Environmental Intolerance, Symptoms and Disability Among Fertile-Aged Women
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Study Population
2.2. Intolerance Attributed to Environmental Factors
2.2.1. Symptoms
2.2.2. Behavioral Changes
2.2.3. Disability
2.3. Definitions of EI
- (A)
- Feeling ill or annoyed (annoyance) by different environmental factors;
- (B)
- Annoyance with symptoms;
- (C)
- Annoyance with symptoms from multiple organ systems including the CNS (at least one CNS symptom and one non-CNS symptom);
- (D)
- Annoyance with multiple organ symptoms including CNS symptoms (=definition C) and behavioral changes (at least one behavioral change, see Table 1);
- (E)
- Annoyance with multiple organ symptoms including CNS symptoms, behavioral changes (=definition D) and disability; and;
- (F)
- Annoyance with multiple organ symptoms including CNS symptoms, behavioral changes (=definition D) and severe disability.
2.4. EI Attributed to Chemicals, Indoor Molds, and EMFs
2.5. Statistical Analysis
3. Results
3.1. Annoyance Attributed to Environmental Factors
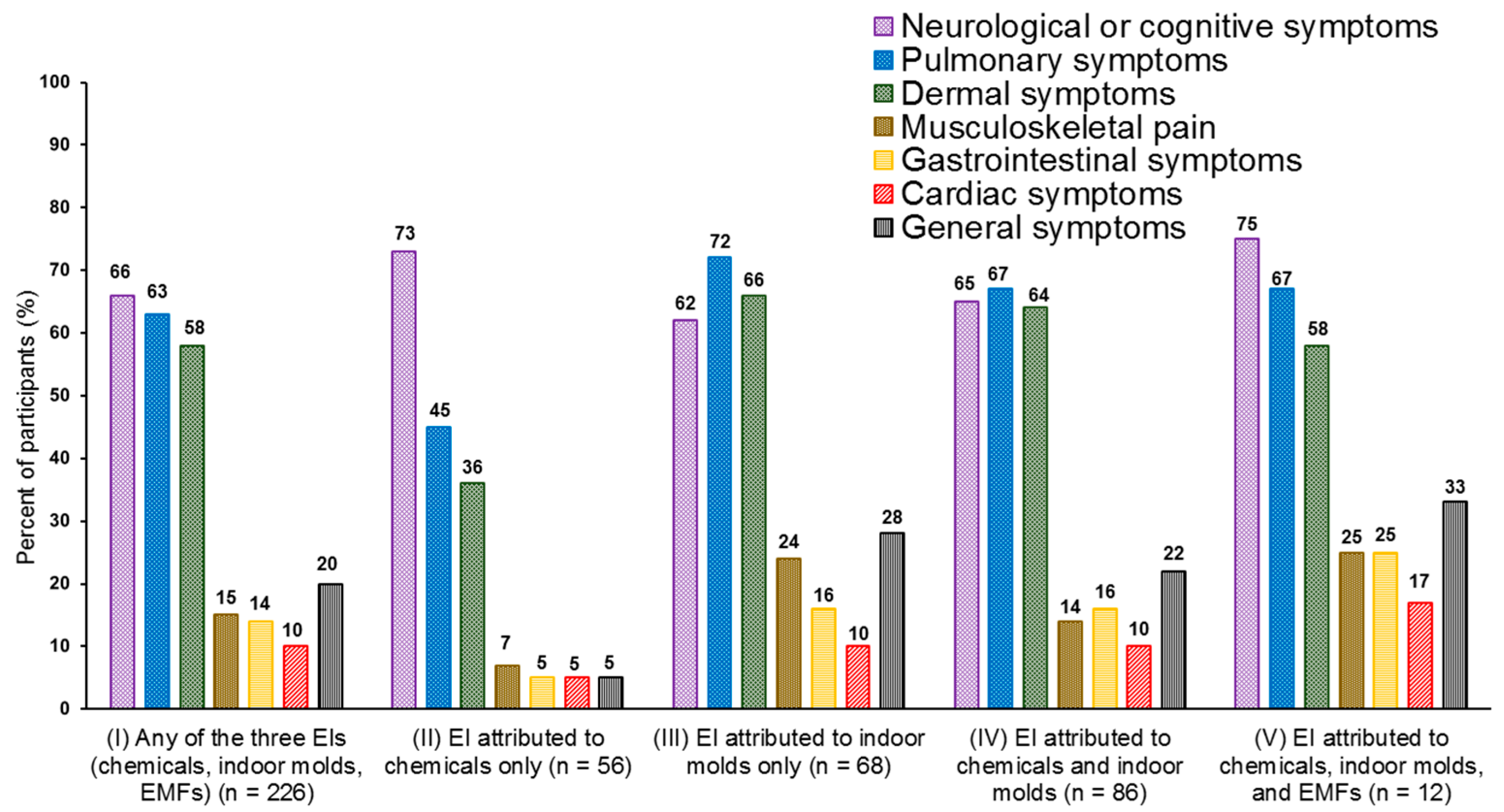
3.2. Annoyance and Symptoms Attributed to Environmental Factors
3.3. Behavioral Changes Due to EI
3.4. Disability Due to EI
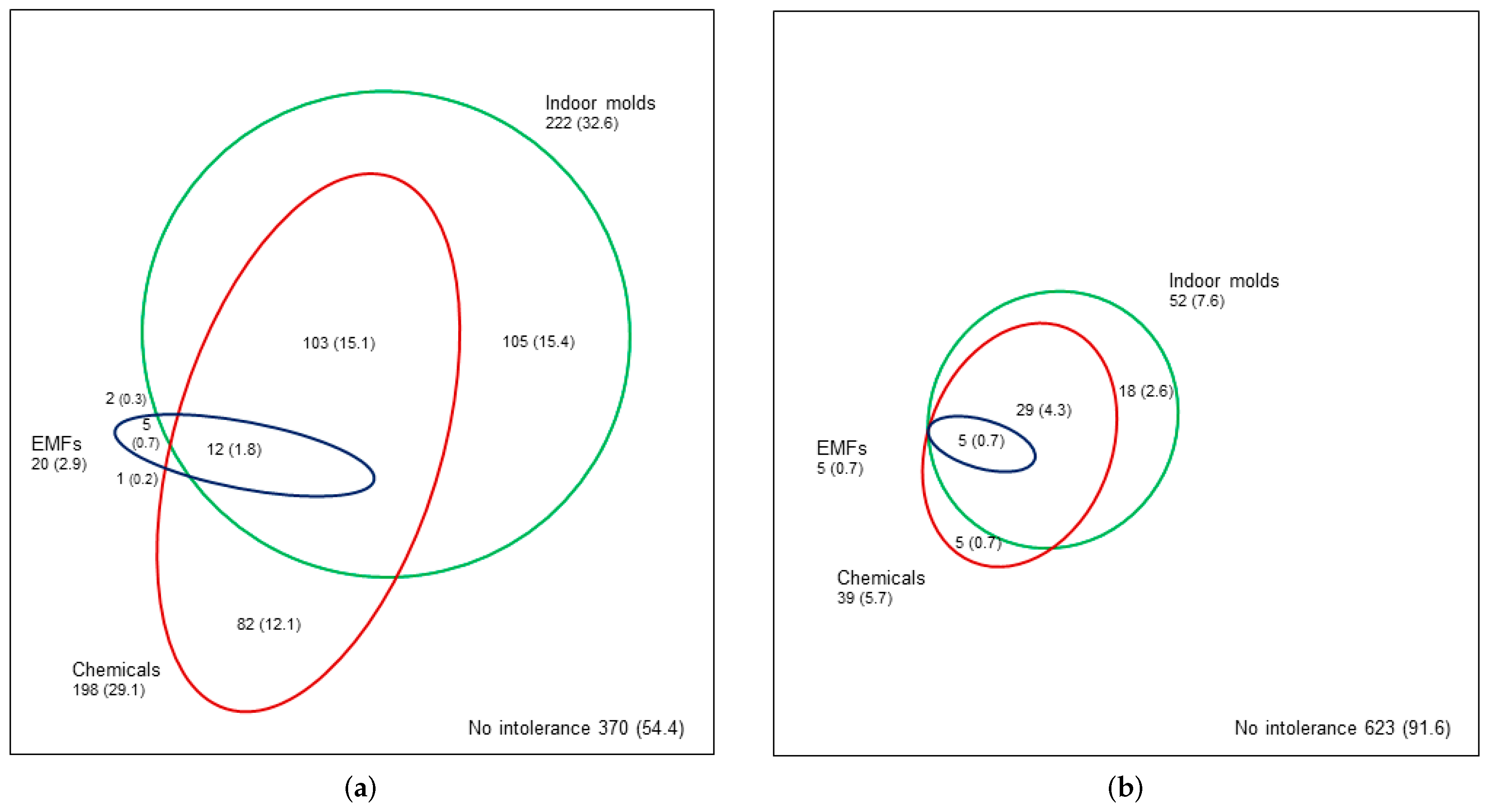
3.5. Co-Occurrence of EIs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berg, N.D.; Linneberg, A.; Dirksen, A.; Elberling, J. Prevalence of self-reported symptoms and consequences related to inhalation of airborne chemicals in a Danish general population. Int. Arch. Occup. Environ. Health 2008, 81, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Andersson, L.; Nordin, S.; Skovbjerg, S. Chemical intolerance. Curr. Rheumatol. Rev. 2015, 11, 167–184. [Google Scholar] [CrossRef] [PubMed]
- IPCS/WHO (International Programme on Chemical Safety/World Health Organization). Conclusions and recommendations of a workshop on Multiple Chemical Sensitivities (MCS). Regul. Toxicol. Pharmacol. 1996, 24, 188–189. [Google Scholar]
