato, G.; La Vecchia, C. Dietary Factors and Risk of Spontaneous Abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 132–136. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Yang, L.; Yuan, L.; Guo, H.; Zhang, F.; Guan, Y.; Yao, W. Risk Factors for Early Miscarriage among Chinese: A Hospital-Based Case-Control Study. Fertil. Steril. 2014, 101, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Prepregnancy Dietary Patterns and Risk of Developing Hypertensive Disorders of Pregnancy: Results from the Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 2015, 102, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, B.; Thanopoulou, A.; Anastasiou, E.; Assaad-Khalil, S.; Albache, N.; Bachaoui, M.; Slama, C.B.; El Ghomari, H.; Jotic, A.; Lalic, N.; et al. Relation of the Mediterranean Diet with the Incidence of Gestational Diabetes. Eur. J. Clin. Nutr. 2014, 68, 8–13. [Google Scholar] [CrossRef] [PubMed]
- He, J.R.; Yuan, M.Y.; Chen, N.N.; Lu, J.H.; Hu, C.Y.; Mai, W.B.; Zhang, R.F.; Pan, Y.H.; Qiu, L.; Wu, Y.F.; et al. Maternal Dietary Patterns and Gestational Diabetes Mellitus: A Large Prospective Cohort Study in China. Br. J. Nutr. 2015, 113, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Hillesund, E.R.; Bere, E.; Haugen, M.; Overby, N.C. Development of a New Nordic Diet Score and its Association with Gestational Weight Gain and Fetal Growth—A Study Performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr. 2014, 17, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Silva-del Valle, M.A.; Sanchez-Villegas, A.; Serra-Majem, L. Association between the Adherence to the Mediterranean Diet and Overweight and Obesity in Pregnant Women in Gran Canaria. Nutr. Hosp. 2013, 28, 654–659. [Google Scholar] [PubMed]
- Vujkovic, M.; Steegers, E.A.; Looman, C.W.; Ocke, M.C.; van der Spek, P.J.; Steegers-Theunissen, R.P. The Maternal Mediterranean Dietary Pattern is Associated with a Reduced Risk of Spina Bifida in the Offspring. BJOG 2009, 116, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Krikov, S.; Carmichael, S.L.; Munger, R.G.; Shaw, G.M.; Feldkamp, M.L. Lower Rate of Selected Congenital Heart Defects with Better Maternal Diet Quality: A Population-Based Study. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F43–F49. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Mendez, M.; Garcia, R.; Roumeliotaki, T.; Ibarluzea, J.; Tardon, A.; Amiano, P.; Lertxundi, A.; Iniguez, C.; Vioque, J.; et al. Mediterranean Diet Adherence during Pregnancy and Fetal Growth: INMA (Spain) and RHEA (Greece) Mother-Child Cohort Studies. Br. J. Nutr. 2012, 107, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Halldorsson, T.I.; Willett, W.C.; Knudsen, V.K.; Gillman, M.W.; Mikkelsen, T.B.; Olsen, J.; NUTRIX Consortium. Milk Consumption during Pregnancy is Associated with Increased Infant Size at Birth: Prospective Cohort Study. Am. J. Clin. Nutr. 2007, 86, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Birgisdottir, B.E.; Meltzer, H.M.; Kvalem, H.E.; Alexander, J.; Magnus, P.; Haugen, M. Maternal Seafood Consumption and Infant Birth Weight, Length and Head Circumference in the Norwegian Mother and Child Cohort Study. Br. J. Nutr. 2012, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Heppe, D.H.; Medina-Gomez, C.; Hofman, A.; Franco, O.H.; Rivadeneira, F.; Jaddoe, V.W. Maternal First-Trimester Diet and Childhood Bone Mass: The Generation R Study. Am. J. Clin. Nutr. 2013, 98, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The Association between Maternal Diet during Pregnancy and Bone Mass of the Children at Age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef] [PubMed]
