Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study
Abstract
1. Introduction
2. Materials and Methods
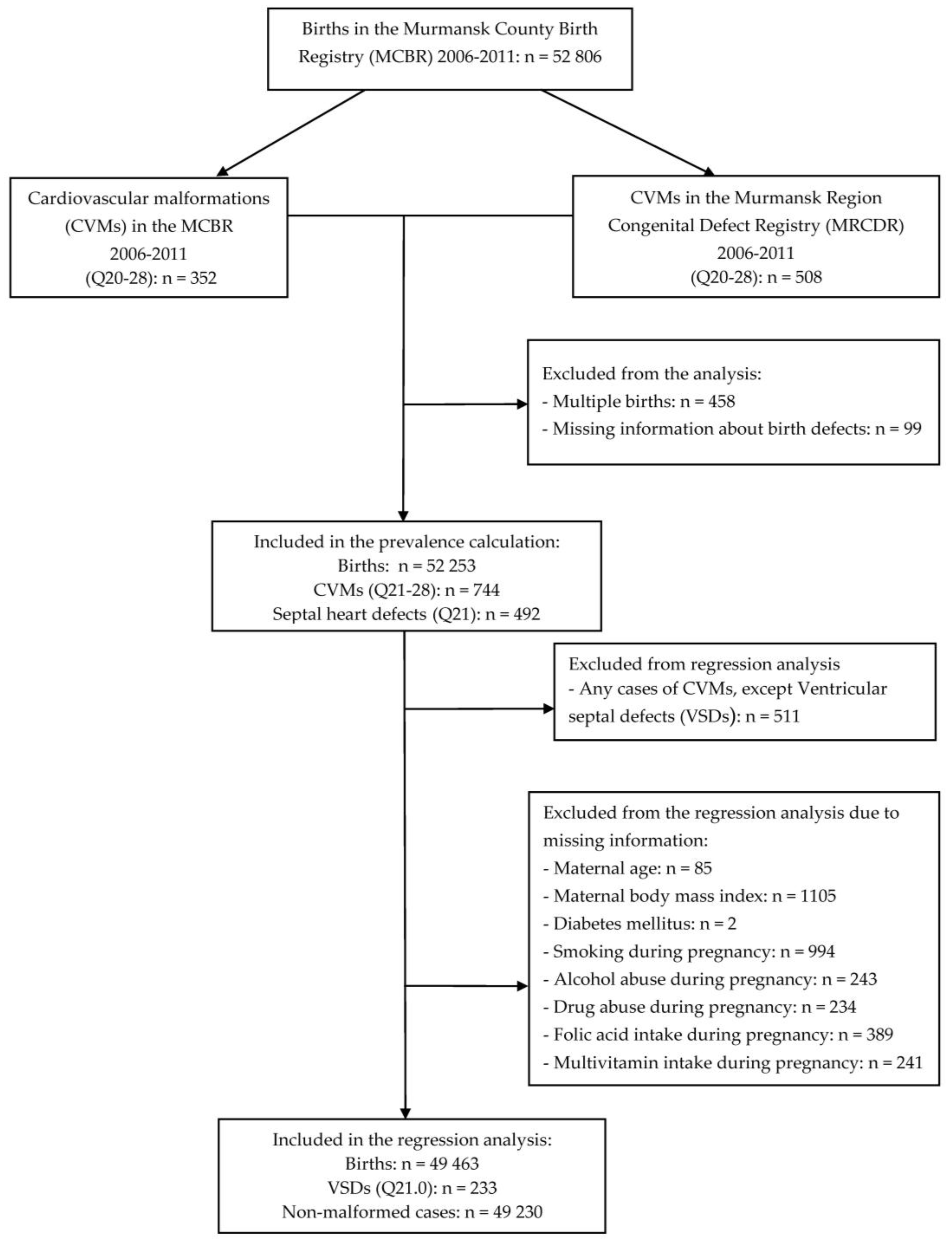
2.1. Data
2.2. Ethical Considerations
2.3. Variables
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Selected Risk Factors
4.1.1. Smoking during Pregnancy
4.1.2. Sex of the Baby
4.1.3. Alcohol Abuse during Pregnancy
4.1.4. Drug Abuse During Pregnancy
4.1.5. Diabetes
4.1.6. Folic Acid and Multivitamins
4.2. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ou, Y.; Mai, J.; Zhuang, J.; Liu, X.; Wu, Y.; Gao, X.; Nie, Z.; Qu, Y.; Chen, J.; Kielb, C.; et al. Risk factors of different congenital heart defects in Guangdong, China. Pediatr. Res. 2016, 79, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Jortveit, J.; Oyen, N.; Leirgul, E.; Fomina, T.; Tell, G.S.; Vollset, S.E.; Eskedal, L.; Dohlen, G.; Birkeland, S.; Holmstrom, H. Trends in mortality of congenital heart defects. Congenit. Heart Dis. 2016, 11, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Postoev, V.A.; Talykova, L.V.; Vaktskjold, A. Epidemiology of cardiovascular malformations among newborns in monchegorsk (North-West Russia): A register-based study. J. Public Health Res. 2014, 3, 270. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Kang, Y.; Zhao, Y.; Yan, H. Prevalence and risk factors of congenital heart defects among live births: A population-based cross-sectional survey in Shaanxi province, Northwestern China. BMC Pediatr. 2017, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.A. Epidemiology of cardiovascular malformations: Prevalence and risk factors. Am. J. Med. Genet. 2000, 97, 319–325. [Google Scholar] [CrossRef]
- Wilson, P.D.; Loffredo, C.A.; Correa-Villasenor, A.; Ferencz, C. Attributable fraction for cardiac malformations. Am. J. Epidemiol. 1998, 148, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Gelb, B.D.; Chung, W.K. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb. Perspect. Med. 2014, 4, a013953. [Google Scholar] [CrossRef] [PubMed]
- Van der Bom, T.; Zomer, A.C.; Zwinderman, A.H.; Meijboom, F.J.; Bouma, B.J.; Mulder, B.J. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 2011, 8, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, D.; Yang, L.; Da, M.; Wang, Z.; Lin, Y.; Ni, B.; Wang, S.; Mo, X. Maternal lifestyle factors in pregnancy and congenital heart defects in offspring: Review of the current evidence. Ital. J. Pediatr. 2014, 40, 85. [Google Scholar] [CrossRef] [PubMed]
- Batra, M.; Heike, C.L.; Phillips, R.C.; Weiss, N.S. Geographic and occupational risk factors for ventricular septal defects: Washington State, 1987–2003. Arch. Pediatr. Adolesc. Med. 2007, 161, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.A.; Jaimes, C.; Abbara, S. Ventricular septal defects: Embryology and imaging findings. J. Thorac. Imaging 2013, 28, W28–W34. [Google Scholar] [CrossRef] [PubMed]
- Minette, M.S.; Sahn, D.J. Ventricular septal defects. Circulation 2006, 114, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.P., Jr.; Gutgesell, H.P. Ventricular Septal Defects. In Moss and Andrews Heart Disease in Infants, Children and Adolescents: Including the Fetus and Young Adult, 5th ed.; Emmanouilides, G.C., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1995; pp. 724–744. [Google Scholar]
