Study on Cr(VI) Leaching from Cement and Cement Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Cements
2.2. Cement Composites
2.3. Leaching Experiments
2.4. Analytical Methods
3. Results and Discussion
3.1. Chemical Compositions of the OPCs and Cement Composites
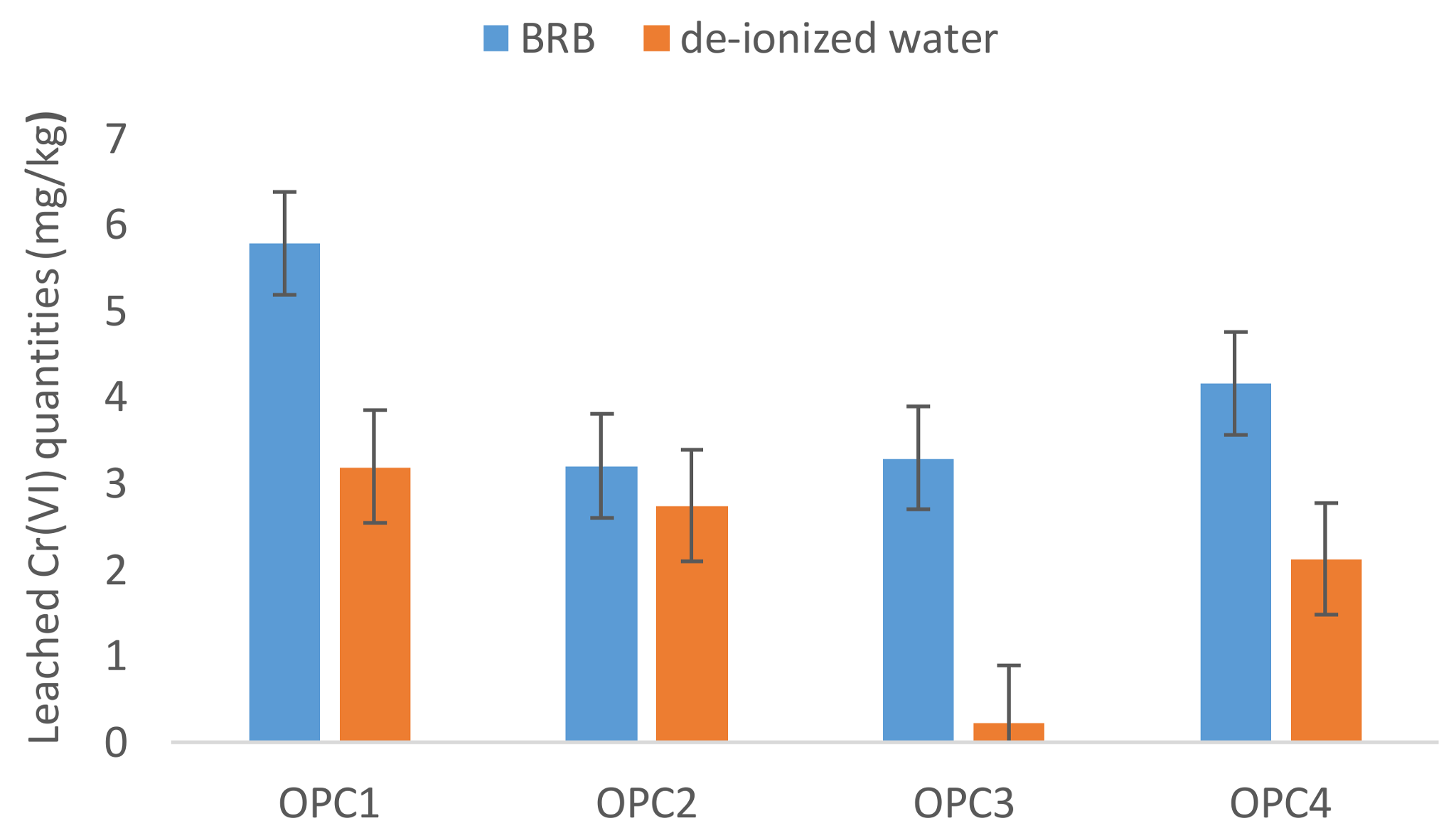
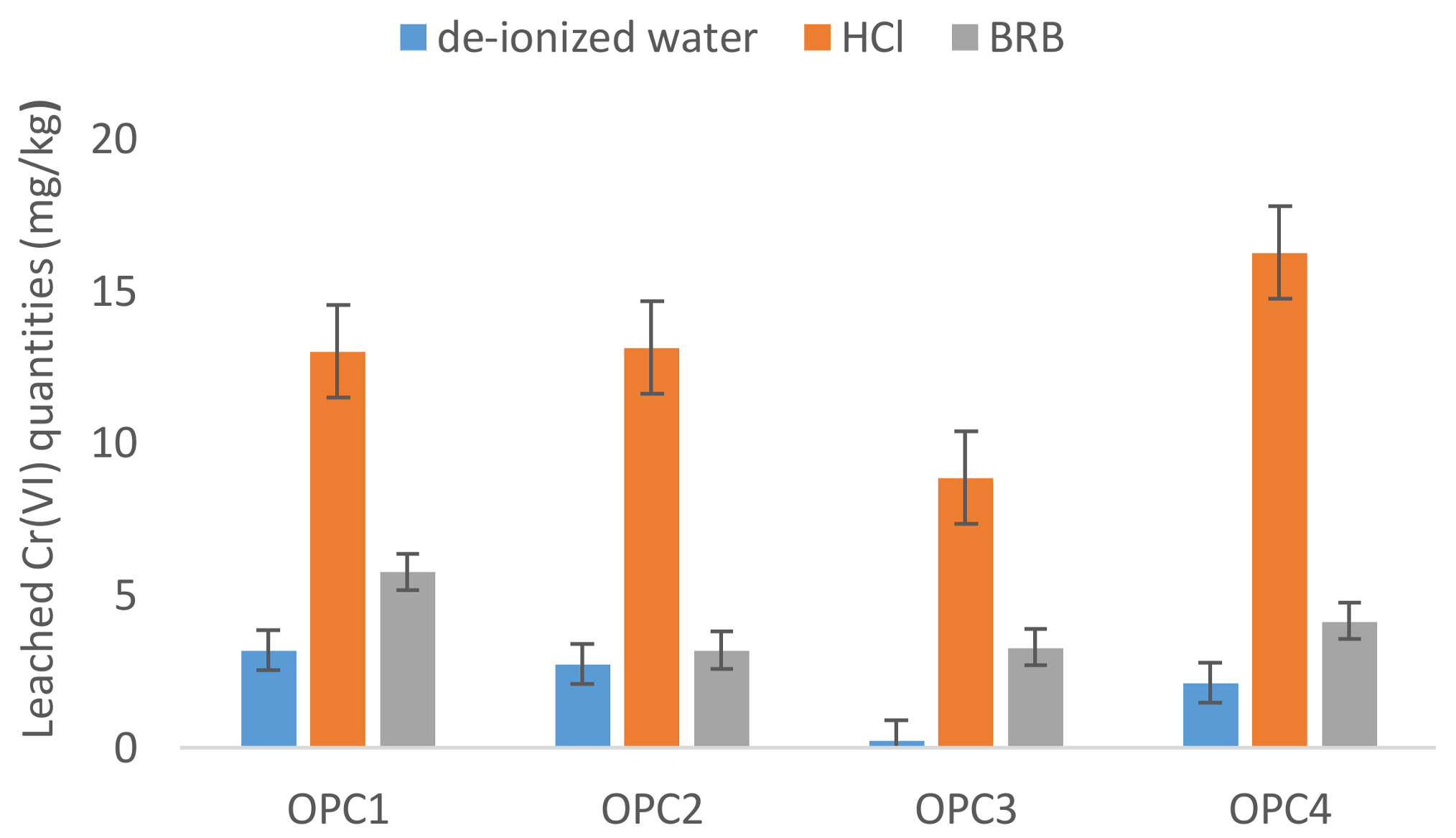
3.2. Influence of the Leaching Agent on Hexavalent Chromium Leaching
3.2.1. Deionized Water
3.2.2. BRB
3.2.3. HCl
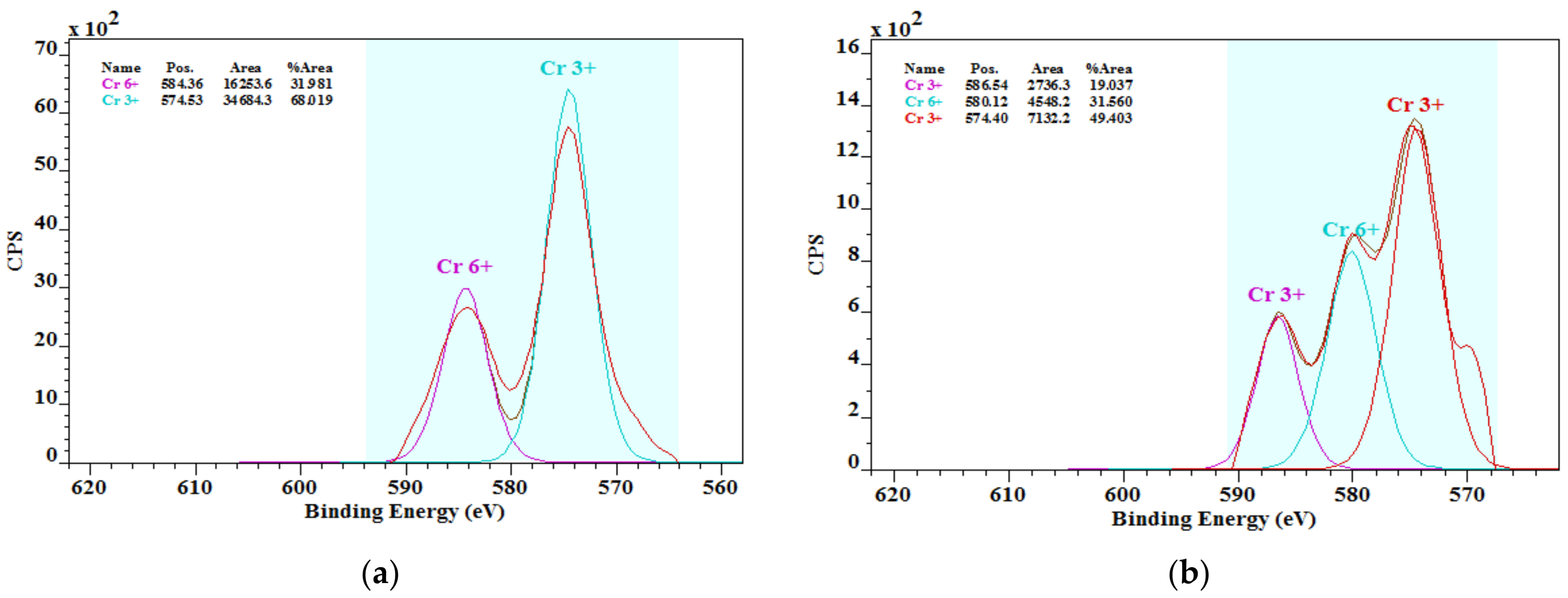
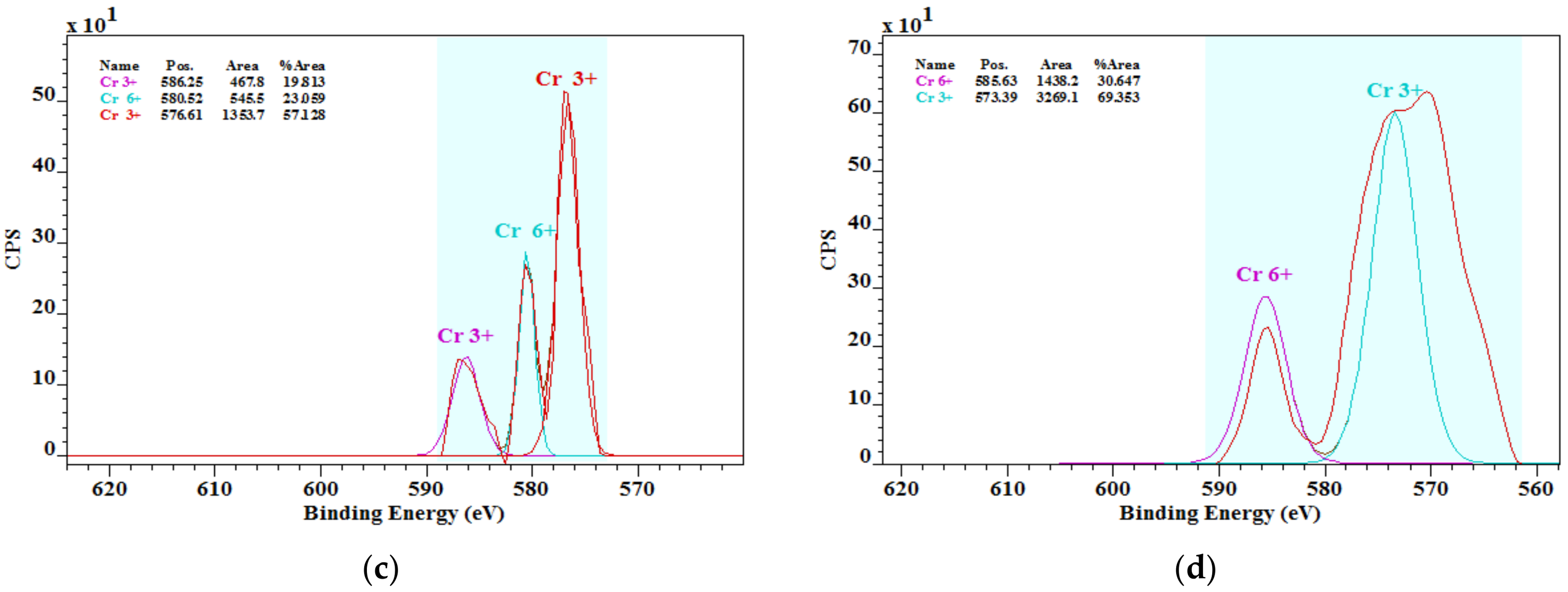
3.3. Chromium Species in Cements
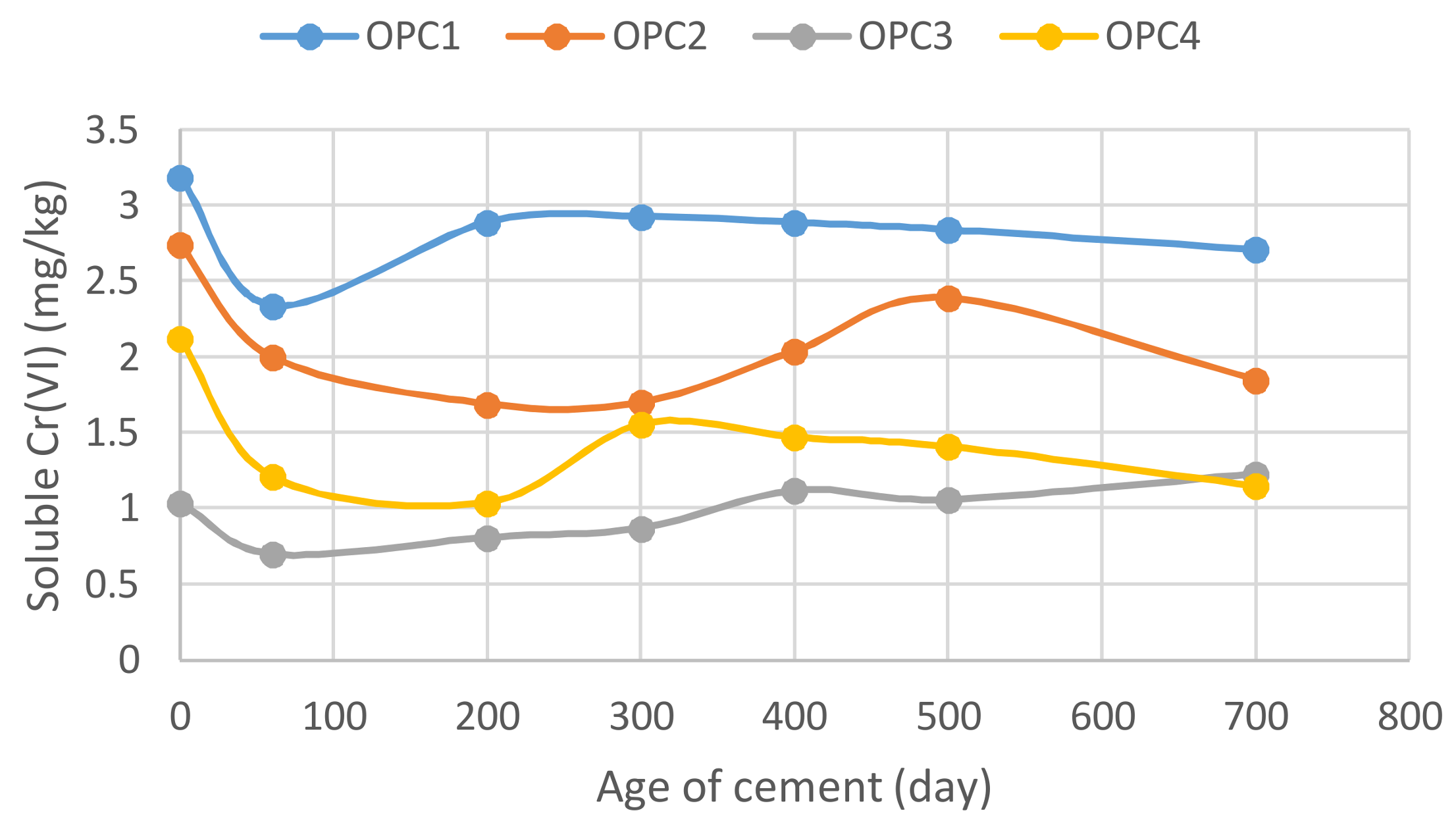
3.4. Dependence of Hexavalent Chromium Leaching on the Cement Age
4. Conclusions
- The OPCs average value of Cr(VI) dissolved in deionized water was lower than those reported in the literature in the past, which indicated that most manufacturers use reduction agents in the cement manufacturing process now. In spite of that, the EU limit value of 2 mg/kg for water-soluble Cr(VI) was exceeded in 27% of the analysed OPCs.
- The current recommended procedures for testing water-soluble chromium using extraction by deionized water seem not to predict the real portion of Cr(VI) dissolved from cement materials into the environment.