- De Batlle, J.; Garcia-Aymerich, J.; Barraza-Villarreal, A.; Anto, J.M.; Romieu, I. Mediterranean Diet is Associated with Reduced Asthma and Rhinitis in Mexican Children. Allergy 2008, 63, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Netting, M.J.; Middleton, P.F.; Makrides, M. Does Maternal Diet during Pregnancy and Lactation Affect Outcomes in Offspring? A Systematic Review of Food-Based Approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Barres, S.; Romaguera, D.; Valvi, D.; Martinez, D.; Vioque, J.; Navarrete-Munoz, E.M.; Amiano, P.; Gonzalez-Palacios, S.; Guxens, M.; Pereda, E.; et al. Mediterranean Dietary Pattern in Pregnant Women and Offspring Risk of Overweight and Abdominal Obesity in Early Childhood: The INMA Birth Cohort Study. Pediatr. Obes. 2016, 11, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Leon-Munoz, L.M.; Guallar-Castillon, P.; Graciani, A.; Lopez-Garcia, E.; Mesas, A.E.; Aguilera, M.T.; Banegas, J.R.; Rodriguez-Artalejo, F. Adherence to the Mediterranean Diet Pattern has Declined in Spanish Adults. J. Nutr. 2012, 142, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Varela-Moreiras, G.; Ruiz, E.; Valero, T.; Avila, J.M.; del Pozo, S. The Spanish Diet: An Update. Nutr. Hosp. 2013, 28 (Suppl. 5), 13–20. [Google Scholar] [PubMed]
- Hu, E.A.; Toledo, E.; Diez-Espino, J.; Estruch, R.; Corella, D.; Salas-Salvado, J.; Vinyoles, E.; Gomez-Gracia, E.; Aros, F.; Fiol, M.; et al. Lifestyles and Risk Factors Associated with Adherence to the Mediterranean Diet: A Baseline Assessment of the PREDIMED Trial. PLoS ONE 2013, 8, e60166. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carrasco, L.; Brugarolas, M.; Martinez-Poveda, A. Análisis De Las Tendencias Actuales En La Alimentación De Los Españoles: Posibilidades De Difusión De La Dieta Mediterránea. Rev. Esp. Estud. Agrosoc. Pesq. 2004, 201, 151–164. [Google Scholar]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. 2016, 100, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Promoting Optimal Fetal Development: Report of a Technical Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Edmond, K.; Bahl, R.; World Health Organization. Optimal Feeding of Low-Birth-Weight Infants: Technical Review; Worls Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Cunningham, F.G.; Leveno, K.J.; Bloom, S.L.; Spong, C.Y.; Dashe, J.S.; Hoffman, B.L.; Casey, B.M.; Sheffield, J.S. Obstetrícia De Williams; McGraw Hill: Sao Paulo, Brasil, 2016. [Google Scholar]
- Kjøllesdal, M.K.; Holmboe-Ottesen, G. Dietary Patterns and Birth Weight—A Review. AIMS Public Health 2014, 1, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; Garcia, A.; Perez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; López-Nicolás, R.; Rodríguez-Tadeo, A.; Ros-Berruezo, G.; Martínez-Marín, M.; Doménech-Asensi, G. Adherence to the Mediterranean Diet by Nursing Students of Murcia (Spain). Nutr. Hosp. 2014, 30, 165–172. [Google Scholar] [PubMed]
- San Mauro-Martin, I.; Onrubia-Gonzalez-De la Aleja, J.; Garicano-Vilar, E.; Cadenato-Ruiz, C.; Hernandez-Villa, I.; Rodriguez-Alonso, P.; Pina-Ordunez, D.; Fortunez-Garrido, E.; Villacorta-Perez, P.; Sanz-Guisado, C.; et al. Analysis of the Nutritional Status and Body Composition of Persons with Intellectual Disability. Rev. Neurol. 2016, 62, 493–501. [Google Scholar] [PubMed]
- Rodriguez, F.; Palma, X.; Romo, A.; Escobar, D.; Aragu, B.; Espinoza, L.; McMillan, N.; Galvez, J. Eating Habits, Physical Activity and Socioeconomic Level in University Students of Chile. Nutr. Hosp. 2013, 28, 447–455. [Google Scholar] [PubMed]
- Alacid, F.; Vaquero-Cristobal, R.; Sanchez-Pato, A.; Muyor, J.M.; Lopez-Minarro, P.A. Habit Based Consumptions in the Mediterranean Diet and the Relationship with Anthropometric Parameters in Young Female Kayakers. Nutr. Hosp. 2014, 29, 121–127. [Google Scholar] [PubMed]
