Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Chemical Analysis
2.3. Data Analyses
3. Results and Discussion
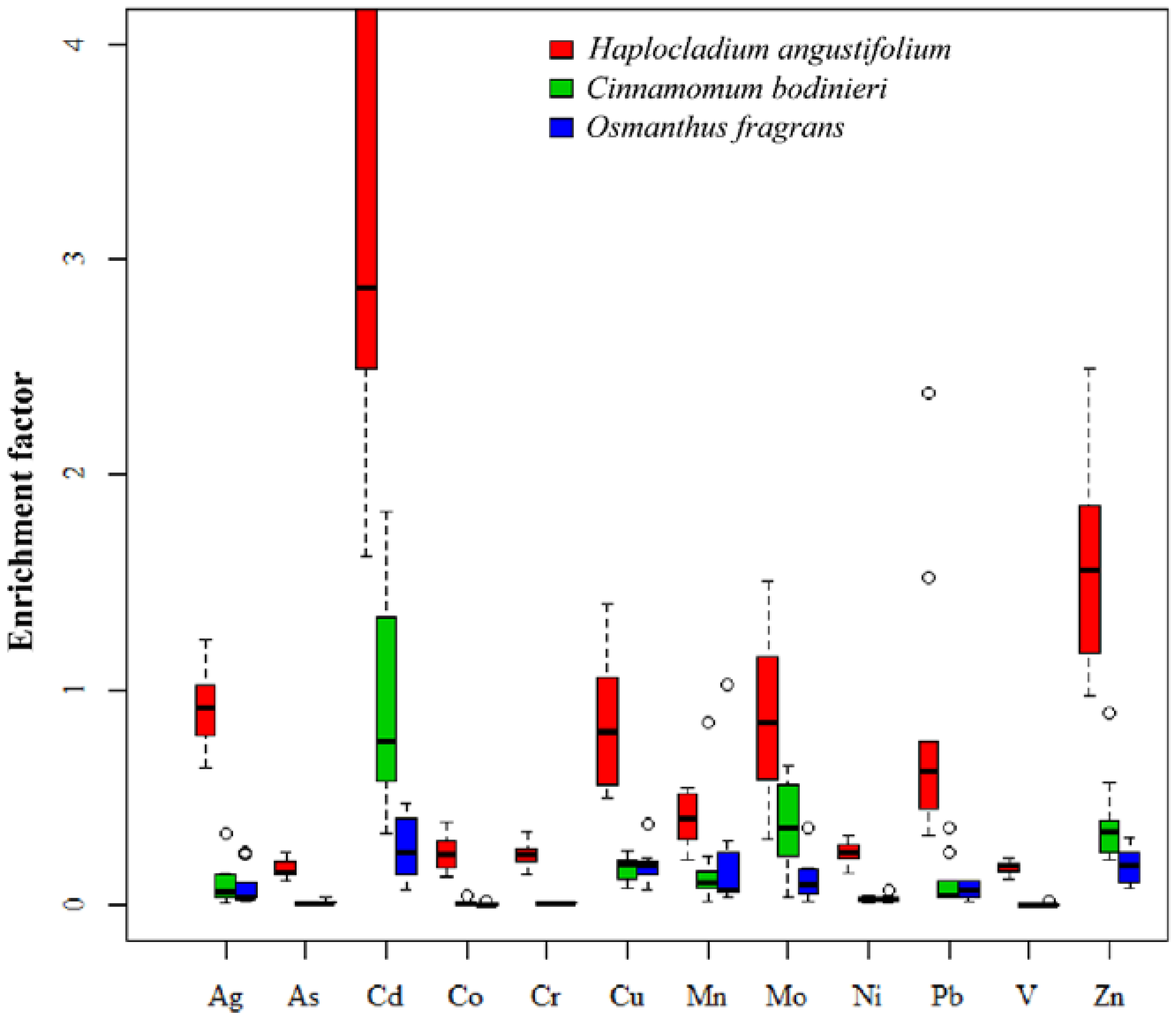
3.1. Comparisons of Trace Element Concentrations in Moss with That in Leaves of Vascular Species
3.2. Differentiation of Metal Accumulation in Moss Species H. angustifolium
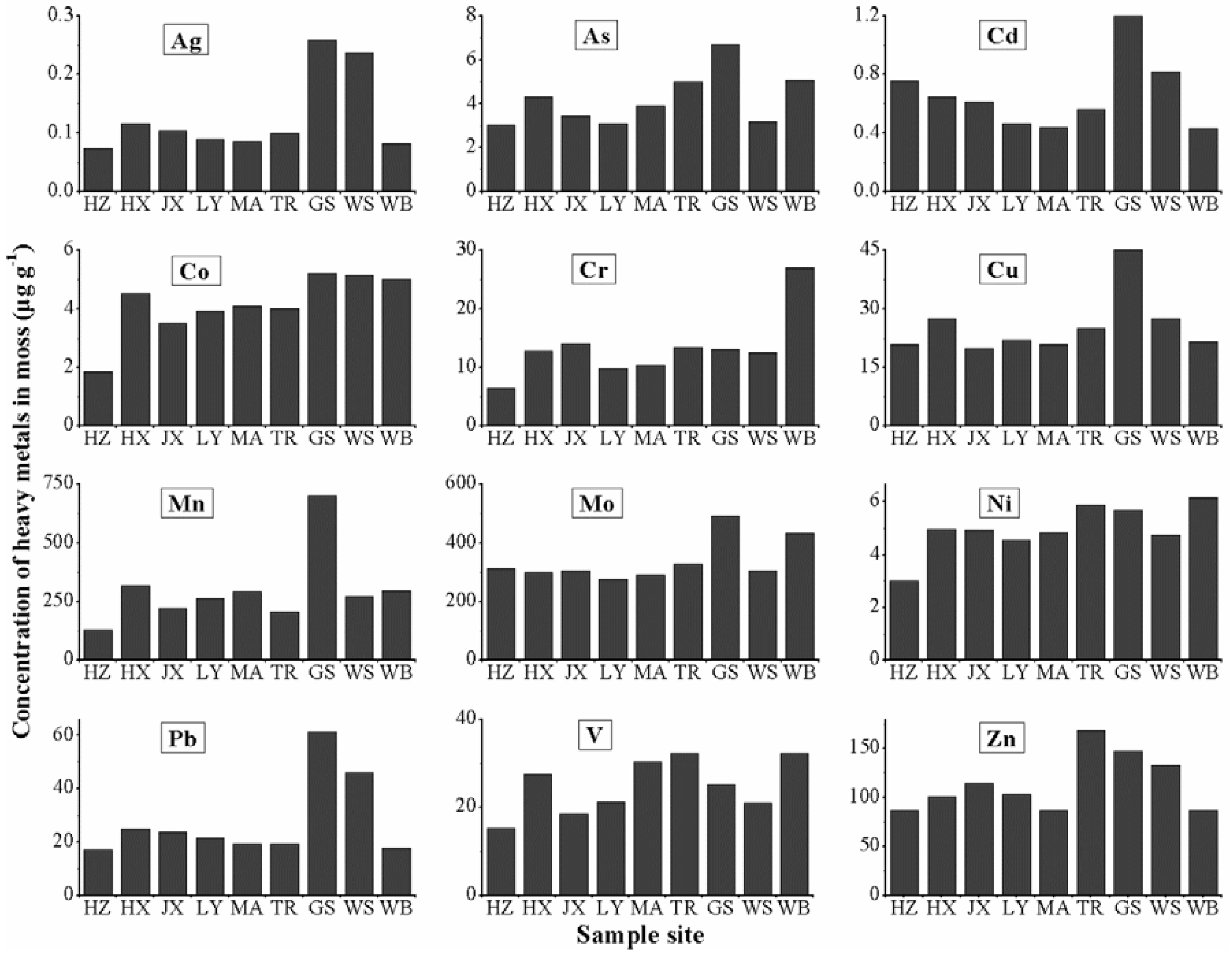
3.3. Atmospheric Heavy Metal Pollution Assessing by CF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, O.H.; Tan, B.C.; Obbard, J.P. Lichens as bioindicators of atmospheric heavy metal pollution in Singapore. Environ. Monit. Assess. 2006, 123, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, Y.; Rui, X.; Li, Y.X.; Li, Y.; Wang, H.Z.; Zuo, J.; Tong, Y.D. Sources of atmospheric pollution: A bibliometric analysis. Scientometrics 2017, 112, 1025–1045. [Google Scholar] [CrossRef]
- Ram, S.S.; Majumder, S.; Chaudhuri, P.; Chanda, S.; Santra, S.C.; Chakraborty, A.; Sudarshan, M. A review on air pollution monitoring and management using plants with special reference to foliar dust adsorption and physiological stress responses. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2489–2522. [Google Scholar] [CrossRef]
- Harmens, H.; Norris, D.A.; Koerber, G.R.; Buse, A.; Steinnes, E.; Ruhling, A. Temporal trends in the concentration of arsenic, chromium, copper, iron, nickel, vanadium and zinc in mosses across Europe. Atmos. Environ. 2007, 41, 6673–6687. [Google Scholar] [CrossRef]
- Stankovic, S.; Kalaba, P.; Stankovic, A.R. Biota as toxic metal indicators. Environ. Chem. Lett. 2014, 12, 63–84. [Google Scholar] [CrossRef]
- Ayodele, J.T.; Ahmed, A. Monitoring air pollution in Kano municipality by chemical analysis of Scots Pine (Pinus sylvestris L.) needles for sulphur content. Environmentalist 2001, 21, 145–151. [Google Scholar] [CrossRef]
- Brignole, D.; Drava, G.; Minganti, V.; Giordani, P.; Samson, R.; Vieira, J.; Pinho, P.; Branquinho, C. Chemical and magnetic analyses on tree bark as an effective tool for biomonitoring: A case study in Lisbon (Portugal). Chemosphere 2018, 195, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Wojtun, B.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J.; Skrzypek, G. Metals in some dominant vascular plants, mosses, lichens, algae, and the biological soil crust in various types of terrestrial tundra, SW Spitsbergen, Norway. Polar Biol. 2013, 36, 1799–1809. [Google Scholar] [CrossRef]