- Lacour, M.; Zunder, T.; Schmidtke, K.; Vaith, P.; Scheidt, C. Multiple chemical sensitivity syndrome (MCS)—Suggestions for an extension of the U.S. MCS-case definition. Int. J. Hyg. Environ. Health 2005, 208, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D. An update on sick building syndrome. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, E.; Claeson, A.S.; Neely, G.; Stenberg, B.; Nordin, S. Overlap in prevalence between various types of environmental intolerance. Int. J. Hyg. Environ. Health 2014, 217, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Baliatsas, C.; Van Kamp, I.; Lebret, E.; Rubin, G.J. Idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF): A systematic review of identifying criteria. BMC Public Health 2012, 12, 643. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, O.; Brown, R.J.; Petersen, S.; Witthöft, M. Idiopathic environmental intolerance: A comprehensive model. Clin. Psychol. Sci. 2017, 5, 551–567. [Google Scholar] [CrossRef]
- Hetherington, L.; Battershill, J. Review of evidence for a toxicological mechanism of idiopathic environmental intolerance. Hum. Exp. Toxicol. 2013, 32, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Todaka, E.; Seto, H.; Saito, I.; Hanazato, M.; Watanabe, M.; Mori, C. Correlating the symptoms of sick-building syndrome to indoor VOCs concentration levels and odour. Indoor Built Environ. 2014, 23, 804–813. [Google Scholar] [CrossRef]
- Tietjen, G.E.; Khubchandani, J.; Ghosh, S.; Bhattacharjee, S.; Kleinfelder, J. Headache symptoms and indoor environmental parameters: Results from the EPA BASE study. Ann. Indian Acad. Neurol. 2012, 15 (Suppl. S1), S95–S99. [Google Scholar] [PubMed]
- Lu, C.Y.; Tsai, M.C.; Muo, C.H.; Kuo, Y.H.; Sung, F.C.; Wu, C.C. Personal, psychosocial and environmental factors related to sick building syndrome in official employees of Taiwan. Int. J. Environ. Res. Public Health 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tonori, H.; Aizawa, Y. Multiple chemical sensitivity and idiopathic environmental intolerance (part one). Environ. Health Prev. Med. 2003, 7, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Karvala, K.; Sainio, M.; Palmquist, E.; Nyback, M.H.; Nordin, S. Prevalence of various environmental intolerances in a Swedish and Finnish general population. Environ. Res. 2018, 161, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Caress, S.M.; Steinemann, A.C. A national population study of the prevalence of