- The literature knowledge that the chromium in cements is mostly in the chromate form was not confirmed. In addition, the presence of chromium chromate Cr2(CrO4)3 was found by the XPS analysis. More studies in this field on chromium species and their solubility are important.
- The assumption of the lower Cr(VI) leaching rate of monolithic concrete blocks compared to that of the OPCs was confirmed. The increased leaching in deionized water of chromium from cement composites with silica fume and zeolite was confirmed. Cementitious materials made of OPC and secondary materials show very systematic leaching behaviours.
- The prediction of leachability from the tank leaching test could be a basis for geochemical modelling.
- A minimum concentration was observed for all cement samples when studying the relationship between the soluble Cr(VI) and cement storage time.
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Emsley, J. Chromium. In Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Roskovic, R.; Oslakovic, I.S.; Radic, J.; Serdar, M. Effects of chromium (VI) reducing agents in cement on corrosion of reinforcing steel. Cem. Concr. Compos. 2011, 33, 1020–1025. [Google Scholar] [CrossRef]
- Laforest, G.; Duchesne, J. Immobilization of chromium (VI) evaluated by binding isotherms for ground granulated blast furnace slag and ordinary Portland cement. Cem. Concr. Res. 2005, 35, 2322–2332. [Google Scholar] [CrossRef]