- Paoli, L.; Munzi, S.; Guttova, A.; Senko, D.; Sardella, G.; Loppi, S. Lichens as suitable indicators of the biological effects of atmospheric pollutants around a municipal solid waste incinerator (S Italy). Ecol. Indic. 2015, 52, 362–370. [Google Scholar] [CrossRef]
- Aprile, G.G.; Di Salvatore, M.; Carratu, G.; Mingo, A.; Carafa, A.M. Comparison of the suitability of two lichen species and one higher plant for monitoring airborne heavy metals. Environ. Monit. Assess. 2010, 162, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Naeth, M.A.; Wilkinson, S.R. Lichens as biomonitors of air quality around a diamond mine, Northwest Territories, Canada. J. Environ. Qual. 2008, 37, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Scerbo, R.; Ristori, T.; Possenti, L.; Lampugnani, L.; Barale, R.; Barghigiani, C. Lichen (Xanthoria parietina) biomonitoring of trace element contamination and air quality assessment in Pisa Province (Tuscany, Italy). Sci. Total Environ. 2002, 286, 27–40. [Google Scholar] [CrossRef]
- Coskun, M.; Steinnes, E.; Coskun, M.; Cayir, A. Comparison of epigeic moss (Hypnum cupressiforme) and lichen (Cladonia rangiformis) as biomonitor species of atmospheric metal deposition. Bull. Environ. Contam. Toxicol. 2009, 82, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pesch, R.; Schroeder, W. Mosses as bioindicators for metal accumulation: Statistical aggregation of measurement data to exposure indices. Ecol. Indic. 2006, 6, 137–152. [Google Scholar] [CrossRef]
- Pata, I.M.C.; Balan, C.D.; Pata, S.M.; Macoveanu, M. Passive biomonitoring of atmospheric pollution with heavy metals using native Epigeic moss. Environ. Eng. Manag. J. 2009, 8, 1281–1286. [Google Scholar]
- Bargagli, R.; Monaci, F.; Borghini, F.; Bravi, F.; Agnorelli, C. Mosses and lichens as biomonitors of trace metals. A comparison study on Hypnum cupressiforme and Parmelia caperata in a former mining district in Italy. Environ. Pollut. 2002, 116, 279–287. [Google Scholar] [CrossRef]