- Abdulla, R. Perspective in pediatric cardiology. Volume 5. Genetic and environmental risk factors of major cardiovascular malformations. Pediatr. Cardiol. 1998, 19, 435. [Google Scholar] [CrossRef] [PubMed]
- Brickner, M.E.; Hillis, L.D.; Lange, R.A. Congenital heart disease in adults. Second of two parts. N. Engl. J. Med. 2000, 342, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.A. Atrial and Ventricular Septal Defects: Molecular Determinants, Impact of Environmental Factors and Non-Surgical Interventions; Nova Biomedical: Waltham, MA, USA, 2013; pp. 36–50. [Google Scholar]
- Tikkanen, J.; Heinonen, O.P. Risk factors for ventricular septal defect in Finland. Public Health 1991, 105, 99–112. [Google Scholar] [CrossRef]
- Tikkanen, J.; Heinonen, O.P. Risk factors for atrial septal defect. Eur. J. Epidemiol. 1992, 8, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.J.; Correa, A.; Rasmussen, S. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res. A 2004, 70, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Greenlees, R.; Neville, A.; Addor, M.C.; Amar, E.; Arriola, L.; Bakker, M.; Barisic, I.; Boyd, P.A.; Calzolari, E.; Doray, B.; et al. Paper 6: EUROCAT member registries: Organization and activities. Birth Defects Res. A 2011, 91 (Suppl. 1), S51–S100. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Robert-Gnansia, E.; Siffel, C.; Harris, J.; Borman, B.; Mastroiacovo, P. Fostering international collaboration in birth defects research and prevention: A perspective from the International Clearinghouse for Birth Defects Surveillance and Research. Am. J. Public Health 2006, 96, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Russian Institute of Public Health. Report of Federal Informational Center of Gene Registering and Birth Defects’ Monitoring; Russian Institute of Public Health: Moscow, Russia, 2015. (In Russian) [Google Scholar]
- Jacobs, J.P.; Jacobs, M.L.; Mavroudis, C.; Chai, P.J.; Tchervenkov, C.I.; Lacour-Gayet, F.G.; Walters, H., 3rd; Quintessenza, J.A. Atrioventricular septal defects: Lessons learned about patterns of practice and outcomes from the congenital heart surgery database of the society of thoracic surgeons. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.A.; Brenn, T.; Odland, J.O.; Nieboer, E.; Krettek, A.; Anda, E.E. Under-reporting of major birth defects in northwest russia: A registry-based study. Int. J. Circumpolar Health 2017, 76, 1366785. [Google Scholar] [CrossRef] [PubMed]
- Kuciene, R.; Dulskiene, V. Selected environmental risk factors and congenital heart defects. Medicina (Kaunas) 2008, 44, 827–832. [Google Scholar] [PubMed]