multiple chemical sensitivity. Arch. Environ. Health 2004, 59, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, F.; Karlson, B.; Ørbӕk, P.; Österberg, K.; Östergren, P.O. Prevalence of annoyance attributed to electrical equipment and smells in a Swedish population, and relationship with subjective health and daily functioning. Public Health 2005, 119, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Dantoft, T.M.; Ebstrup, J.F.; Linneberg, A.; Skovbjerg, S.; Madsen, A.L.; Mehlsen, J.; Brinth, L.; Eplov, L.F.; Carstensen, T.W.; Schroder, A.; et al. Cohort description: The Danish study of Functional Disorders. Clin. Epidemiol. 2017, 23, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner, C.; Bornschein, S.; Hansen, J.; Zilker, T.; Förstl, H. Self-reported chemical sensitivity in Germany: A population-based survey. Int. J. Hyg. Environ. Health 2005, 208, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Brämerson, A.; Millqvist, E.; Nordin, S.; Bende, M. Prevalence and risk factors for self-reported odour intolerance: The Skovde population-based study. Int. Arch. Occup. Environ. Health 2005, 78, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, R.; Neutra, R.R.; Lashuay, N. Prevalence of people reporting sensitivities to chemicals in a population-based survey. Am. J. Epidemiol. 1999, 150, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Uchiyama, I.; Katoh, T.; Ogata, H.; Arashidani, K.; Kunugita, N. Prevalence and characteristics of chemical intolerance: A Japanese populations-based study. Arch. Environ. Occup. Health 2015, 70, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.; Frei, P.; Braun-Fahrländer, C.; Fröhlich, J.; Neubauer, G.; Röösli, M.; Qualifex Team. Effects of everyday radiofrequency electromagnetic-field exposure on sleep quality: A cross-sectional study. Radiat. Res. 2010, 174, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Runeson-Broberg, R.; Norbäck, D. Sick building syndrome (SBS) and sick house syndrome (SHS) in relation to psychosocial stress at work in the Swedish workforce. Int. Arch. Occup. Environ. Health 2013, 86, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, E.; Janson, C.; Norbäck, D.; Boman, G. Symptoms related to the sick building syndrome in a general population sample: Associations with atopy, bronchial hyper-responsiveness and anxiety. Int. J. Tuberc. Lung Dis. 1998, 2, 1023–1028. [Google Scholar] [PubMed]