- Potgieter, S.S.; Panichev, N.; Potgieter, J.H.; Panicheva, S. Determination of hexavalent chromium in South African cements and cement-related materials with electrothermal atomic absorption spectrometry. Cem. Concr. Res. 2003, 33, 1589–1593. [Google Scholar] [CrossRef]
- Frías, M.; Sánchez De Rojas, M.I. Total and soluble chromium, nickel and cobalt content in the main materials used in the manufacturing of Spanish commercial cements. Cem. Concr. Res. 2002, 32, 435–440. [Google Scholar] [CrossRef]
- Barceloux, D.G. Chromium. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef]
- Guttmann, D.; Poage, G.; Johnston, T.; Zhitkovich, A. Reduction with glutathione is a weakly mutagenic pathway in chromium (VI) metabolism. Chem. Res. Toxicol. 2008, 21, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Wu, J.D.; Sheu, S.C.; Shih, T.S.; Chang, H.Y.; Guo, Y.L.; Wang, Y.-J.; Chou, T.C. Occupational hand dermatitis among cement workers in Taiwan. J. Formos. Med. Assoc. 2011, 110, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Eugeniusz, S. Chromium in Portland cement. Cem. Wapno Gips 1985, 1, 1–16. [Google Scholar]
- Sazakli, E.; Villanueva, C.M.; Kogevinas, M.; Maltezis, K.; Mouzaki, A.; Leotsinidis, M. Chromium in drinking water: Association with biomarkers of exposure and effect. Int. J. Environ. Res. Public Health 2014, 11, 10125–10145. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Xiao, J.; Wang, Y.; Chen, L. Assessment of ecological and human health risks of heavy metal contamination in agriculture soils disturbed by pipeline construction. Int. J. Environ. Res. Public Health 2014, 11, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Van der Sloot, H.A.; Meeussen, J.C.L.; Garrabrants, A.C.; Kosson, D.S.; Fuhrmann, M. Review of the Physical and Chemical Aspects of Leaching Assessment; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2009. [Google Scholar]
- Van der Sloot, H.A.; Van Zomeren, A.; Meeussen, J.C.L.; Hoede, D.; Rietra, R.P.J.J.; Stenger, R.; Lang, T.; Schneider, M.; Spanka, G.; Stoltenberg-Hansson, E.; Lerat, A.; Dath, P. Environmental Criteria for Cement Based Products; ECRICEM: Phase I: Ordinary Portland Cements, Phase II: Blended Cements: ECN: E-Series; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2011. [Google Scholar]
- Lackovic, J.A.; Nikolaidis, N.P.; Cheeda, P.; Carley, R.J.; Patton, E. Evaluation of batch leaching procedures for estimating metal mobility in glaciated soils. Groundw. Monit. Retried 1997, 17, 231–240. [Google Scholar] [CrossRef]
