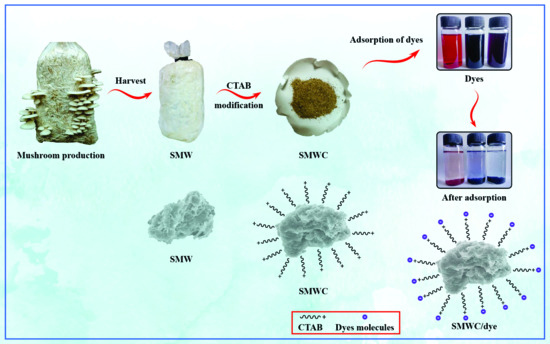
Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Adsorbents
2.3. Dye Adsorption Study of SMWC
2.4. Modeling Study
2.5. Application of SMWC for the Removal of Dyes from Urban and Industrial Water
3. Results and Discussion
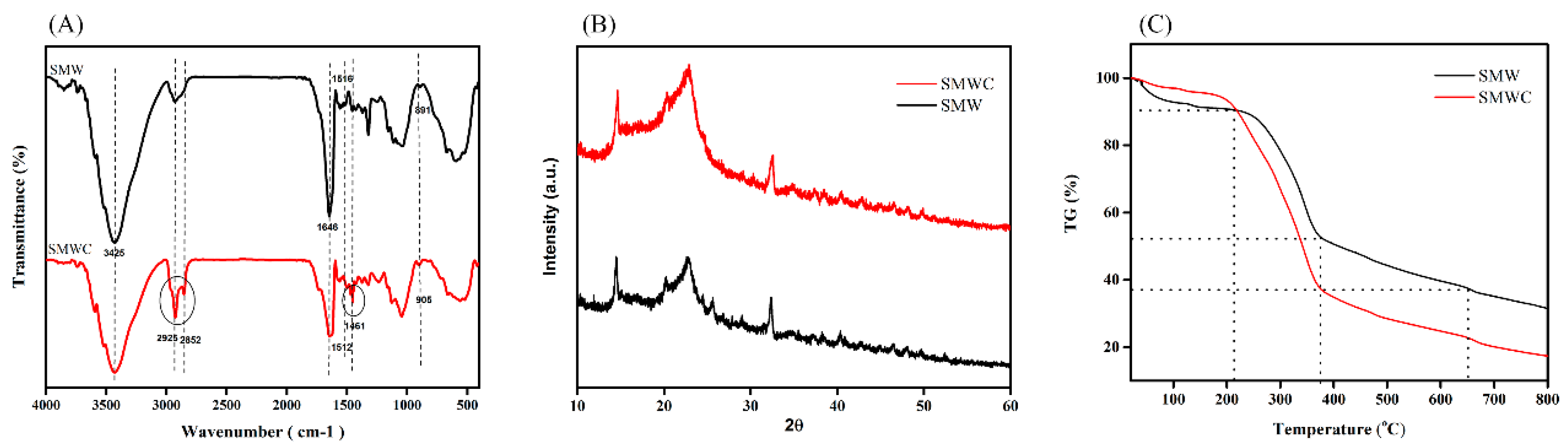
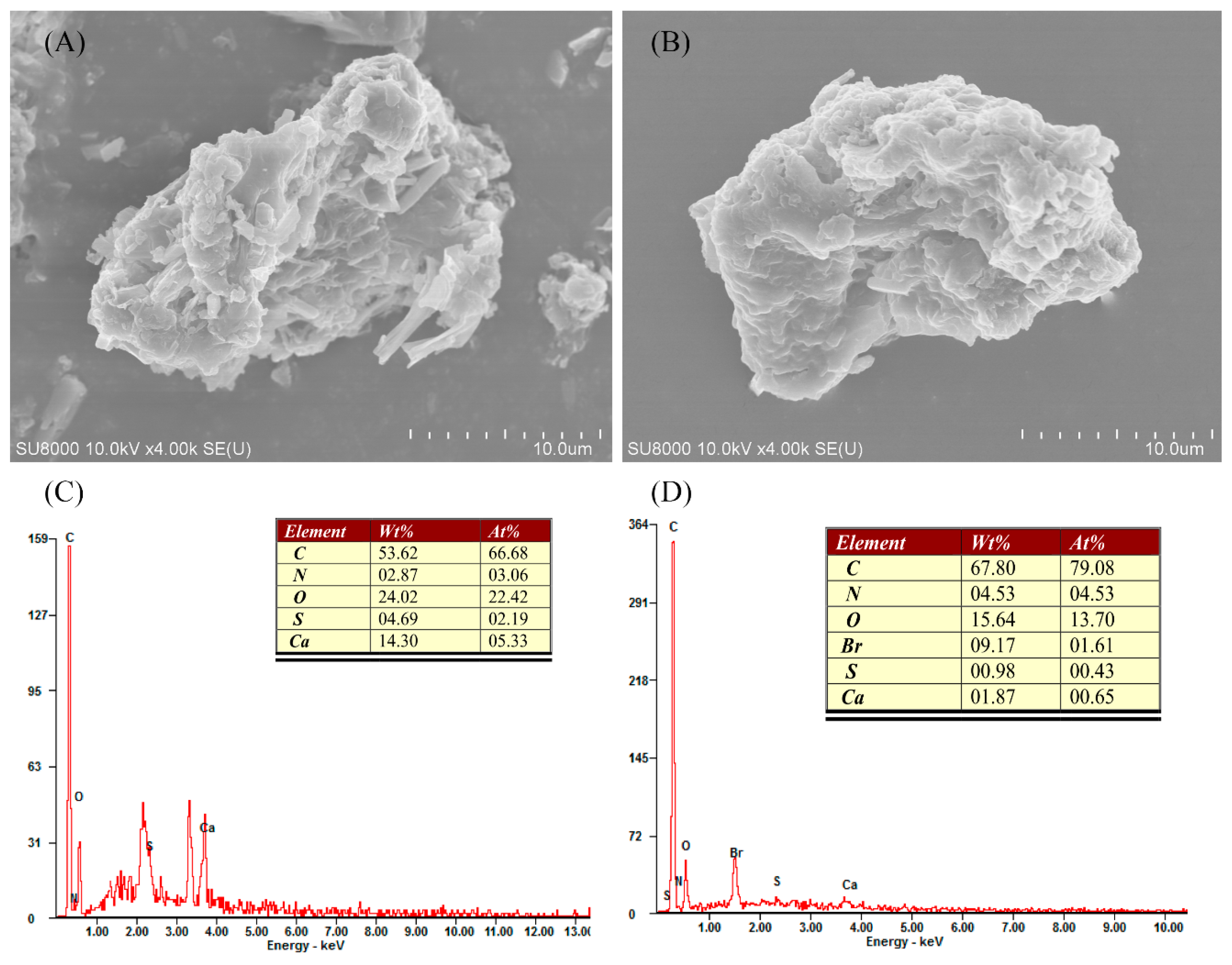
3.1. Characterization of SMW and SMWC
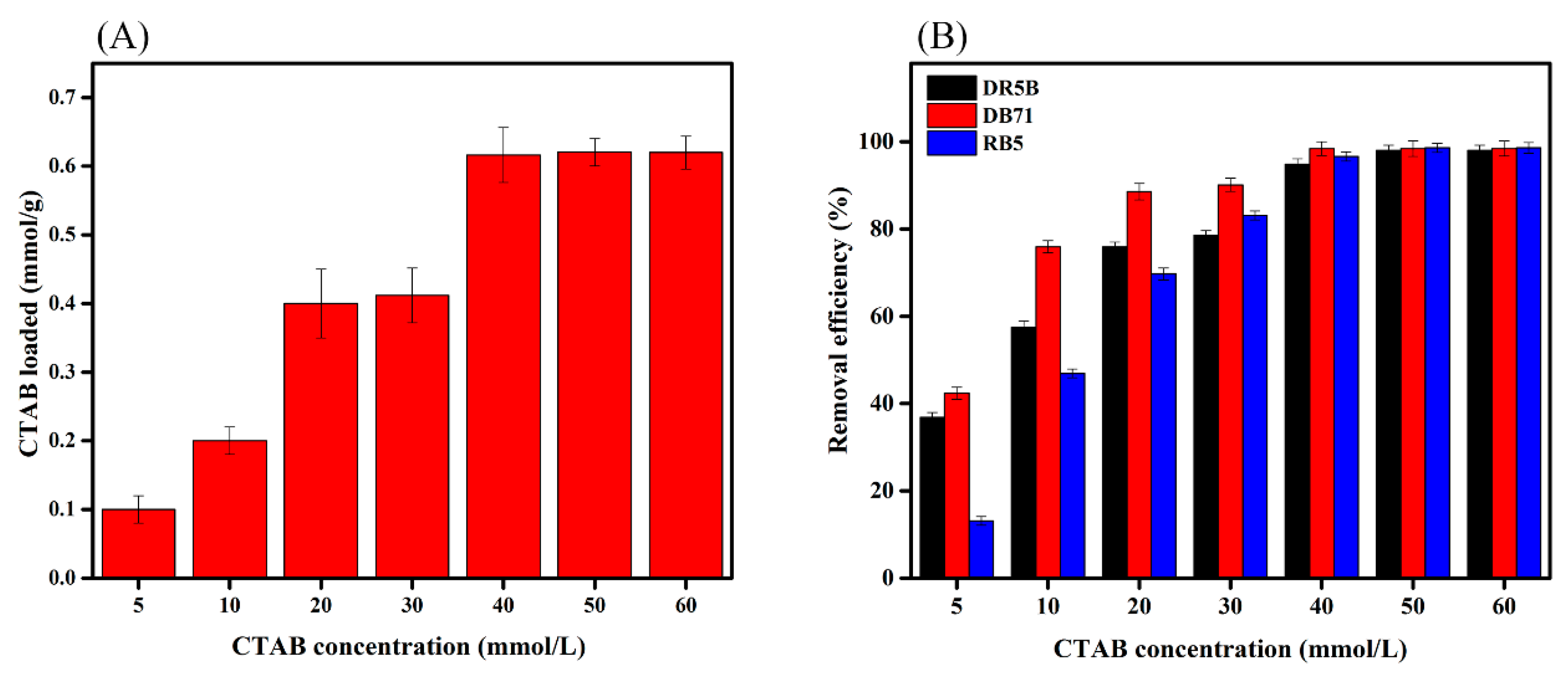
3.2. Effect of Surfactant Concentration on the SMW Surface and Its Impact on the Removal of Dyes
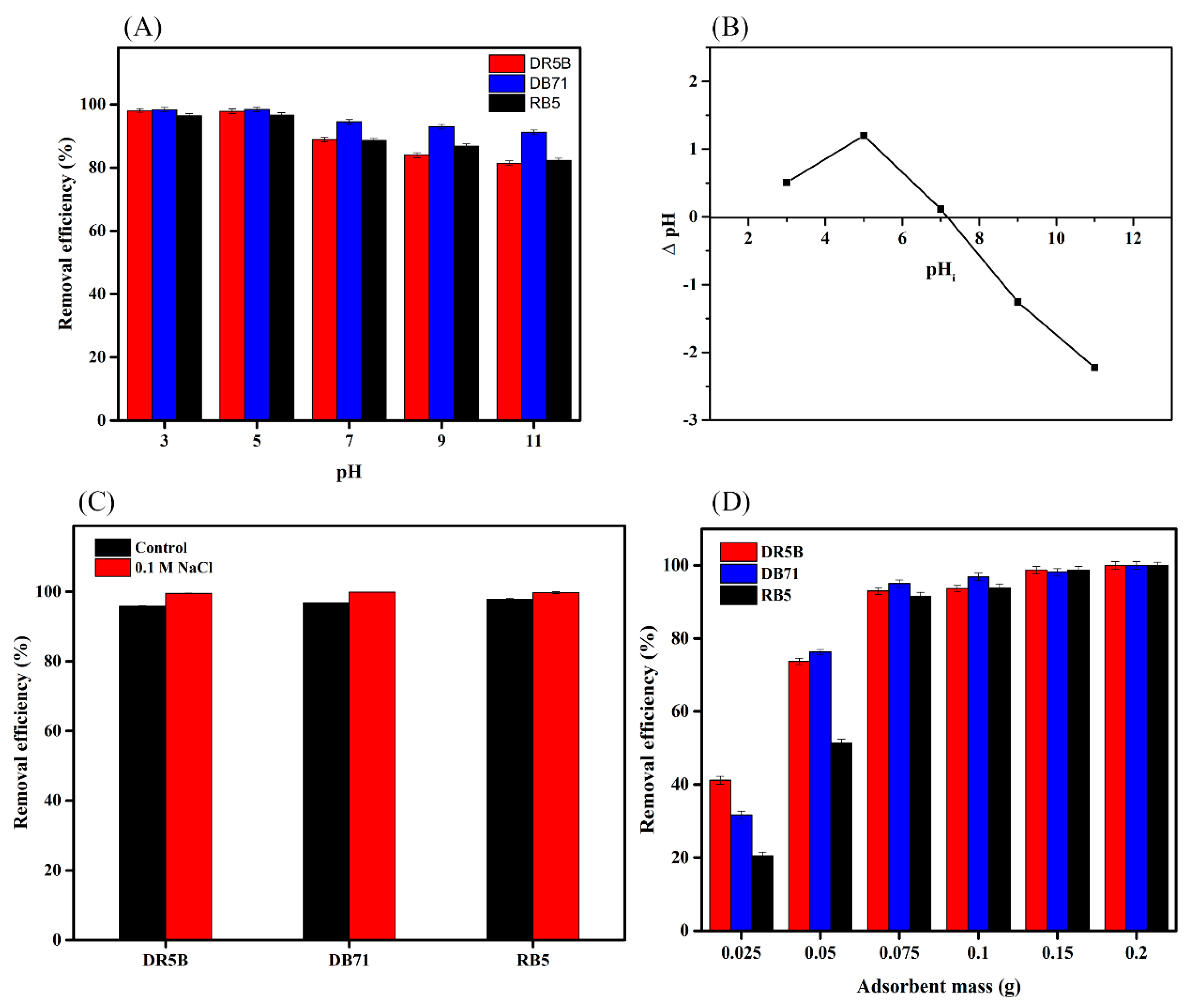
3.3. Effects of pH and Ionic Strength on the Dye Adsorption Capacity of SMWC
3.4. Effect of the Adsorbent Dose on Dye Removal
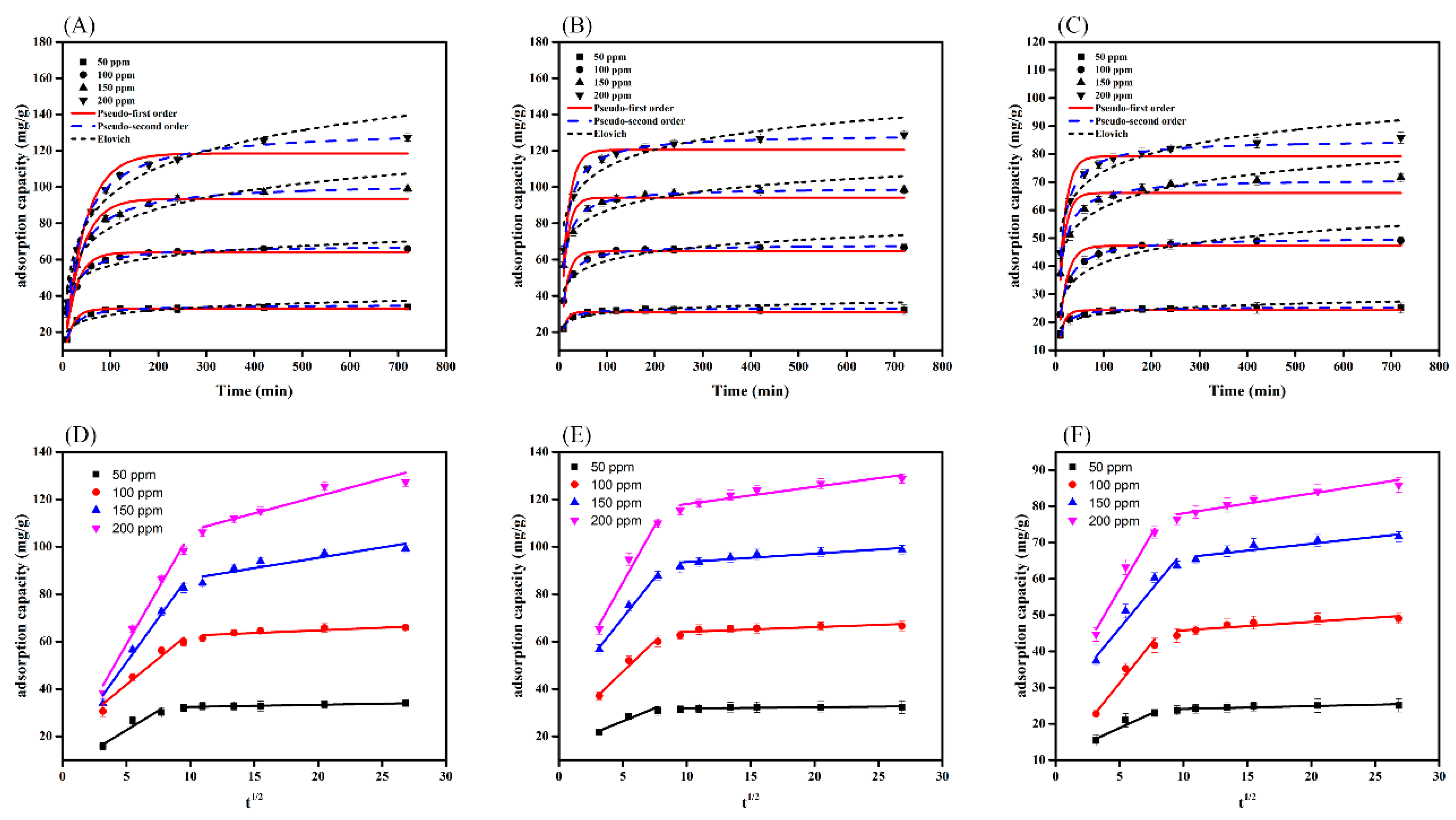
3.5. Adsorption Kinetics
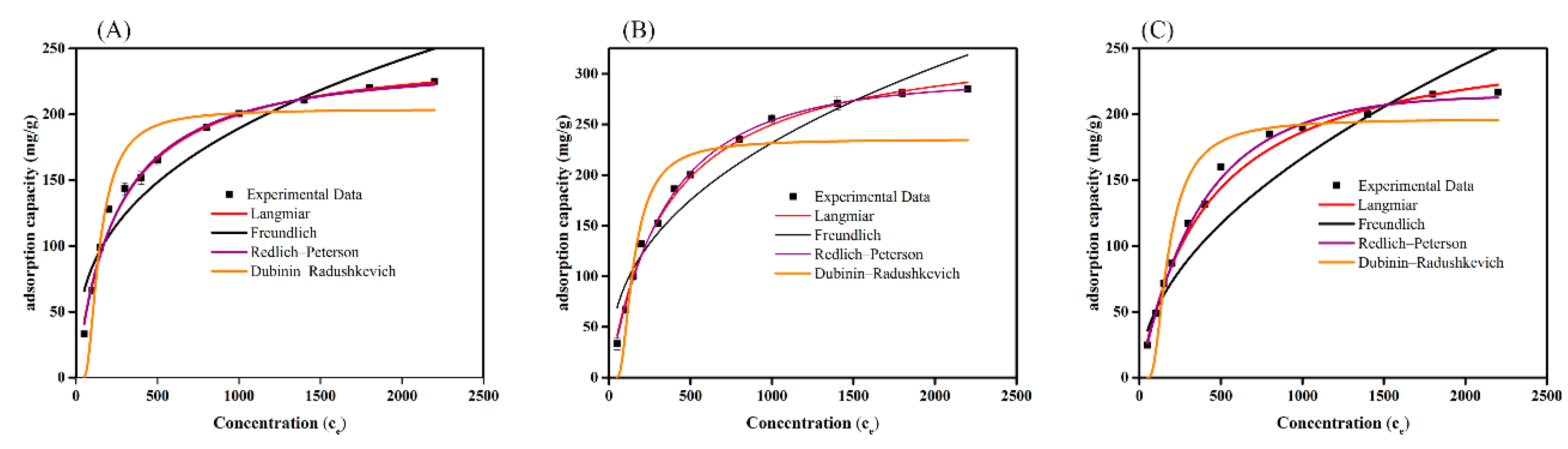
3.6. Adsorption Isotherms
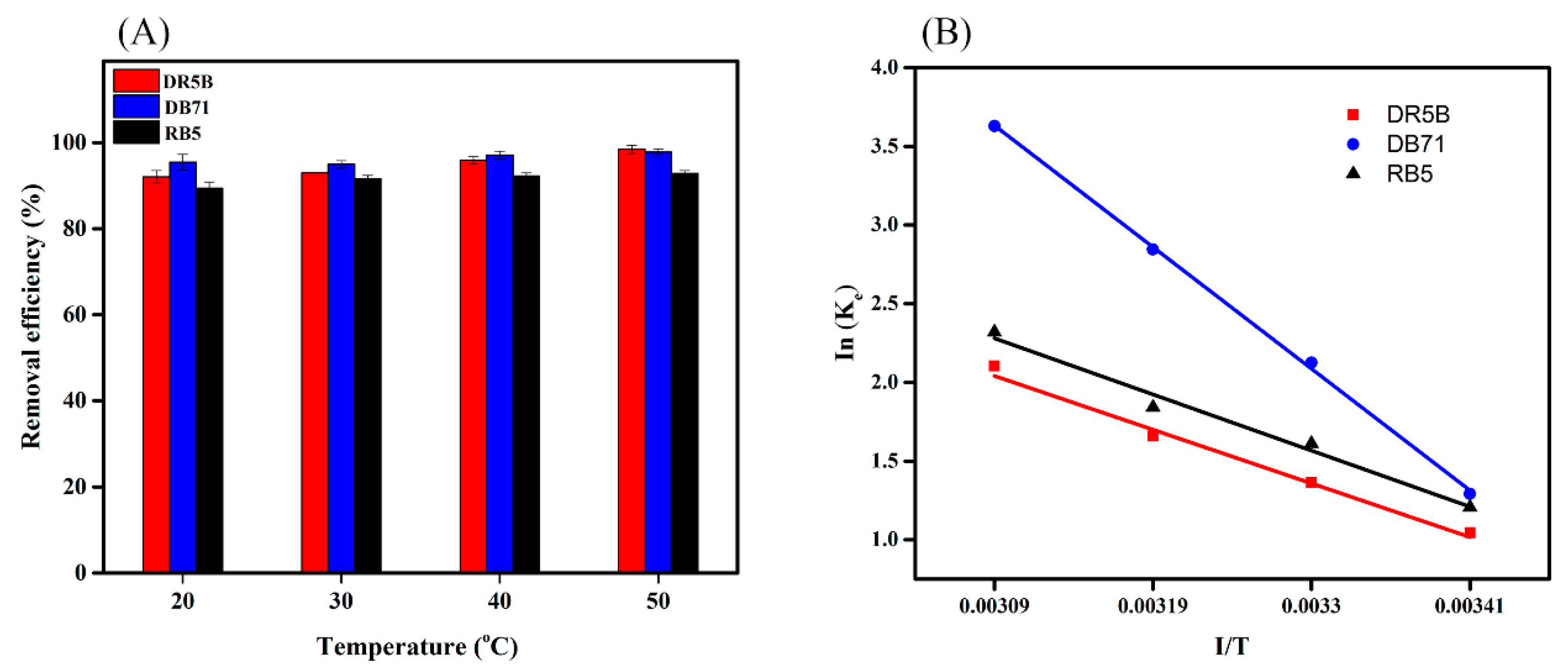
3.7. Temperature Effect on Dye Removal and Thermodynamic Analysis
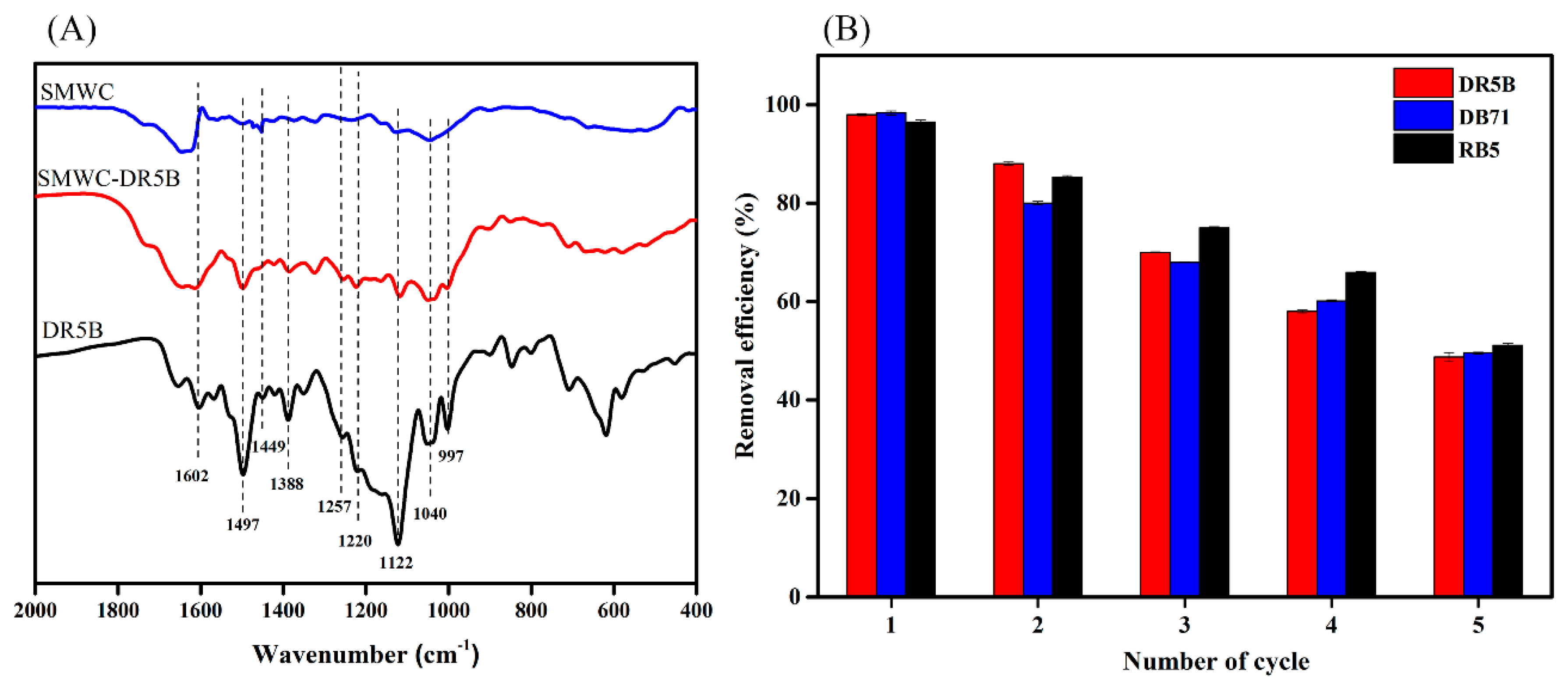
3.8. Adsorption Mechanism
3.9. Dye Removal from Different Water Samples
3.10. Recyclability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanghi, R.; Bhattacharya, B.; Singh, V. Seed gum polysaccharides and their grafted co-polymers for the effective coagulation of textile dye solutions. React. Funct. Polym. 2007, 67, 495–502. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Q.; Lu, K.; Huang, W.; Lü, Z.; Zhou, C.; Yu, S.; Gao, C. High efficient removal of dyes from aqueous solution through nanofiltration using diethanolamine-modified polyamide thin-film composite membrane. Sep. Purif. Technol. 2017, 173, 135–143. [Google Scholar] [CrossRef]
