Immune Responses to Dengue and Zika Viruses—Guidance for T Cell Vaccine Development
Abstract
1. History
2. Antibody Cross-Reactivity between Zika and Dengue Viruses
3. T Cell Responses against DENV and Prospects for a Vaccine
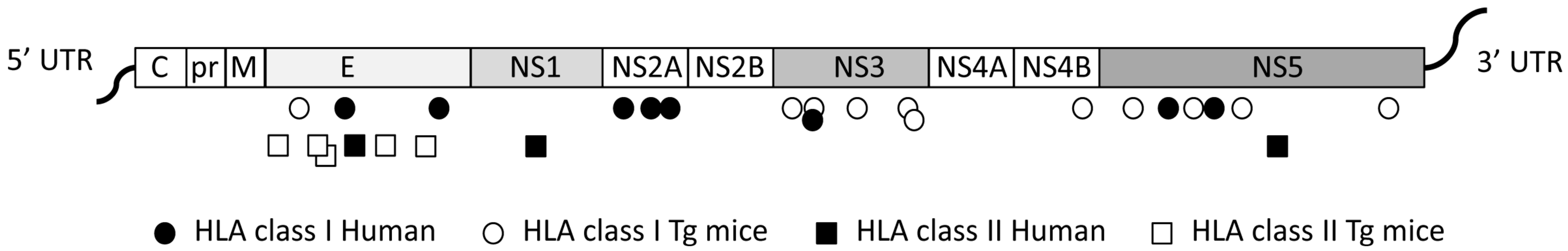
4. Identification ZIKV-Specific and DENV/ZIKV Cross-Reactive T Cells
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika virus, French polynesia, South pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika virus outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Rouzeyrol, M.; O’Connor, O.; Calvez, E.; Daurès, M.; John, M.; Grangeon, J.-P.; Gourinat, A.-C. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg. Infect. Dis. 2015, 21, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, O.; Beltrán, M.; Nelson, C.A.; Valencia, D.; Tolosa, N.; Farr, S.L.; Padilla, A.V.; Tong, V.T.; Cuevas, E.L.; Espinosa-Bode, A.; et al. Zika virus disease in Colombia—Preliminary report. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastère, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Soares De Araujo, J.S.; Teixeira Regis, C.; Silva Gomes, R.G.; Riberio Tavares, T.; Rocha dos Santos, C.; Melo Assunção, P.; Nóbrega, R.V.; Alves Pinto, D.F.; Dantas Bezerra, B.V.; da Silva Mattos, S. Microcephaly in north-east Brazil: A retrospective study on neonates born between 2012 and 2015. Bull. World Health Organ. 2016, 94, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Enfissi, A.; Codrington, J.; Roosblad, J.; Kazanji, M.; Rousset, D. Zika virus genome from the Americas. Lancet 2016, 387, 227–228. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.-F.; Zhang, R.; Wang, T.; Qin, C.-F.; et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Huang, X.-Y.; Liu, Z.-Y.; Zhang, F.; Zhu, X.-L.; Yu, J.-Y.; Ji, X.; Xu, Y.-P.; Li, G.; Li, C.; et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 2017, 358, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvniski, A.; Vaney, M.-C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Haouz, A.; et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 A resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, H.; Qi, J.; Liu, Y.; Wang, H.; Su, C.; Shi, Y.; Gao, G.F. Contribution of intertwined loop to membrane association revealed by Zika virus full-length NS1 structure. EMBO J. 2016, 35, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.V.; Harris, E. Recent advances in understanding the adaptive immune response to Zika virus and the effect of previous flavivirus exposure. Virus Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.F.; Goodwin, E.C.; Briney, B.; Sok, D.; Beutler, N.; Strubel, A.; Nedellec, R.; Le, K.; Brown, M.E.; Burton, D.R.; et al. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Hudson, W.; Ahmed, R.; Wrammert, J. Humoral cross-reactivity between Zika and dengue viruses: Implications for protection and pathology. Emerg. Microbes Infect. 2017, 6, e33. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Vaughan, K.; Weiskopf, D.; Grifoni, A.; Diamond, M.S.; Sette, A.; Peters, B. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Nisalak, A.; Anderson, K.B.; Libraty, D.H.; Kalayanarooj, S.; Vaughn, D.W.; Putnak, R.; Gibbons, R.V.; Jarman, R.; Endy, T.P. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 2009, 81, 825–833. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, R.; de Silva, A.M. Measuring antibody neutralization of dengue virus (DENV) using a flow cytometry-based technique. Methods Mol. Biol. 2014, 1138, 27–39. [Google Scholar] [PubMed]
- Calvo, E.P.; Sanchez-Quete, F.; Duran, S.; Sandoval, I.; Castellanos, J.E. Easy and inexpensive molecular detection of dengue, chikungunya and zika viruses in febrile patients. Acta Trop. 2016, 163, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.-L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg. Inf. Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.P.B.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E.; et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar] [PubMed]
- Patel, B.; Longo, P.; Miley, M.J.; Montoya, M.; Harris, E.; de Silva, A.M. Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Neglect. Trop. Dis. 2017, 11, e0005554. [Google Scholar] [CrossRef] [PubMed]