- Icel, Y.; Cobanoglu, G. Biomonitoring of atmospheric heavy metal pollution using lichens and mosses in the city of Istanbul, Turkey. Fresen. Environ. Bull. 2009, 18, 2066–2071. [Google Scholar]
- Zhou, X.L.; Chen, Q.; Liu, C.; Fang, Y.M. Using moss to assess airborne heavy metal pollution in Taizhou, China. Int. J. Environ. Res. Public Health 2017, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Zhang, Z.H.; Wang, Z.H. Bryophyte communities as biomonitors of environmental factors in the Goujiang karst bauxite, southwestern China. Sci. Total Environ. 2015, 538, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.; Ugulu, I.; Baslar, S. Turkish red pine as a biomonitor: A comparative study of the accumulation of trace elements in the needles and bark. Ekoloji 2010, 19, 88–96. [Google Scholar] [CrossRef]
- Sun, F.F.; Wen, D.Z.; Kuang, Y.W.; Li, J.O.; Li, J.L.; Zuo, W.D. Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson pine (Pinus massoniana L.) growing nearby different industrial sources. J. Environ. Sci. China 2010, 22, 1006–1013. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. A preliminary investigation in using Pohlia nutans and Larix decidua as biomonitors of air pollution by the coke industry in Walbrzych (SW Poland). Pol. J. Environ. Stud. 2008, 17, 121–128. [Google Scholar]
- Szczepaniak, K.; Biziuk, M. Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environ. Res. 2003, 93, 221–230. [Google Scholar] [CrossRef]
- Sun, S.Q.; Wang, D.Y.; He, M.; Zhang, C. Monitoring of atmospheric heavy metal deposition in Chongqing, China—Based on moss bag technique. Environ. Monit. Assess. 2009, 148, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Q.; Wang, G.G.; Fang, Y.M. Atmospheric deposition of heavy metals in Wuxi, China: estimation based on native moss analysis. Environ. Monit. Assess. 2016, 188, 360. [Google Scholar] [CrossRef] [PubMed]
- Maxhuni, A.; Lazo, P.; Kane, S.; Qarri, F.; Marku, E.; Harmens, H. First survey of atmospheric heavy metal deposition in Kosovo using moss biomonitoring. Environ. Sci. Pollut. Res. 2016, 23, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L. Epiphytic bryophytes as indicators of changes in background levels of airborne metals from 1951–1975. Environ. Pollut. 1977, 14, 37–45. [Google Scholar] [CrossRef]