- Nekouei, F.; Kargarzadeh, H.; Nekouei, S.; Keshtpour, F.; Makhlouf, A.S. Efficient method for determination of methylene blue dye in water samples based on a combined dispersive solid phase and cloud point extraction using Cu(OH)2 nanoflakes: Central composite design optimization. Anal. Bioanal. Chem. 2017, 409, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Lombraña, J.I.; de Luis, A.; Sanz, J. Oxidizing efficiency analysis of an ozonation process to degrade the dye rhodamine 6G. J. Chem. Technol. Biotechnol. 2017, 92, 674–683. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, Y.; Sun, W.; Shen, H.; Zheng, H.; Xu, Y.; Zhao, J.; Wu, H.; Liu, C. Advanced treatment of actual textile dye wastewater by fenton-flocculation process. Can. J. Chem. Eng. 2017, 95, 1245–1252. [Google Scholar] [CrossRef]
- Fahimirad, B.; Asghari, A.; Rajabi, M. Photo-degradation of basic green 1 and basic red 46 dyes in their binary solution by La2O3-Al2O3 nanocomposite using first-order derivative spectra and experimental design methodology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.A.; Souza, R.; Soler, J.; Silva, T.F.C.V.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Remediation of a synthetic textile wastewater from polyester-cotton dyeing combining biological and photochemical oxidation processes. Sep. Purif. Technol. 2017, 172, 450–462. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Zabihollahi, S.; Pourzamani, H.R. Removal of a common textile dye, navy blue (NB), from aqueous solutions by combined process of coagulation–flocculation followed by adsorption. Desalin. Water Treat. 2016, 57, 5200–5211. [Google Scholar] [CrossRef]