- Olkowski, S.; Forshey, B.M.; Morrison, A.C.; Rocha, C.; Vilcarromero, S.; Halsey, E.S.; Kochel, T.J.; Scott, T.W.; Stoddard, S.T. Reduced risk of disease during postsecondary dengue virus infections. J. Infect. Dis. 2013, 208, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; Macagno, A.; Simonelli, L.; Ha Quyen, N.T.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; de Silva, A.M.; et al. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Zhou, Y.; Olivarez, N.P.; Broadwater, A.H.; de Silva, A.M.; Crowe, J.E., Jr. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 2012, 86, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Cho, A.; Onlamoon, N.; Zheng, N.-Y.; Huang, M.; Kovalenkov, Y.; Chokephaibulkit, K.; Angkasekwinai, N.; Pattanapanyasat, K.; Ahmed, R.; et al. B cell responses during secondary dengue virus infection are dominated by highly cross-reactive, memory-derived plasmablasts. J. Virol. 2016, 90, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Ros, S.; Nogueira, L.; Patel, R.; et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017, 169, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Puschnik, A.; Lau, L.; Cromwell, E.A.; Balmaseda, A.; Zompi, S.; Harris, E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS Neglect. Trop. Dis. 2013, 7, e2274. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Katzelnick, L.; Tissera, H.; Amerasinghe, A.; Dharshan de Silva, A.; de Silva, A.M. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J. Infect. Dis. 2015, 211, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Clapham, H.E.; Rodriguez-Barraquer, I.; Azman, A.S.; Althouse, B.M.; Salje, H.; Gibbons, R.V.; Rothman, A.L.; Jarman, R.G.; Nisalak, A.; Thaisomboonsuk, B.; et al. Dengue virus (DENV) neutralizing antibody kinetics in children after symptomatic primary and postprimary DENV infection. J. Infect. Dis. 2016, 213, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1952, 1, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Casals, J.; Shotwell, H.; Palumbo, N. Studies on the immunization of monkeys against dengue. I. Protection derived from single and sequential virus infections. Am. J. Trop. Med. Hyg. 1973, 22, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Forshey, B.M.; Reiner, R.C.; Olkowski, S.; Morrison, A.C.; Espinoza, A.; Long, K.C.; Vilcarromero, S.; Casanova, W.; Wearing, H.J.; Halsey, E.S.; et al. Incomplete protection against dengue virus type 2 re-infection in Peru. PLoS Neglect. Trop. Dis. 2016, 10, e0004398. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Balmaseda, A.; Gresh, L.; Sahoo, M.K.; Montoya, M.; Wang, C.; Abeynayake, J.; Kuan, G.; Pinsky, B.A.; Harris, E. Homotypic dengue virus reinfections in Nicaraguan children. J. Infect. Dis. 2016, 214, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Culshaw, A.; Mongkolsapaya, J.; Screaton, G.R. The immunopathology of dengue and Zika virus infections. Curr. Opin. Immunol. 2017, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L. T cell immunity to dengue virus and implications for vaccine design. Expert Rev. Vaccines 2016, 15, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Harris, E. Participants in the Summit on Dengue Immune Correlates of P Immune correlates of protection for dengue: State of the art and research agenda. Vaccine 2017, 35, 4659–4669. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.-N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yachi, P.P.; Ampudia, J.; Zal, T.; Gascoigne, N.R. Altered peptide ligands induce delayed CD8-T cell receptor interaction—A role for CD8 in distinguishing antigen quality. Immunity 2006, 25, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Limpitikul, W.; Tangthawornchaikul, N.; Malasit, P.; Mongkolsapaya, J.; Screaton, G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. USA 2010, 107, 16922–16927. [Google Scholar] [CrossRef] [PubMed]
- Bashyam, H.S.; Green, S.; Rothman, A.L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J. Immunol. 2006, 176, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Rothman, A.L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J. Immunol. 2005, 175, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsapaya, J.; Duangchinda, T.; Dejnirattisai, W.; Vasanawathana, S.; Avirutnan, P.; Jairungsri, A.; Khemnu, N.; Tangthawornchaikul, N.; Chotiyarnwong, P.; Sae-Jang, K.; et al. T cell responses in dengue hemorrhagic fever: Are cross-reactive T cells suboptimal? J. Immunol. 2006, 176, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Zivny, J.; DeFronzo, M.; Jarry, W.; Jameson, J.; Cruz, J.; Ennis, F.A.; Rothman, A.L. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J. Immunol. 