- Bureau for Standardisation. Methods of Testing Cement—Part 10: Determination of the Water Soluble Chromium (VI) Content of Cement; EN 196-10; Bureau for Standardisation: Brussels, Belgium, 2006. [Google Scholar]
- European Commission. Development of a Leaching Method for the Determination of the Environmental Quality of Concrete; EUR 17869 EN; European Commission: Brussels, Belgium, 1997. [Google Scholar]
- Bobirica, C.; Bobirica, L.; Stănescu, R.; Constantinescu, I. Leaching behavior of cement-based solidified wastes containing hexavalent chromium. UPB Sci. Bull. Ser. B 2010, 72, 121–128. [Google Scholar]
- Yamaguchi, O.; Ida, M.; Uchiyama, Y.; Hanehara, S. A method for the determination of total Cr(VI) in cement. J. Eur. Ceram. Soc. 2006, 26, 785–790. [Google Scholar] [CrossRef]
- Menéndez, E.; Alvaro, A.M.; Hernández, M.T.; Parra, J.L. New methodology for assessing the environmental burden of cement mortars with partial replacement of coal bottom ash and fly ash. J. Environ. Manag. 2014, 133, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Sharma, R. Reduction of water soluble hexavalent chromium in hydrated Portland cement. Indian J. Sci. Technol. 2015, 8, 56862. [Google Scholar] [CrossRef][Green Version]
- Mohammadi, J.; South, W. Measuring chromium in general purpose (GP) cement. J. Test Eval. 2016, 45, 1362–1377. [Google Scholar] [CrossRef]