- Kadam, A.A.; Lade, H.S.; Patil, S.M.; Govindwar, S.P. Low cost CaCl2 pretreatment of sugarcane bagasse for enhancement of textile dyes adsorption and subsequent biodegradation of adsorbed dyes under solid state fermentation. Bioresour. Technol. 2013, 132, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mittal, A.; Gajbe, V.; Mittal, J. Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: Bottom ash and de-oiled soya. Ind. Eng. Chem. Res. 2006, 45, 1446–1453. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; He, P.W.; Luo, Y.; Lu, X.; Liang, Y.; Fu, J. Adsorption of phosphate by biomass char deriving from fast pyrolysis of biomass waste. Clean-Soil Air Water 2012, 40, 493–498. [Google Scholar] [CrossRef]
- Haque, E.; Jun, J.W.; Jhung, S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, R.; Rao, N.N.; Pande, S.P.; Kaul, S.N. Removal of basic dyes from aqueous medium using a novel polymer: Jalshakti. Bioresour. Technol. 2006, 97, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Humelnicu, I.; Băiceanu, A.; Ignat, M.-E.; Dulman, V. The removal of basic blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: Kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 105, 274–287. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173–174, 152–161. [Google Scholar] [CrossRef]
- Tian, X.L.; Li, C.; Yang, H.J.; Ye, Z.X.; Xu, H. Spent mushroom: A new low-cost adsorbent for removal of congo red from aqueous solutions. Desalin. Water Treat. 2011, 27, 319–326. [Google Scholar] [CrossRef]
- Toptas, A.; Demierege, S.; Ayan, E.M.; Yanik, J. Spent mushroom compost as biosorbent for dye biosorption. Clean-Soil Air Water 2014, 42, 1721–1728. [Google Scholar] [CrossRef]