- Cai, G.J.; Sun, X.X.; Zhang, L.; Hong, Q. Association between maternal body mass index and congenital heart defects in offspring: A systematic review. Am. J. Obstet. Gynecol. 2014, 211, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.M.; Dervan, L.A.; Reiger, S.; Buddhe, S.; Schwartz, S.M. Risk of congenital heart defects in the offspring of smoking mothers: A population-based study. J. Pediatr. 2015, 166, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Rodeck, C.; Boniface, S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Hum. Reprod. Update 2011, 17, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Kallen, K. Maternal smoking and congenital heart defects. Eur. J. Epidemiol. 1999, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Lupo, P.J. Maternal smoking during pregnancy and the risk of congenital heart defects in offspring: A systematic review and metaanalysis. Pediatr. Cardiol. 2013, 34, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Levis, D.M.; Tinker, S.C.; Cragan, J.D. Maternal cigarette smoking and congenital heart defects. J. Pediatr. 2015, 166, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Alverson, C.J.; Strickland, M.J.; Gilboa, S.M.; Correa, A. Maternal smoking and congenital heart defects in the baltimore-washington infant study. Pediatrics 2011, 127, e647–e653. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, O.A.; Grjibovski, A.M.; Krettek, A.; Nieboer, E.; Odland, J.O. Effect of Smoking Behavior before and during Pregnancy on Selected Birth Outcomes among Singleton Full-Term Pregnancy: A Murmansk County Birth Registry Study. Int. J. Environ. Res. Public Health 2017, 14, E867. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, R.L.; Bergmann, K.E.; Schumann, S.; Richter, R.; Dudenhausen, J.W. [Smoking during pregnancy: Rates, trends, risk factors]. Z. Geburtshilfe Neonatol. 2008, 212, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Nembhard, W.N.; Wang, T.; Loscalzo, M.L.; Salemi, J.L. Variation in the prevalence of congenital heart defects by maternal race/ethnicity and infant sex. J. Pediatr. 2010, 156, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Engelfriet, P.; Mulder, B.J. Gender differences in adult congenital heart disease. Neth. Heart J. 2009, 17, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Mercuro, G.; Bassareo, P.P.; Mariucci, E.; Deidda, M.; Zedda, A.M.; Bonvicini, M. Sex differences in congenital heart defects and genetically induced arrhythmias. J. Cardiovasc. Med. (Hagerstown) 2014, 15, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Yazici, A.B.; Uslu Yuvaci, H.; Yazici, E.; Halimoglu Caliskan, E.; Cevrioglu, A.S.; Erol, A. Smoking, alcohol, and substance use and rates of quitting during pregnancy: Is it hard to quit? Int. J. Womens Health 2016, 8, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Tsang, T.W.; Elliott, E.J. High global prevalence of alcohol use during pregnancy and fetal alcohol syndrome indicates need for urgent action. Lancet Glob. Health 2017, 5, e232–e233. [Google Scholar] [CrossRef]
- Shillingford, A.J.; Weiner, S. Maternal issues affecting the fetus. Clin. Perinatol. 2001, 28, 31–70. [Google Scholar] [CrossRef]
- Menegola, E.; Broccia, M.L.; Di Renzo, F.; Giavini, E. Acetaldehyde in vitro exposure and apoptosis: A possible mechanism of teratogenesis. Alcohol 2001, 23, 35–39. [Google Scholar] [CrossRef]