- Strigáč, J.; Števulová, N. Influence of Alternative Fuels and Secondary Materials to Clinker´s and Cement´s Quality; Technical University of Kosice: Košice, Slovakia, 2016. (In Slovak) [Google Scholar]
- Frías, M.; Sánchez De Rojas, M.I. Determination and quantification of total chromium and water soluble chromium contents in commercial cements. Cem. Concr. Res. 1995, 25, 433–439. [Google Scholar] [CrossRef]
- Lu, H.; Wei, F.; Tang, J.; Giesy, J.P. Leaching of metals from cement under simulated environmental conditions. J. Environ. Manag. 2016, 169, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Strigáč, J.; Martauz, P. Fungistatic properties of granulated blastfurnace slag and related slag-containing cements. Mater. Constr. 2016, 66, e087. [Google Scholar] [CrossRef]
- Tandon, R.; Aarts, B. Chromium, nickel and cobalt contents of some Australian cements. Contact Dermat. 1993, 28, 201–205. [Google Scholar] [CrossRef]
- Directive 2003/53/EC of the European Parliament and of the Council of 18 June 2003 Amending for the 26th Time Council Directive 76/769/EEC relating to Restrictions on the Marketing and use of Certain Dangerous Substances and Preparations (Nonylphenol, Nonylphenol Ethoxylate and Cement). Directive 2003/53/EC. 2003.