- Štefan, L.; Prosoli, R.; Juranko, D.; Čule, M.; Milinović, I.; Novak, D.; Sporiš, G. The Reliability of the Mediterranean Diet Quality Index (KIDMED) Questionnaire. Nutrients 2017, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Dura Trave, T.; Castroviejo Gandarias, A. Adherence to a Mediterranean Diet in a College Population. Nutr. Hosp. 2011, 26, 602–608. [Google Scholar] [PubMed]
- Schlaudecker, E.P.; Munoz, F.M.; Bardají, A.; Boghossian, N.S.; Khalil, A.; Mousa, H.; Nesin, M.; Nisar, M.I.; Pool, V.; Spiegel, H.M. Small for Gestational Age: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Maternal Immunisation Safety Data. Vaccine 2017, 35, 6518–6528. [Google Scholar] [PubMed]
- Apgar, V. A Proposal for a New Method of Evaluation of the Newborn Infant. Curr. Res. Anesth. Analg. 2015, 32, 250–259, reprinted in Anesth. Analg. 2015, 120, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Finster, M.; Wood, M. The Apgar Score has Survived the Test of Time. Anesthesiol. J. Am. Soc. Anesthesiol. 2005, 102, 855–857. [Google Scholar] [CrossRef]
- Casey, B.M.; McIntire, D.D.; Leveno, K.J. The Continuing Value of the Apgar Score for the Assessment of Newborn Infants. N. Engl. J. Med. 2001, 344, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Alegria, X.; Cerda, M. Gases En Cordón Umbilical. Rev. Obstet. Ginecol. Hosp. Santiago Oriente Dr. Luis Tisné Brousse 2009, 4, 78–81. [Google Scholar]
- Engelhardt, B.; König, J.; Blettner, M.; Wild, P.; Münzel, T.; Lackner, K.; Blankenberg, S.; Pfeiffer, N.; Beutel, M.; Zwiener, I. Combining Cross-Sectional Data on Prevalence with Risk Estimates from a Prediction Model. Methods Inf. Med. 2014, 53, 371–379. [Google Scholar] [PubMed]
- National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M. A Short Screener is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Tur, J.A.; Romaguera, D.; Pons, A. Adherence to the Mediterranean Dietary Pattern among the Population of the Balearic Islands. Br. J. Nutr. 2004, 92, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Bamia, C.; Pons, A.; Tur, J.A.; Trichopoulou, A. Food Patterns and Mediterranean Diet in Western and Eastern Mediterranean Islands. Public Health Nutr. 2009, 12, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Arriscado, D.; Muros, J.J.; Zabala, M.; Dalmau, J.M. Factors Associated with Low Adherence to a Mediterranean Diet in Healthy Children in Northern Spain. Appetite 2014, 80, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean Diet in Pregnancy is Protective for Wheeze and Atopy in Childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall Survival in Elderly People. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Arcas, M.; Lopez-Martinez, C.; Granada, A.; Olea, N.; Lorenzo-Tovar, M.; Olea-Serrano, F. Organochlorine Pesticides in Umbilical Cord Blood Serum of Women from Southern Spain and Adherence to the Mediterranean Diet. Food Chem. Toxicol. 2010, 48, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Requena, R.; Fernández, J.G.; Prieto, C.A.; Moreno, J.M.; Bueno-Cavanillas, A.; Jiménez-Moleón, J.J. Factors Associated with a Low Adherence to a Mediterranean Diet Pattern in Healthy Spanish Women before Pregnancy. Public Health Nutr. 2014, 17, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Gómez Roig, M.D.; Mazarico, E.; Ferrero, S.; Montejo, R.; Ibáñez, L.; Grima, F.; Vela, A. Differences in Dietary and Lifestyle Habits between Pregnant Women with Small Fetuses and Appropriate-for-gestational-age Fetuses. J. Obstet. Gynaecol. Res. 2017, 43, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; de la Torre, N.G.; Durán, A.; Fuentes, M.; Bordiú, E.; del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A. A Mediterranean Diet with Additional Extra Virgin Olive Oil and Pistachios Reduces the Incidence of Gestational Diabetes Mellitus (GDM): A Randomized Controlled Trial: The St. Carlos GDM Prevention Study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Plana, E.; Guxens, M.; Foradada Morillo, C.M.; Albareda, R.M.; Garcia-Esteban, R.; Goni, F.; Kogevinas, M.; Sunyer, J. Seafood Consumption in Pregnancy and Infant Size at Birth: Results from a Prospective Spanish Cohort. J. Epidemiol. Community Health 2010, 64, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Poon, A.K.; Yeung, E.; Boghossian, N.; Albert, P.S.; Zhang, C. Maternal Dietary Patterns during Third Trimester in Association with Birthweight Characteristics and Early Infant Growth. Scientifica (Cairo) 2013, 2013, 786409. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.H.; Fairfield, K.M. Vitamins for Chronic Disease Prevention in Adults: Clinical Applications. JAMA 2002, 287, 3127–3129. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Nutrient Adequacy and Mediterranean Diet in Spanish School Children and Adolescents. Eur. J. Clin. Nutr. 2003, 57, S35. [Google Scholar] [CrossRef] [PubMed]
- Schenker, S.; Yang, Y.; Perez, A.; Acuff, R.V.; Papas, A.M.; Henderson, G.; Lee, M.P. Antioxidant Transport by the Human Placenta. Clin. Nutr. 1998, 17, 159–167. [Google Scholar] [CrossRef]
- Rodriguez-Bernal, C.L.; Rebagliato, M.; Iniguez, C.; Vioque, J.; Navarrete-Munoz, E.M.; Murcia, M.; Bolumar, F.; Marco, A.; Ballester, F. Diet Quality in Early Pregnancy and its Effects on Fetal Growth Outcomes: The Infancia Y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am. J. Clin. Nutr. 2010, 91, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Estruch, R.; Toledo, E.; Sanchez-Tainta, A.; Corella, D.; Bullo, M.; Fiol, M.; Iglesias, P.; Gomez-Gracia, E.; Aros, F.; et al. Predictors of Adherence to a Mediterranean-Type Diet in the PREDIMED Trial. Eur. J. Nutr. 2010, 49, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Alvarez, I.; Aguinaga Ontoso, I.; Marin Fernandez, B.; Guillen Grima, F.; Niu, H. Cross-Sectional Study of Factors Influencing Adherence to the Mediterranean Diet in Pregnancy. Nutr. Hosp. 2015, 31, 1845–1852. [Google Scholar] [PubMed]
- Chen, L.; Bell, E.M.; Browne, M.L.; Druschel, C.M.; Romitti, P.A.; National Birth Defects Prevention Study. Exploring Maternal Patterns of Dietary Caffeine Consumption before Conception and during Pregnancy. Matern. Child Health J. 2014, 18, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.C.; LeMasters, G.K.; Wilson, K.A. Changes in Caffeine Consumption as a Signal of Pregnancy. Reprod. Toxicol. 2004, 18, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Sengpiel, V.; Elind, E.; Bacelis, J.; Nilsson, S.; Grove, J.; Myhre, R.; Haugen, M.; Meltzer, H.M.; Alexander, J.; Jacobsson, B.; et al. Maternal Caffeine Intake during Pregnancy is Associated with Birth Weight but Not with Gestational Length: Results from a Large Prospective Observational Cohort Study. BMC Med. 2013, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal Total Caffeine Intake, mainly from Japanese and Chinese Tea, during Pregnancy was Associated with Risk of Preterm Birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Bech, B.H.; Obel, C.; Henriksen, T.B.; Olsen, J. Effect of Reducing Caffeine Intake on Birth Weight and Length of Gestation: Randomised Controlled Trial. BMJ 2007, 334, 409. [Google Scholar] [CrossRef] [PubMed]
- Hollins Martin, C. Higher Coffee Intake in Pregnancy Linked to Prolonged Gestation, and Higher Caffeine Intake Linked with Babies being Small for Gestational Age. Evid Based. Nurs. 2014, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of Soft Drink Consumption on Nutrition and Health: A Systematic Review and Meta-Analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Haider, B.A.; Bhutta, Z.A. Multiple-Micronutrient Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2015, 11, CD004905. [Google Scholar]
- Rodríguez, M.L.; Méndez, J.S.; Martínez, M.S.; Domínguez, M.C. Suplementos En Embarazadas: Controversias, Evidencias Y Recomendaciones. Inf. Ter. Sist. Nac. Salud 2010, 34, 117–128. [Google Scholar]