- Dedov, I.; Maslova, O.; Suntsov, Y.; Bolotskaia, L.; Milenkaia, T.; Besmertnaia, L. Prevalence of diabetic retinopathy and cataract in adult patients with type 1 and type 2 diabetes in russia. Rev. Diabet. Stud. 2009, 6, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.L.; Norgard, B.; Puho, E.; Rothman, K.J.; Sorensen, H.T.; Czeizel, A.E. Risk of specific congenital abnormalities in offspring of women with diabetes. Diabet. Med. 2005, 22, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; O’Brien, T.E.; Chan, W.S. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: A meta-analysis. QJM 2001, 94, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E. Periconceptional folic acid-containing multivitamin supplementation for the prevention of neural tube defects and cardiovascular malformations. Ann. Nutr. Metab. 2011, 59, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Krikov, S.; Carmichael, S.L.; Munger, R.G.; Shaw, G.M.; Feldkamp, M.L. National Birth Defects Prevention Study. Lower rate of selected congenital heart defects with better maternal diet quality: A population-based study. Arch. Dis. Child. Fetal Neonatal. Ed. 2016, 101, F43–F49. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Mulinare, J.; Erickson, J.D. Do multivitamin or folic acid supplements reduce the risk for congenital heart defects? Evidence and gaps. Am. J. Med. Genet. A 2003, 121, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, S.; Werler, M.M.; Walker, A.M.; Mitchell, A.A. Folic acid antagonists during pregnancy and the risk of birth defects. New Engl. J. Med. 2000, 343, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Anda, E.E.; Nieboer, E.; Voitov, A.V.; Kovalenko, A.A.; Lapina, Y.M.; Voitova, E.A.; Kovalenko, L.F.; Odland, J.O. Implementation, quality control and selected pregnancy outcomes of the murmansk county birth registry in russia. Int. J. Circumpolar Health 2008, 67, 318–334. [Google Scholar] [CrossRef] [PubMed]
| ICD-10 Code b | CVM | Cases | Prevalence c | |
|---|---|---|---|---|
| n | % | |||
| Q20 | Congenital malformations of cardiac chambers and connections | 14 | 1.9 | 0.27 (0.2–0.5) |
| Q21 | Septal defects | 492 | 66.1 | 9.4 (8.6–10.3) |
| Q22–23 | Valves defects | 32 | 4.3 | 0.6 (0.4–0.9) |
| Q24 | Other congenital malformations of the heart | 51 | 6.9 | 1.0 (0.8–1.3) |
| Q25–27 | Vessels anomalies | 88 | 11.8 | 1.7 (1.4–2.1) |
| Q28 | Other congenital malformations of the circulatory system | 2 | 0.3 | 0.038 (0.037–0.040) |
| Multiple | Two or more | 65 | 8.7 | 1.2 (1.0–1.7) |
| Q20–28 | All | 744 | 100 | 14.2 (13.2–15.3) |
| ICD-10 Code a | CVM | Cases | |
|---|---|---|---|
| n | % | ||
| Q21.0 | Ventricular septal defects | 233 | 47.4 |
| Q21.1 | Atrial septal defects | 112 | 22.8 |
| Q21.2 | Atrio-ventricular septal defects | 10 | 2.0 |
| Q21.3 | Tetralogy of Fallot | 6 | 1.2 |
| Q21.4 | Aorto-pulmonary septal defects | 9 | 1.8 |
| Q21.8 | Other | 5 | 1.0 |
| Q21.9 | Unspecified | 117 | 23.8 |
| Q21 | All | 492 | 100 |
| Variables | Cases, n = 233 a | Non-Cases, n = 49,230 a | p-Value b | ||
|---|---|---|---|---|---|
| X | SD or % | X | SD or % | ||
| Infant characteristics | |||||
| Birth weight (g), mean ± SD | 3244.4 | 677.6 | 3377.2 | 546.5 | <0.001 |
| <2500 | 30 | 12.9 | 2211 | 4.5 | |
| 2500–3999 | 179 | 76.8 | 41,958 | 85.2 | |