- Eštoková, A.; Palaščáková, L.; Singovszká, E.; Holub, M. Analysis of the chromium concentrations in cement materials. Procedia Eng. 2012, 42, 146–154. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, J.E.; Lai, Y.C.; Ko, M.S. Effects of sintering atmosphere on cement clinkers produced from chromium-bearing sludge. J. Air Waste Manag. Assoc. 2012, 62, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.M.C.; Tang, C.I.; Li, X.D.; Poon, C.S. Leaching and microstructural analysis of cement-based solidified wastes. Environ. Sci. Technol. 2000, 34, 5038–5042. [Google Scholar] [CrossRef]
- Ogunbileje, J.O.; Sadagoparamanujam, V.M.; Anetor, J.I.; Farombi, E.O.; Akinosun, O.M.; Okorodudu, A.O. Lead, mercury, cadmium, chromium, nickel, copper, zinc, calcium, iron, manganese and chromium (VI) levels in Nigeria and United States of America cement dust. Chemosphere 2013, 90, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Bentaieb, N.; Lachemet, A.; Zirour, R.; Belaadi, S.; Frances, C. Thermal analysis of composite cements. Chem. Eng. Trans. 2011, 24, 685–690. [Google Scholar]
- European Commission, Joint Research Centre, Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide, 2013. Available online: http://eippcb.jrc.ec.europa.eu/reference/BREF/CLM_Published_def.pdf (accessed on 10 March 2018).
- Sinyoung, S.; Songsiriritthigul, P.; Asavapisit, S.; Kajitvichyanukul, P. Chromium behavior during cement-production processes: A clinkerization, hydration, and leaching study. J. Hazard. Mater. 2011, 191, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, A.; Quast, K.; Xu, D.F.; Skinner, W.; Robinson, D.; Addai-Mensah, J. Agglomeration and column leaching behaviour of nickel laterite ores: Effect of ore mineralogy and particle size distribution. Hydrometallurgy 2014, 146, 29–39. [Google Scholar] [CrossRef]
- Rodrfguez-Piñero, M.; Fernández Pereira, C.; Ruiz de Elvira Francoy, C.; Vale Parapar, J.F. Stabilization of a chromium-containing solid waste: Immobilization of hexavalent chromium. J. Air Waste Manag. Assoc. 1998, 48, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Nagataki, S.; Jinmei, L.; Saeki, T.; Hisada, M. The leachability of heavy metals in hardened fly ash cement and cement-solidified fly ash. Cem. Concr. Res. 2005, 35, 1056–1063. [Google Scholar] [CrossRef]
- Lee, J.F.; Bait, S.; Clark, S.B.; Lamble, G.M.; Langton, C.A.; Oji, L. Chromium speciation in hazardous, cement-based waste forms. Physica B 1995, 208–209, 577–578. [Google Scholar] [CrossRef]
- Iacobescu, R.I.; Koumpouri, D.; Pontike, Y.; Angelopoulos, G.N. Hydraulic and leaching behaviour of belite cements produced with electric arc furnace steel slag as raw material. Ceram. Silikáty 2013, 57, 126–132. [Google Scholar]
- Vaity, R.S.; Verma, J.K. Stability study and impact of the Cr(VI) reducing additives on cement performance. Chem. Miner. Res. 2013, 3, 102–113. [Google Scholar]

| Cement Composite | Components Per 1 m3 of Cement Composite | |||||||