- Feart, C.; Alles, B.; Merle, B.; Samieri, C.; Barberger-Gateau, P. Adherence to a Mediterranean Diet and Energy, Macro-, and Micronutrient Intakes in Older Persons. J. Physiol. Biochem. 2012, 68, 691–700. [Google Scholar] [CrossRef] [PubMed]
- De trabajo de la Guía, Grupo. De Práctica Clínica De Atención En El Embarazo Y Puerperio. In Guía de Práctica Clínica de Atención en el Embarazo y Puerperio; Ministerio de Sanidad, Servicios Sociales e Igualdad; Agencia de Evaluación de Tecnologías Sanitarias de Andalucía: Sevilla, Spain, 2014; pp. 394–404. [Google Scholar]
- Doyle, W.; Srivastava, A.; Crawford, M.A.; Bhatti, R.; Brooke, Z.; Costeloe, K.L. Inter-Pregnancy Folate and Iron Status of Women in an Inner-City Population. Br. J. Nutr. 2001, 86, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Shand, A.W.; Walls, M.; Chatterjee, R.; Nassar, N.; Khambalia, A.Z. Dietary Vitamin, Mineral and Herbal Supplement use: A Cross-Sectional Survey of before and during Pregnancy use in Sydney, Australia. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.; Cardoso, S. Routine Iron Supplementation in Pregnancy. Acta Med. Port. 2010, 23, 785–792. [Google Scholar] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean Diet and Mild Cognitive Impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Weiss, N.S.; Sacks, D.A.; Pettitt, D.J.; Selby, J.V.; Quesenberry, C.P.; Ferrara, A. Pregnancy Weight Gain and Risk of Neonatal Complications: Macrosomia, Hypoglycemia, and Hyperbilirubinemia. Obstet. Gynecol. 2006, 108, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- DeVader, S.R.; Neeley, H.L.; Myles, T.D.; Leet, T.L. Evaluation of Gestational Weight Gain Guidelines for Women with Normal Prepregnancy Body Mass Index. Obstet. Gynecol. 2007, 110, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.E. The Increasing Impact of Maternal Obesity on Obstetric Practice. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.; Santos, P.C.; Moreira, P.; Santos, R.; Moreira, C.; Montenegro, N.; Mota, J. Predictors of Adherence to the Mediterranean Diet from the First to the Second Trimester of Pregnancy. Nutr. Hosp. 2014, 31, 1403–1412. [Google Scholar] [PubMed]
- Northstone, K.; Emmett, P.; Rogers, I. Dietary Patterns in Pregnancy and Associations with Socio-Demographic and Lifestyle Factors. Eur. J. Clin. Nutr. 2008, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Álvarez, I.; Aguinaga Ontoso, I.; Marín Fernández, B.; Guillén Grima, F.; Niu, H. Estudio Transversal De Los Factores Que Influyen En La Adhesión a La Dieta Mediterránea En El Embarazo. Nutr. Hosp. 2015, 31, 1845–1852. [Google Scholar] [PubMed]
- Ferland, S.; O’Brien, H.T. Maternal Dietary Intake and Pregnancy Outcome. J. Reprod. Med. 2003, 48, 86–94. [Google Scholar] [PubMed]
- Gresham, E.; Byles, J.E.; Bisquera, A.; Hure, A.J. Effects of Dietary Interventions on Neonatal and Infant Outcomes: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2014, 100, 1298–1321. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fetus and Newborn, American Academy of Pediatrics, and Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Use and Abuse of the Apgar Score. Pediatrics 1996, 98, 141–142. [Google Scholar]
- Leuthner, S.R.; Das, U.G. Low Apgar Scores and the Definition of Birth Asphyxia. Pediatr. Clin. N. Am. 2004, 51, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Tejerina Morató, H. Asfixia Neonatal. Rev. Soc. Boliv. Pediatr. 2007, 46, 145–150. [Google Scholar]
- Cuco, G.; Fernandez-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary Patterns and Associated Lifestyles in Preconception, Pregnancy and Postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef] [PubMed]
| Low Adherence N = 198 (40.2%) | Optimal Adherence N = 294 (59.7%) | pa | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Maternal age | |||||