| ≥4000 | 24 | 10.3 | 5061 | 10.3 | |
| Sex, male | 98 | 42.1 | 25,571 | 52.0 | 0.003 |
| Maternal characteristics | |||||
| Age at delivery (years), mean ± SD | 26.06 | 5.44 | 26.79 | 5.27 | 0.43 |
| <18 | 3 | 1.3 | 727 | 1.5 | |
| 18–35 | 209 | 89.7 | 45,429 | 92.3 | |
| >35 | 21 | 9.0 | 3074 | 6.2 | |
| Gestational age (weeks), mean ± SD | 39.2 | 2.3 | 39.5 | 2.2 | 0.05 |
| BMI (kg/cm2), mean ± SD | 23.37 | 4.57 | 23.49 | 4.28 | 0.67 |
| <18.5 | 16 | 6.9 | 3103 | 6.3 | |
| 18.5–24.9 | 157 | 67.4 | 32,325 | 65.7 | |
| 25.0–29.9 | 40 | 17.2 | 9712 | 19.7 | |
| 30.0–34.9 | 15 | 6.4 | 3084 | 6.3 | |
| 35.0–39.9 | 3 | 1.3 | 782 | 1.6 | |
| ≥40 | 2 | 0.9 | 224 | 0.5 | |
| Smoking during pregnancy | 74 | 31.8 | 12,234 | 24.9 | 0.02 |
| Alcohol abuse during pregnancy | 6 | 2.6 | 178 | 0.4 | <0.001 |
| Drugs abuse during pregnancy | 4 | 1.7 | 173 | 0.4 | 0.01 |
| Folic acid intake during pregnancy | 175 | 75.1 | 36,545 | 74.2 | 0.76 |
| Multivitamins intake during pregnancy | 211 | 90.6 | 45,568 | 92.6 | 0.25 |
| Diabetes mellitus Type 1 or 2 | 4 | 1.7 | 94 | 0.2 | 0.001 |
| Variables | Crude | Adjusted b | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Maternal age at delivery (years) | ||||
| <18 | 0.90 | 0.29–2.81 | 0.84 | 0.27–2.65 |
| 18–35 | 1 | Reference | 1 | Reference |
| >35 | 1.49 | 0.95–2.33 | 1.53 | 0.97–2.41 |
| Maternal BMI (kg/cm2) c | ||||
| <18.5 | 1.06 | 0.63–1.78 | 1.10 | 0.66–1.85 |
| 18.5–24.9 | 1 | Reference | 1 | Reference |
| >25 | 0.90 | 0.66–1.21 | 0.86 | 0.63–1.16 |
| Smoking during pregnancy | 1.41 | 1.07–1.86 | 1.35 | 1.02–1.80 |
| Alcohol abuse during pregnancy | 7.28 | 3.20–16.60 | 4.83 | 1.88–12.42 |
| Drugs abuse during pregnancy | 4.95 | 1.82–13.46 | 2.39 | 0.77–7.44 |
| Folic acid intake during pregnancy | 1.05 | 0.78–1.41 | 1.14 | 0.84–1.55 |
| Multivitamins intake during pregnancy | 0.77 | 0.50–1.20 | 0.99 | 0.69–1.43 |
| Diabetes mellitus type 1 or 2 | 9.13 | 3.33–25.04 | 8.72 | 3.16–24.07 |
| Sex (male) | 0.67 | 0.52–0.87 | 0.67 | 0.52–0.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalenko, A.A.; Anda, E.E.; Odland, J.Ø.; Nieboer, E.; Brenn, T.; Krettek, A. Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study. Int. J. Environ. Res. Public Health 2018, 15, 1320. https://doi.org/10.3390/ijerph15071320
Kovalenko AA, Anda EE, Odland JØ, Nieboer E, Brenn T, Krettek A. Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study. International Journal of Environmental Research and Public Health. 2018; 15(7):1320. https://doi.org/10.3390/ijerph15071320
Chicago/Turabian StyleKovalenko, Anton A., Erik Eik Anda, Jon Øyvind Odland, Evert Nieboer, Tormod Brenn, and Alexandra Krettek. 2018. "Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study" International Journal of Environmental Research and Public Health 15, no. 7: 1320. https://doi.org/10.3390/ijerph15071320
APA StyleKovalenko, A. A., Anda, E. E., Odland, J. Ø., Nieboer, E., Brenn, T., & Krettek, A. (2018). Risk Factors for Ventricular Septal Defects in Murmansk County, Russia: A Registry-Based Study. International Journal of Environmental Research and Public Health, 15(7), 1320. https://doi.org/10.3390/ijerph15071320