- Scott, G.V. Spectrophotometric determination of cationic surfactants with orange II. Anal. Chem. 1968, 40, 768–773. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Nabil, G.M.; El-Mallah, N.M.; Bassiouny, H.I.; Kumar, S.; Abdel-Fattah, T.M. Kinetics, isotherm, and thermodynamic studies of the adsorption of reactive red 195 a dye from water by modified switchgrass biochar adsorbent. J. Ind. Eng. Chem. 2016, 37, 156–167. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: Ftir analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gong, W.; Xie, C.; Yang, D.; Ling, X.; Yuan, X.; Chen, S.; Liu, X. Removal of neutral red from aqueous solution by adsorption on spent cottonseed hull substrate. J. Hazard. Mater. 2011, 185, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, A. Adsorption properties of congo red from aqueous solution onto surfactant-modified montmorillonite. J. Hazard. Mater. 2008, 160, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Structure and properties of natural cellulose fibers obtained from sorghum leaves and stems. J. Agric. Food. Chem. 2007, 55, 5569–5574. [Google Scholar] [CrossRef] [PubMed]
- Putun, A.E.; Ozbay, N.; Onal, E.P.; Putun, E. Fixed-bed pyrolysis of cotton stalk for liquid and solid products. Fuel Process. Technol. 2005, 86, 1207–1219. [Google Scholar] [CrossRef]
- Sharma, H. Biochemical and thermal analyses of mushroom compost during preparation. In Proceedings of the 13th International Congress on the Science and Cultivation of Edible Fung; Maher, E.M.J., Ed.; National Centre for Horticulture: Dublin, Ireland, 1991; Volume 13, pp. 169–179. [Google Scholar]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene blue adsorption onto swede rape straw (brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Wu, J.; Dai, G. Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J. Hazard. Mater. 2011, 192, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Chen, W.-F.; Cheng, M.-T.; Li, Q. Investigation of factors that affect cationic surfactant loading on activated carbon and perchlorate adsorption. Colloids Surf. Physicochem. Eng. Asp. 2013, 434, 236–242. [Google Scholar] [CrossRef]
- Sullivan, E.J.; Hunter, D.B.; Bowman, R.S. Topological and thermal properties of surfactant-modified clinoptilolite studied by tapping-mode atomic force microscopy and high-resolution thermogravimetric analysis. Clays Clay Miner. 1997, 45, 42–53. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]
- Aldegs, Y.; Elbarghouthi, M.; Elsheikh, A.; Walker, G. Effect of solution ph, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Germán-Heins, J.; Flury, M. Sorption of brilliant blue fcf in soils as affected by ph and ionic strength. Geoderma 2000, 97, 87–101. [Google Scholar] [CrossRef]
- Jayasantha Kumari, H.; Krishnamoorthy, P.; Arumugam, T.K.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto tlac/chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Kayranli, B. Adsorption of textile dyes onto iron based waterworks sludge from aqueous solution; isotherm, kinetic and thermodynamic study. Chem. Eng. J. 2011, 173, 782–791. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Kan, X.; Dong, L.; Yan, H.; Jiang, Z.; Yang, H.; Li, A.; Cheng, R. Adsorption of anionic dyes from aqueous solutions using chemically modified straw. Bioresour. Technol. 