| Years (Mean ± SD) | 31.1 ± 5.6 | 33.3 ± 4.8 | 0.001 | ||
| ≤24 | 34 | 17.2% | 18 | 6.1% | 0.001 |
| 25–29 | 40 | 20.2% | 43 | 14.6% | |
| 30–34 | 70 | 40,5% | 103 | 35.0% | |
| ≥35 | 54 | 27.3% | 130 | 44.2% | |
| Marital status (Single) | 92 | 46.5% | 120 | 40.8% | 0.126 |
| Maternal origin | |||||
| Africa | 6 | 3.0% | 14 | 4.8% | 0.489 |
| Asia | 1 | 0.5% | 1 | 0.3% | |
| America | 15 | 7.6% | 31 | 10.5% | |
| Europe | 176 | 88.9% | 248 | 84.4% | |
| Maternal education | |||||
| Without studies | 11 | 5.6% | 2 | 0.7% | 0.001 |
| Primary education | 46 | 23.2% | 34 | 11.6% | |
| Secondary education | 97 | 49% | 125 | 42.5% | |
| University or postgraduate education | 44 | 22.2% | 133 | 45.3% | |
| Maternal physical activity | |||||
| None | 70 | 35.4% | 65 | 22.2% | 0.006 |
| Light | 97 | 49.0% | 166 | 56.7% | |
| Moderate | 31 | 15.2% | 62 | 21.2% | |
| Maternal employment status (Employed) | 106 | 53.8% | 206 | 70.1% | 0.001 |
| Maternal smoking before pregnancy (Yes) | 76 | 50.7% | 63 | 30.3% | 0.198 |
| Maternal smoking during pregnancy (Yes) | 45 | 30,6% | 30 | 14.6% | 0.001 |
| Maternal drug use (Yes) | 2 | 1.5% | 0 | 0% | 0.319 |
| Maternal alcohol use (Yes) | 1 | 0.8% | 2 | 1.0% | 0.640 |
| Low Adherence N = 198 (40.2%) | Optimal Adherence N = 294 (59.7%) | pa | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Coffee | 68 | 42.8% | 99 | 42.7% | 0.507 |
| Tea | 12 | 8.6% | 26 | 12.9% | 0.139 |
| Caffeinated drinks b | 68 | 43.9% | 63 | 28.9% | 0.002 |
| Hot chocolate | 65 | 35.5% | 105 | 39.6% | 0.307 |
| Dietary supplement intake | |||||
| Prenatal vitamins | 33 | 25.4% | 58 | 33.5% | 0.080 |
| Folic acid c supplement | 36 | 25.2% | 65 | 34.2% | 0.048 |
| Iron d supplement | 100 | 60.2% | 165 | 70.2% | 0.025 |
| Dairy product at breakfast | 163 | 82.3% | 268 | 91.2% | 0.001 |
| Daily dairy intake | |||||
| <2 yogurts and/or 40 g cheese per day | 85 | 42.9% | 51 | 17.3% | 0.003 |
| ≥2 yogurts and/or 40 g cheese per day | 113 | 57.1% | 243 | 82.7% | |
| Fish | |||||
| <2 times per week | 111 | 56.1% | 87 | 29.6% | 0.001 |
| ≥2–3 times per week | 87 | 43.9% | 207 | 70.4% | |
| Low Adherence N = 198 (40.2%) | ||||
|---|---|---|---|---|
| Crude OR | 95% CI | Adjusted OR | 95% CI | |
| Small for Gestational Age | ||||
| No | 1.0 Reference | 1.0 Reference | ||
| Yes | 0.97 | 0.5–2.31 | 1.68 a | 1.02–5.46 |
| 2.32 b | 0.69–7.78 | |||
| 2.65 c | 0.66–10.65 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peraita-Costa, I.; Llopis-González, A.; Perales-Marín, A.; Sanz, F.; Llopis-Morales, A.; Morales-Suárez-Varela, M. A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn. Int. J. Environ. Res. Public Health 2018, 15, 1530. https://doi.org/10.3390/ijerph15071530
Peraita-Costa I, Llopis-González A, Perales-Marín A, Sanz F, Llopis-Morales A, Morales-Suárez-Varela M. A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn. International Journal of Environmental Research and Public Health. 2018; 15(7):1530. https://doi.org/10.3390/ijerph15071530
Chicago/Turabian StylePeraita-Costa, Isabel, Agustín Llopis-González, Alfredo Perales-Marín, Ferran Sanz, Agustín Llopis-Morales, and María Morales-Suárez-Varela. 2018. "A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn" International Journal of Environmental Research and Public Health 15, no. 7: 1530. https://doi.org/10.3390/ijerph15071530
APA StylePeraita-Costa, I., Llopis-González, A., Perales-Marín, A., Sanz, F., Llopis-Morales, A., & Morales-Suárez-Varela, M. (2018). A Retrospective Cross-Sectional Population-Based Study on Prenatal Levels of Adherence to the Mediterranean Diet: Maternal Profile and Effects on the Newborn. International Journal of Environmental Research and Public Health, 15(7), 1530. https://doi.org/10.3390/ijerph15071530