- Tyler, G. Bryophytes and heavy metals: A literature review. Bot. J. Linn. Soc. 1990, 104, 231–253. [Google Scholar] [CrossRef]
- Sawidis, T.; Marnasidis, A.; Zachariadis, G.; Stratis, J. A study of air pollution with heavy metals in Thessaloniki City (Greece) using trees as biological indicators. Arch. Environ. Contam. Toxicol. 1995, 28, 118–124. [Google Scholar] [CrossRef]
- Sawidis, T.; Krystallidis, P.; Veros, D.; Chettri, M. A study of air pollution with heavy metals in Athens City and Attica basin using evergreen trees as biological indicators. Biol. Trace Elem. Res. 2012, 148, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Dongarra, G.; Sabatino, G.; Triscari, M.; Varrica, D. The effects of anthropogenic particulate emissions on roadway dust and Nerium oleander leaves in Messina (Sicily, Italy). J. Environ. Monit. 2003, 5, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Piraga, D.; Tabors, G.; Nikodemus, O.; Zigure, Z.; Brumelis, G. Current content of selected pollutants in moss, humus, soil and bark and long-term radial growth of pine trees in the Mezaparks forest in Riga. Environ. Sci. Pollut. Res. 2017, 24, 11964–11977. [Google Scholar] [CrossRef] [PubMed]
- Tsikritzis, L.I.; Ganatsios, S.S.; Duliu, O.G.; Sawidis, T.D. Natural and artificial radionuclides distribution in some lichens, mosses, and trees in the vicinity of lignite power plants from West Macedonia, Greece. J. Trace Microprobe Tech. 2003, 21, 543–554. [Google Scholar] [CrossRef]
- Salemaa, M.; Derome, J.; Helmisaari, H.S.; Nieminen, T.; Vanha-Majamaa, I. Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulfur deposition in Finland. Sci. Total Environ. 2004, 324, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, M.; An, L.; Yu, Y.H.; Lou, Y.X.; Guo, S.L.; Zuo, B.R.; Liu, Y.; Wu, J.M.; Cao, Y.; et al. Air quality for metals and sulfur in Shanghai, China, determined with moss bags. Environ. Pollut. 2009, 157, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Wu, Y.; Hu, R.; Jiang, Y. Diversity and distribution of bryophytes and their relationship with environmental factors in Wuhan. Plant Sci. J. 2017, 35, 825–834. [Google Scholar]
- Fernández, J.A.; Carballeira, A. A comparison of indigenous mosses and topsoils for use in monitoring atmospheric heavy metal deposition in Galicia (northwest Spain). Environ. Pollut. 2001, 114, 431–441. [Google Scholar]