2012, 117, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Wang, H.B.; Ifthikar, J.; Khan, A.; Wang, T.; Zhan, K.; Shahzad, A.; Chen, Z.L.; Chen, Z.Q. Efficient, stable and selective adsorption of heavy metals by thio-functionalized layered double hydroxide in diverse types of water. Chem. Eng. J. 2018, 332, 387–397. [Google Scholar] [CrossRef]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Equilibrium modeling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: Coconut (cocos nucifera) bunch waste. J. Hazard. Mater. 2008, 158, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, F.; Gu, C.; Stedtfeld, R.; Bian, Y.; Liu, G.; Wu, W.; Jiang, X. Sorption characteristics of n-acyl homserine lactones as signal molecules in natural soils based on the analysis of kinetics and isotherms. RSC Adv. 2018, 8, 9364–9374. [Google Scholar] [CrossRef]
- Garcia, E.R.; Medina, R.L.; Lozano, M.M.; Hernandez Perez, I.; Valero, M.J.; Franco, A.M.M. Adsorption of azo-dye orange II from aqueous solutions using a metal-organic framework material: Iron- benzenetricarboxylate. Materials 2014, 7, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, orange G from aqueous solutions. Arabian J. Chem. 2017, in press. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-laqtah, N.A.; Walker, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Bulut, Y.; Gozubenli, N.; Aydin, H. Equilibrium and kinetics studies for adsorption of direct blue 71 from aqueous solution by wheat shells. J. Hazard. Mater. 2007, 144, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Suresh, S. Kinetic and equilibrium studies on the biosorption of reactive black 5 dye by aspergillus foetidus. Bioresour. Technol. 2008, 99, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.S.A.; Jawad, A.H.; Nawawi, W.I. Equilibrium, kinetic and thermodynamic studies of reactive red 120 dye adsorption by chitosan beads from aqueous solution. Energy Ecol. Environ. 