1999, 163, 2754–2760. [Google Scholar] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silverea, C.G.T.; Costa, P.R.; et al. Human CD8+ T-cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J. Infect. Dis. 2015, 212, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus-specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011, 203, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Loke, H.; Bethell, D.B.; Phuong, C.X.T.; Dung, M.; Scheinder, J.; White, N.J.; Day, N.P.; Farrar, J.; Hill, A.V.S. Strong HLA class I-restricted T cell responses in dengue hemorrhagic fever: A double-edged sword? J. Infect. Dis. 2001, 184, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.A.; Klaythong, R.; Sirikong, M.; Vaughn, D.W.; Green, S.; Kalayanaooj, S.; Endy, T.P.; Libraty, D.H.; Nisalak, A.; Innis, B.L.; et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 2002, 60, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Sierra, B.; Alegre, R.; Pérez, A.B.; García, G.; Sturn-Ramirez, K.; Obasanjo, O.; Aguirre, E.; Alvarez, M.; Rodriguez-Roche, R.; Valdés, L.; et al. HLA-A, -B, -C, and -DRB1 allele frequencies in Cuban individuals with antecedents of dengue 2 disease: Advantages of the Cuban population for HLA studies of dengue virus infection. Hum. Immunol. 2007, 68, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kikuchi, M.; Huong, V.T.Q.; Ha, D.Q.; Thuy, T.T.; Tham, V.D.; Tuan, H.M.; Tuong, V.V.; Nga, C.T.P.; Dat, T.V.; et al. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS Neglect. Trop. Dis. 2008, 2, e304. [Google Scholar]
- Coffey, L.L.; Mertens, E.; Brehin, A.-C.; Fernandez-Garcia, M.D.; Amara, A.; Després, P.; Sakuntabhai, A. Human genetic determinants of dengue virus susceptibility. Microbes Infect. 2009, 11, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Rostron, T.; Rohanachandra, L.T.; Fernando, N.; Dharshan de Silva, A.; Liyanage, M.; Ogg, G. HLA class I and class II associations in dengue viral infections in a Sri Lankan population. PLoS ONE 2011, 6, e20581. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A protective role for dengue virus-specific CD8+ T cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; de Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef] [PubMed]
- Simon-Lorière, E.; Duong, V.; Tawfik, A.; Ung, S.; Ly, S.; Casadémont, I.; Prot, M.; Courtejoie, N.; Bleakley, K.; Buchy, P.; et al. Increased adaptive immune responses and proper feedback regulation protect against clinical dengue. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Livingston, P.G.; Kurane, I.; Dai, L.C.; Okamoto, Y.; Lai, C.J.; Men, R.; Karaki, S.; Takiguchi, M.; Ennis, F.A. Dengue virus-specific, HLA-B35-restricted, human CD8+ cytotoxic T lymphocyte (CTL) clones. Recognition of NS3 amino acids 500 to 508 by CTL clones of two different serotype specificities. J. Immunol. 1995, 154, 1287–1295. [Google Scholar] [PubMed]
- Kurane, I.; Zeng, L.; Brinton, M.A.; Ennis, F.A. Definition of an epitope on NS3 recognized by human CD4+ cytotoxic T lymphocyte clones cross-reactive for dengue virus types 2, 3, and 4. Virology 1998, 240, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Kumaran, E.A.P.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Hao Gan, V.C.; Lye, D.C.; et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Tan, A.T.; Chia, A.; Kumaran, E.A.; Grotenbreg, G.M.; MacAry, P.A.; Bertoletti, A. Defining CD8+ T cell determinants during human viral infection in populations of Asian ethnicity. J. Immunol. 2013, 191, 4010–4019. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; de Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Kurane, I.; Okamoto, Y.; Ennis, F.A.; Brinton, M.A. Identification of amino acids involved in recognition by dengue virus NS3-specific, HLA-DR15-restricted cytotoxic CD4+ T-cell clones. J. Virol. 1996, 70, 3108–3117. [Google Scholar] [PubMed]
- Okamoto, Y.; Kurane, I.; Leporati, A.M.; Ennis, F.A. Definition of the region on NS3 which contains multiple epitopes recognized by dengue virus serotype-cross-reactive and flavivirus-cross-reactive, HLA-DPw2-restricted CD4+ T cell clones. J. Gen. Virol. 1998, 79, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Phuong Dung, N.T.; Bich Chau, T.N.; Thu Thao, L.T.; Dung, N.T.; Hien, T.T.; Rowland-Jones, S.; Farrar, J. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 2005, 79, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, E.J.; Mailliard, R.B.; Khan, A.M.; Sidney, J.; Sette, A.; Guzman, N.; Paulaitis, M.; de Melo, A.B.; Cordeiro, M.T.; Gil, L.V.G.; et al. Identification of conserved and HLA promiscuous DENV3 T-cell epitopes. PLoS Neglect. Trop. Dis. 2013, 7, e2497. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Ali, S.; Ahamad, S.; Malik, M.Z.; Ishrat, R. From ZikV genome to vaccine: In silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology 2016, 149, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.; Zaheer, T.; Talha Rehman, M.; Ali, A.; Javed, A.; Ayub Khan, G.; Mujtaba Babar, M.; Waheed, Y. Prediction of promiscuous T-cell epitopes in the Zika virus polyprotein: An in silico approach. Asian Pac. J. Trop. Med. 2016, 9, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Usman Mirza, M.; Rafique, S.; Ali, A.; Munir, M.; Ikram, N.; Manan, A.; Salo-Ahen, O.M.H.; Idrees, M. Towards peptide vaccines against Zika virus: Immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci. Rep. 2016, 6, 37313. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Lim, M.Q. CD4+ and CD8+ T-cell immunity to Dengue—Lessons for the study of Zika virus. Immunology 2016, 150, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Weihao Tang, W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Sulyeman, O.M.; Ortega-Prieto, A.M.; Skelton, J.K.; Bonnesoeur, P.; Blohm, A.; Carregaro, V.; Silva, J.S.; James, E.A.; Maillère, B.; et al. T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 2018, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Zust, R.; Toh, Y.-X.; Valdés, I.; Cerny, D.; Heinrich, J.; Hermida, L.; Marcos, E.; Guillén, G.; Kalinke, U.; Shi, P.-Y.; et al. Type I Interferon signals in macrophages and dendritic cells control dengue virus infection: Implications for a new mouse model to test dengue vaccines. J. Virol. 2014, 88, 7276–7285. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.S.; Zanotto, P.M.A.; Iampietro, M.J.; Badamchi-Zadech, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; de la Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.S.; Rolland, M.; Modjarrad, K.; Trautmann, L. T Cell immunity and Zika virus vaccine development. Trends Immunol. 2017, 38, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, S.; Zhang, Y.; Han, X.; Jia, B.; Liu, H.; Liu, D.; Tan, S.; Wang, Q.; Bi, Y.; et al. CD8+ T cell immune response in immunocompetent mice during Zika virus infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T cell response to Zika virus and identification of a novel CD8+ T cell epitope in immunocompetent mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive immune responses to Zika virus are important for controlling virus infection and preventing infection in brain and testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef] [PubMed]
- Naiyer, M.M.; Cassidy, S.A.; Magri, A.; Cowton, V.; Chen, K.; Mansour, S.; Kranidioti, C.; Mbiribindi, B.; Rettman, P.; Harris, S.; et al. KIR2DS2 recognizes conserved peptides derived from viral helicases in the context of HLA-C. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue virus exposure shapes T cell immunity to Zika virus in humans. J. Virol 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Which Dengue Vaccine Approach Is the Most Promising, and Should We Be Concerned about Enhanced Disease after Vaccination? There Is Only One True Winner. C.S.H. Perspect. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Eddy, W.E.; Tang, W.W.; Miller, R.; Shresta, S. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014, 193, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Tang, W.W.; Eddy, W.E.; King, K.; Sanchez, M.C.; Shresta, S. CD8+ T cells can mediate short-term protection against heterotypic dengue virus reinfection in mice. J. Virol. 2015, 89, 6494–6505. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, C.; Delgado, F.G.; Simon-Lorière, E.; Sakuntabhai, A. Immune Responses to Dengue and Zika Viruses—Guidance for T Cell Vaccine Development. Int. J. Environ. Res. Public Health 2018, 15, 385. https://doi.org/10.3390/ijerph15020385
Roth C, Delgado FG, Simon-Lorière E, Sakuntabhai A. Immune Responses to Dengue and Zika Viruses—Guidance for T Cell Vaccine Development. International Journal of Environmental Research and Public Health. 2018; 15(2):385. https://doi.org/10.3390/ijerph15020385
Chicago/Turabian StyleRoth, Claude, Félix G. Delgado, Etienne Simon-Lorière, and Anavaj Sakuntabhai. 2018. "Immune Responses to Dengue and Zika Viruses—Guidance for T Cell Vaccine Development" International Journal of Environmental Research and Public Health 15, no. 2: 385. https://doi.org/10.3390/ijerph15020385
APA StyleRoth, C., Delgado, F. G., Simon-Lorière, E., & Sakuntabhai, A. (2018). Immune Responses to Dengue and Zika Viruses—Guidance for T Cell Vaccine Development. International Journal of Environmental Research and Public Health, 15(2), 385. https://doi.org/10.3390/ijerph15020385