- Mendil, D.; Celik, F.; Tuzen, M.; Soylak, M. Assessment of trace metal levels in some moss and lichen samples collected from near the motorway in Turkey. J. Hazard. Mater. 2009, 166, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Galuszka, A. The chemistry of soils, rocks and plant bioindicators in three ecosystems of the Holy Cross Mountains, Poland. Environ. Monit. Assess. 2005, 110, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as bioindicator of heavy metal pollution in three European cities. Environ. Pollut. 2011, 159, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Påhlsson, A.-M.B. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. A literature review. Water Air Soil Pollut. 1989, 47, 287–319. [Google Scholar]
- Gough, L.P.; Shacklette, H.T.; Case, A.A. Element concentrations toxic to plants, animals, and man. Geol. Surv. Bull. 1979, 1466, 1–80. [Google Scholar]
- Harmens, H.; Ilyin, I.; Mills, G.; Aboal, J.R.; Alber, R.; Blum, O.; Coskun, M.; De Temmerman, L.; Fernandez, J.A.; Figueira, R.; et al. Country-specific correlations across Europe between modelled atmospheric cadmium and lead deposition and concentrations in mosses. Environ. Pollut. 2012, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, P.; Fang, Y.M. Monitoring Airborne Heavy Metal Using Mosses in the City of Xuzhou, China. Bull. Environ. Contam. Toxicol. 2016, 96, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Du, P.X.; Cao, J.J.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2006, 355, 176–186. [Google Scholar]
- Harmens, H.; Norris, D.A.; Koerber, G.R.; Buse, A.; Steinnes, E.; Ruhling, A. Temporal trends (1990–2000) in the concentration of cadmium, lead and mercury in mosses across Europe. Environ. Pollut. 2008, 151, 368–376. [Google Scholar] [CrossRef] [PubMed]
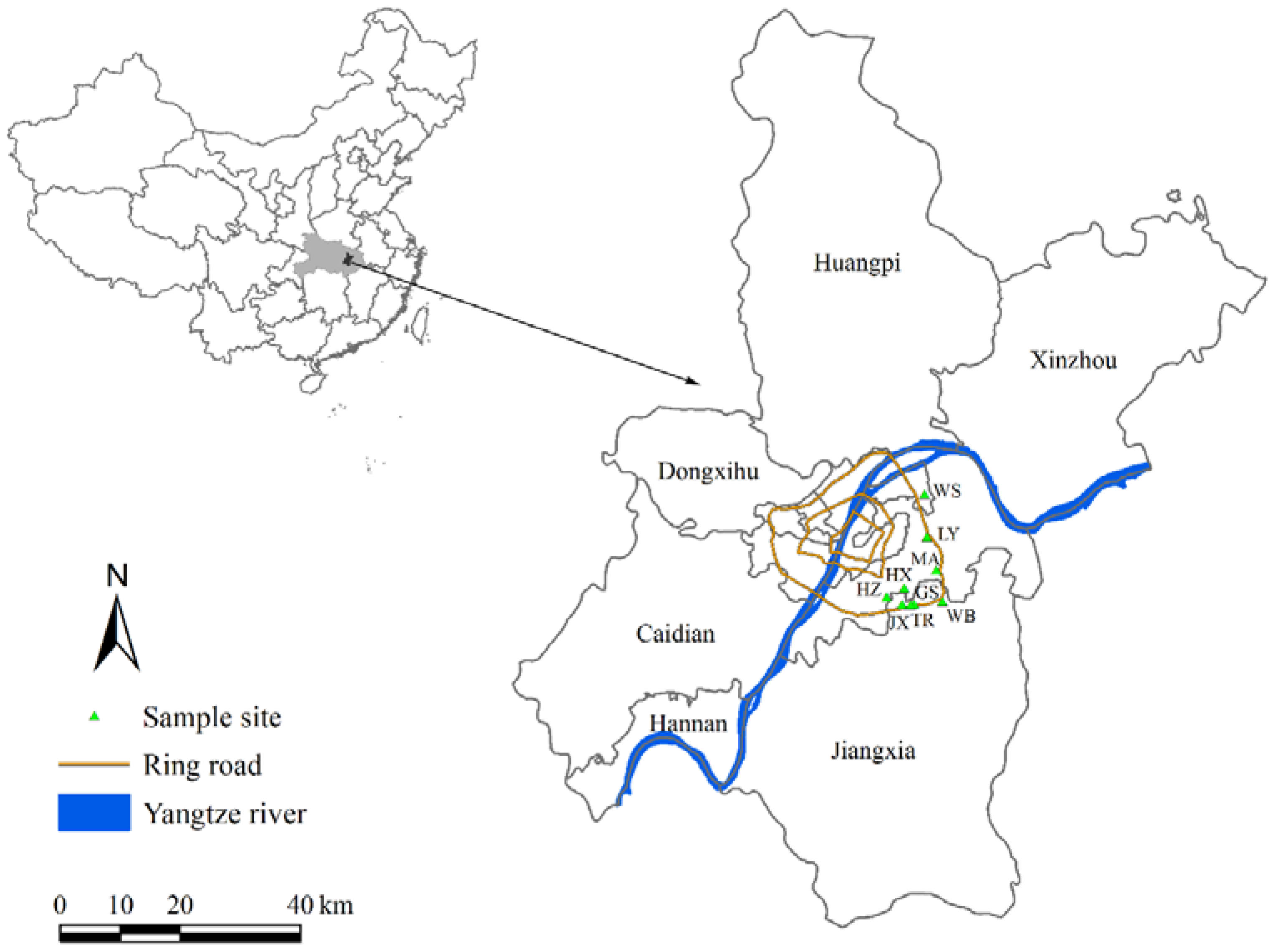
| Site No. | Location (Abbreviation) | Function and Land Use Description | Longitude/E | Latitude/N | Altitude (m) |
|---|---|---|---|---|---|
| 1 | Huazhong Agricultural University (HZ) | Education, University campus | 114°21.682′ | 30°28.625′ | 60 |
| 2 | Hongxiang (HX) | Residential land, residential area | 114°23.588′ | 30°29.285′ | 58 |