2017, 2, 85–93. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.M.; Jin, B.; Chowd, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of congo red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Tabak, A.; Baltas, N.; Afsin, B.; Emirik, M.; Caglar, B.; Eren, E. Adsorption of reactive red 120 from aqueous solutions by cetylpyridinium-bentonite. J. Chem. Technol. Biotechnol. 2010, 85, 1199–1207. [Google Scholar] [CrossRef]
- Osma, J.F.; Saravia, V.; Toca-Herrera, J.L.; Couto, S.R. Sunflower seed shells: A novel and effective low-cost adsorbent for the removal of the diazo dye reactive black 5 from aqueous solutions. J. Hazard. Mater. 2007, 147, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Malekbala, M.R.; Khan, M.A.; Hosseini, S.; Abdullah, L.C.; Choong, T.S.Y. Adsorption/desorption of cationic dye on surfactant modified mesoporous carbon coated monolith: Equilibrium, kinetic and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 369–377. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Fida, H.; Guo, S.; Zhang, G. Preparation and characterization of bifunctional ti-fe kaolinite composite for Cr(VI) removal. J. Colloid Interface Sci. 2015, 442, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Von Oepen, B.; Kördel, W.; Klein, W. Sorption of nonpolar and polar compounds to soils: Processes, measurements and experience with the applicability of the modified oecd-guideline 106. Chemosphere 1991, 22, 285–304. [Google Scholar] [CrossRef]
- Yuen, C.W.M.; Ku, S.K.A.; Choi, P.S.R.; Kan, C.W.; Tsang, S.Y. Determining functional groups of commercially available ink-jet printing reactive dyes using infrared spectroscopy. Res. J. Text. Appar. 2005, 9, 26–38. [Google Scholar] [CrossRef]