|---|---|---|---|---|---|---|---|---|
| Cement (kg) | Water (L) | Silica Fume (kg) | Zeolite (kg) | Aggregate (kg) | Plasticizer (L) | |||
| 0/4 mm | 4/8 mm | 8/16 mm | ||||||
| K1 | 360 | 170 | - | - | 825 | 235 | 740 | 3.1 |
| K2 | 360 | 197 | 20 | - | 800 | 235 | 740 | 3.1 |
| K3 | 360 | 205 | 20 | 20 | 750 | 235 | 740 | 3.1 |
| Sample | Elements in Oxide Form (wt. %) | Cr (Total) (mg/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO2 | MnO | ||
| OPC1 | 58.2 | 19.6 | 4.4 | 3.3 | 3.2 | 3.8 | 0.6 | 0.2 | 0.4 | 178.5 |
| OPC2 | 54.2 | 17.8 | 4.1 | 2.6 | 3.3 | 1.5 | 1.2 | 0.2 | 0.3 | 173.2 |
| OPC3 | 63.6 | 19.8 | 3.9 | 2.7 | 3.1 | 2.1 | 0.5 | 0.2 | 0.3 | 210.1 |
| OPC4 | 63.9 | 19.2 | 4.2 | 2.4 | 3.2 | 2.0 | 0.7 | 0.2 | 0.3 | 218.5 |
| K1 | 31.3 | 30.2 | 5.2 | 4.0 | 2.9 | 3.0 | 0.8 | 0.3 | 0.4 | 180.4 |
| K2 | 26.2 | 45.6 | 5.4 | 3.4 | 2.7 | 2.7 | 0.8 | 0.3 | 0.4 | 233.5 |
| K3 | 25.1 | 39.8 | 5.3 | 4.6 | 2.8 | 2.4 | 0.8 | 0.3 | 0.4 | 485.0 |
| Sample | Cr (VI) Concentrations (mg/kg) | Cr (VI)/Cr (Total) (%) | ||||
|---|---|---|---|---|---|---|
| Number of Leachates | Min | Max | Mean | Standard Deviation | ||
| OPCs | 12 | 0.23 | 3.19 | 2.10 | 0.87 | 0.8–1.79 |
| Composites | 12 | |||||
| K1 | 4 | 0.39 | 0.55 | 0.41 | 0.08 | 0.26 |
| K2 | 4 | 0.80 | 1.04 | 0.85 | 0.18 | 0.36 |
| K3 | 4 | 1.25 | 1.44 | 1.36 | 0.25 | 0.28 |
| Sample | Cr(VI) Concentrations (mg/kg) | Cr(VI)/Cr (Total) (%) | ||||
|---|---|---|---|---|---|---|
| Number of Leachates | Min | Max | Mean | Standard Deviation | ||
| OPCs | 12 | 8.88 | 16.25 | 12.8 | 7.58 | 1.46–9.45 |
| Composites | 12 | |||||
| K1 | 4 | 2.10 | 4.47 | 2.70 | 2.02 | 1.50 |
| K2 | 4 | 1.10 | 3.51 | 1.43 | 1.08 | 0.61 |
| K3 | 4 | 1.43 | 2.45 | 1.73 | 0.90 | 0.36 |
| Sample | Concentration of Chromium Species (%) | |
|---|---|---|
| Cr(III) | Cr(VI) | |
| OPC1 | 68.09 | 31.91 |
| OPC2 | 68.44 | 31.56 |
| OPC3 | 76.94 | 23.06 |
| OPC4 | 69.35 | 30.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estokova, A.; Palascakova, L.; Kanuchova, M. Study on Cr(VI) Leaching from Cement and Cement Composites. Int. J. Environ. Res. Public Health 2018, 15, 824. https://doi.org/10.3390/ijerph15040824
Estokova A, Palascakova L, Kanuchova M. Study on Cr(VI) Leaching from Cement and Cement Composites. International Journal of Environmental Research and Public Health. 2018; 15(4):824. https://doi.org/10.3390/ijerph15040824
Chicago/Turabian StyleEstokova, Adriana, Lenka Palascakova, and Maria Kanuchova. 2018. "Study on Cr(VI) Leaching from Cement and Cement Composites" International Journal of Environmental Research and Public Health 15, no. 4: 824. https://doi.org/10.3390/ijerph15040824
APA StyleEstokova, A., Palascakova, L., & Kanuchova, M. (2018). Study on Cr(VI) Leaching from Cement and Cement Composites. International Journal of Environmental Research and Public Health, 15(4), 824. https://doi.org/10.3390/ijerph15040824