| 3 | Jinxiu (JX) | Residential land, residential area | 114°23.223′ | 30°27.859′ | 48 |
| 4 | Luoyan (LY) | Scenic spot, park nearby East Lake | 114°26.633′ | 30°33.678′ | 33 |
| 5 | Ma’anshan Park (MA) | Scenic spot, park nearby East Lake | 114°27.139′ | 30°30.644′ | 41 |
| 6 | Third Ring Road (TR) | Transportation, roadside of urban expressway | 114°24.093′ | 30°27.729′ | 63 |
| 7 | Guanshan Overpass (GS) | Transportation, roadside of urban overpass | 114°24.439′ | 30°27.805′ | 59 |
| 8 | Wuhan Steel Works (WS) | Industry, manufacturing industry of iron and steel | 114°26.638′ | 30°37.481′ | 38 |
| 9 | Wuhan Boiler Works (WB) | Industry, manufacturing industry of boilers | 114°27.456′ | 30°27.748′ | 39 |
| Sample | Value (μg g−1) | Ag | As | Cd | Co | Cr | Cu | Mn | Mo | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. angustifolium | Minimum | 0.074 | 3.04 | 0.433 | 1.85 | 6.37 | 19.9 | 127 | 277 | 2.99 | 17.3 | 15.3 | 86.4 |
| Maximum | 0.258 | 6.69 | 1.20 | 5.22 | 27.0 | 44.9 | 700 | 491 | 6.17 | 61.3 | 32.2 | 169 | |
| Mean | 0.127 | 4.20 | 0.660 | 4.14 | 13.3 | 25.6 | 300 | 338 | 4.97 | 28.0 | 24.8 | 114 | |
| SD | 0.073 | 1.33 | 0.27 | 1.17 | 6.67 | 8.94 | 189 | 79.2 | 1.05 | 16.6 | 6.39 | 31.1 | |
| Diff | a | a | a | a | a | a | a | a | a | a | a | a | |
| C. bodinieri | Minimum | 0.004 | 0.156 | 0.025 | 0.047 | 0.277 | 3.54 | 39.3 | 17.7 | 0.326 | 1.179 | 0.306 | 8.47 |
| Maximum | 0.021 | 0.871 | 0.092 | 0.345 | 1.29 | 7.41 | 393 | 75.7 | 0.864 | 3.738 | 2.553 | 16.9 | |
| Mean | 0.008 | 0.317 | 0.050 | 0.108 | 0.579 | 5.23 | 120 | 41.1 | 0.549 | 2.361 | 0.735 | 13.4 | |
| SD | 0.006 | 0.222 | 0.023 | 0.091 | 0.339 | 1.35 | 122 | 17.9 | 0.185 | 0.940 | 0.695 | 3.32 | |
| Diff | b | b | b | b | b | b | b | b | b | b | b | b | |
| O. fragrans | Minimum | 0.002 | 0.111 | 0.090 | 0.120 | 0.357 | 3.96 | 13.5 | 42.9 | 0.316 | 1.52 | 0.369 | 17.5 |
| Maximum | 0.018 | 0.318 | 0.358 | 0.330 | 0.759 | 7.17 | 223 | 251 | 0.976 | 10.6 | 0.830 | 40.9 | |
| Mean | 0.009 | 0.196 | 0.182 | 0.224 | 0.538 | 5.14 | 107 | 144 | 0.562 | 3.59 | 0.575 | 27.1 | |
| SD | 0.005 | 0.079 | 0.101 | 0.057 | 0.130 | 1.04 | 75.0 | 71.4 | 0.202 | 3.09 | 0.137 | 7.57 | |
| Diff | b | b | c | b | b | b | b | c | b | b | b | b | |