| Adsorbent | C0 (ppm) | Adsorbent Dose (g) | Adsorbate | Qm (mg·g−1) | Reference |
|---|---|---|---|---|---|
| pine cone acid treated | 10–60 | 0.02 | Congo Red | 40.19 | [49] |
| wheat shells | 50–250 | 0.5 | Direct blue 71 | 46.30 | [50] |
| Fungal biomass | 10-400 | 0.2 | Reactive Black 5 | 106 | [51] |
| Chitosan beads | 30–400 | 0.3 | Reactive Red 120 | 129.9 | [52] |
| Na-Bentonite | 50–1000 | 5 | Congo Red | 36 | [53] |
| Cetylpyridinium-bentonite | 10–70 | 0.01 | Reactive Red 120 | 81.97 | [54] |
| Sunflower seed shells | 15–50 | 5 | Reactive Black 5 | 1.10 | [55] |
| SMWC | 50–2200 | 0.075 | Direct Red 5B | 249.57 | in this study |
| SMWC | 50–2200 | 0.075 | Direct blue 71 | 338.67 | in this study |
| SMWC | 50–2200 | 0.075 | Reactive Black 5 | 265.01 | in this study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes. Int. J. Environ. Res. Public Health 2018, 15, 1421. https://doi.org/10.3390/ijerph15071421
Alhujaily A, Yu H, Zhang X, Ma F. Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes. International Journal of Environmental Research and Public Health. 2018; 15(7):1421. https://doi.org/10.3390/ijerph15071421
Chicago/Turabian StyleAlhujaily, Ahmad, Hongbo Yu, Xiaoyu Zhang, and Fuying Ma. 2018. "Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes" International Journal of Environmental Research and Public Health 15, no. 7: 1421. https://doi.org/10.3390/ijerph15071421
APA StyleAlhujaily, A., Yu, H., Zhang, X., & Ma, F. (2018). Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes. International Journal of Environmental Research and Public Health, 15(7), 1421. https://doi.org/10.3390/ijerph15071421