| Soil | Minimum | 0.064 | 21.5 | 0.113 | 12.5 | 42.4 | 25.1 | 386 | 329 | 16.4 | 28.0 | 102 | 55.9 |
| Maximum | 0.217 | 23.8 | 0.301 | 19.0 | 54.7 | 32.0 | 1035 | 549 | 21.2 | 41.4 | 138 | 81.6 | |
| Mean | 0.107 | 22.7 | 0.185 | 16.4 | 49.3 | 28.0 | 667 | 387 | 18.4 | 32.7 | 124 | 67.8 | |
| SD | 0.047 | 0.876 | 0.056 | 2.14 | 3.56 | 2.54 | 190 | 74 | 1.43 | 4.51 | 10.9 | 9.69 | |
| Diff | a | c | c | c | c | a | c | a | c | a | c | c |
| Metal | H. angustifolium/C. bodinieri | H. angustifolium/O. fragrans |
|---|---|---|
| Ag | 19.8 ± 9.79 | 46.0 ± 18.6 |
| As | 17.4 ± 9.89 | 25.0 ± 11.8 |
| Cd | 15.3 ± 7.76 | 5.05 ± 3.57 |
| Co | 51.3 ± 25.2 | 20.0 ± 7.25 |
| Cr | 29.9 ± 21.3 | 27.8 ± 14.8 |
| Cu | 5.17 ± 1.97 | 5.27 ± 1.18 |
| Mn | 4.08 ± 2.18 | 6.54 ± 5.92 |
| Mo | 10.4 ± 6.62 | 3.30 ± 2.20 |
| Ni | 10.3 ± 4.75 | 10.7 ± 4.05 |
| Pb | 13.6 ± 8.58 | 12.3 ± 10.4 |
| V | 48.5 ± 28.1 | 48.3 ± 19.9 |
| Zn | 9.38 ± 4.94 | 4.78 ± 1.35 |
| Metal | Ag | As | Cd | Co | Cr | Cu | Mn | Mo | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (r) | 0.332 | −0.567 | 0.384 | 0.707 | 0.168 | 0.040 | 0.941 | 0.101 | 0.218 | −0.190 | −0.016 | −0.042 |
| p | 0.384 | 0.112 | 0.307 | 0.033 | 0.665 | 0.919 | 0.000 | 0.797 | 0.573 | 0.624 | 0.967 | 0.916 |
| Metal | Ag | As | Cd | Co | Cr | Cu | Mn | Mo | Ni | Pb | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.340 | ||||||||||
| Cd | 0.753 * | 0.218 | |||||||||
| Co | 0.578 | 0.536 | −0.048 | ||||||||
| Cr | 0.217 | 0.591 | −0.231 | 0.778 * | |||||||
| Cu | 0.842 ** | 0.642 | 0.767 * | 0.471 | 0.153 | ||||||
| Mn | 0.659 | 0.665 | 0.220 | 0.848 ** | 0.551 | 0.700 * | |||||
| Mo | 0.381 | 0.811 ** | 0.399 | 0.320 | 0.500 | 0.610 | 0.525 | ||||
| Ni | 0.351 | 0.721 * | −0.162 | 0.890 ** | 0.897 ** | 0.353 | 0.715 * | 0.456 | |||
| Pb | 0.983 ** | 0.302 | 0.774 * | 0.524 | 0.148 | 0.843 ** | 0.680 * | 0.390 | 0.282 | ||
| V | 0.064 | 0.714 * | −0.373 | 0.727 * | 0.700 ** | 0.225 | 0.551 | 0.324 | 0.835 ** | −0.034 | |
| Zn | 0.674 * | 0.402 | 0.537 | 0.395 | 0.203 | 0.634 | 0.314 | 0.255 | 0.457 | 0.610 | 0.190 |
| Metal | Factor 1 | Factor 2 |
|---|---|---|
| Ag | 0.818 | −0.440 |
| As | 0.854 | 0.310 |
| Cd | 0.712 | −0.659 |
| Co | 0.754 | 0.381 |
| Cr | 0.310 | 0.804 |
| Cu | 0.924 | −0.312 |
| Mn | 0.912 | −0.095 |
| Mo | 0.811 | 0.178 |
| Ni | 0.634 | 0.724 |
| Pb | 0.855 | −0.461 |
| V | 0.323 | 0.834 |
| Zn | 0.593 | −0.127 |
| Variance (%) | 54.2 | 25.9 |
| Metal | Ag | As | Cd | Co | Cr | Cu | Mn | Mo | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HZ | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 2.47 | 1.00 | 1.00 | 1.00 | 1.00 |
| HX | 1.58 | 1.43 | 0.85 | 3.54 | 2.00 | 1.31 | 2.49 | 2.35 | 1.66 | 1.44 | 1.80 | 1.17 |
| JX | 1.40 | 1.14 | 0.81 | 1.89 | 2.22 | 0.95 | 1.74 | 2.41 | 1.64 | 1.38 | 1.21 | 1.32 |
| LY | 1.20 | 1.02 | 0.62 | 2.13 | 1.55 | 1.05 | 2.09 | 2.18 | 1.52 | 1.26 | 1.39 | 1.19 |
| MA | 1.15 | 1.28 | 0.58 | 2.21 | 1.64 | 1.00 | 2.29 | 2.29 | 1.61 | 1.13 | 1.98 | 1.01 |
| TR | 1.33 | 1.65 | 0.74 | 2.17 | 2.10 | 1.19 | 1.62 | 2.58 | 1.96 | 1.13 | 2.11 | 1.95 |
| GS | 3.47 | 2.20 | 1.58 | 2.83 | 2.05 | 2.14 | 5.51 | 3.86 | 1.90 | 3.55 | 1.64 | 1.70 |
| WS | 3.19 | 1.04 | 1.08 | 2.79 | 1.96 | 1.31 | 2.13 | 2.41 | 1.58 | 2.66 | 1.37 | 1.54 |
| WB | 1.11 | 1.67 | 0.57 | 2.71 | 4.24 | 1.03 | 2.33 | 3.40 | 2.06 | 1.04 | 2.11 | 1.00 |
| Mean CF | 3.47 | 2.20 | 1.58 | 3.54 | 4.24 | 2.14 | 5.51 | 3.86 | 2.06 | 3.55 | 2.11 | 1.95 |
| Category | C2 | C2 | C1 | C3 | C3 | C2 | C3 | C2 | C2 | C2 | C2 | C2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Fan, M.; Hu, R.; Zhao, J.; Wu, Y. Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas. Int. J. Environ. Res. Public Health 2018, 15, 1105. https://doi.org/10.3390/ijerph15061105
Jiang Y, Fan M, Hu R, Zhao J, Wu Y. Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas. International Journal of Environmental Research and Public Health. 2018; 15(6):1105. https://doi.org/10.3390/ijerph15061105
Chicago/Turabian StyleJiang, Yanbin, Miao Fan, Ronggui Hu, Jinsong Zhao, and Yupeng Wu. 2018. "Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas" International Journal of Environmental Research and Public Health 15, no. 6: 1105. https://doi.org/10.3390/ijerph15061105
APA StyleJiang, Y., Fan, M., Hu, R., Zhao, J., & Wu, Y. (2018). Mosses Are Better than Leaves of Vascular Plants in Monitoring Atmospheric Heavy Metal Pollution in Urban Areas. International Journal of Environmental Research and Public Health, 15(6), 1105. https://doi.org/10.3390/ijerph15061105