- Black, D.W.; Okiishi, C.; Schlosser, S. A nine-year follow-up of people diagnosed with multiple chemical sensitivities. Psychosomatics 2000, 41, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Black, D.W.; Doebbeling, B.N.; Voelker, M.D.; Clarke, W.R.; Woolson, R.F.; Barrett, D.H.; Schwartz, D.A. Multiple chemical sensitivity syndrome: Symptom prevalence and risk factors in a military population. Arch. Intern. Med. 2000, 160, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Katerndahl, D.A.; Bell, I.R.; Palmer, R.F.; Miller, C.S. Chemical intolerance in primary care settings: Prevalence, comorbidity, and outcomes. Ann. Fam. Med. 2012, 10, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Finell, E.; Seppälä, T. Indoor air problems and experiences of injustice in the workplace: A quantitative and a qualitative study. Indoor Air 2018, 28, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.L. Pregnancy and olfaction: A review. Front. Psychol. 2014, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Fink, P.; Schröder, A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J. Psychosom. Res. 2010, 68, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Baliatsas, C.; van Kamp, I.; Hooiveld, M.; Yzermans, J.; Lebret, E. Comparing non-specific physical symptoms in environmentally sensitive patients: Prevalence, duration, functional status and illness behavior. J. Psychosom. Res. 2014, 76, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner, C.; Bornschein, S.; Zilker, T.; Henningsen, P.; Förstl, H. Dysfunctional cognitions in idiopathic environmental intolerances (IEI)—An integrative psychiatric perspective. Toxicol. Lett. 2007, 171, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bailer, J.; Witthöft, M.; Rist, F. Modern health worries and idiopathic environmental intolerance. J. Psychosom. Res. 2008, 65, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, E. Environmental Intolerance—Psychological Risk and Health Factors. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2017. [Google Scholar]
- Black, D.W.; Okiishi, C.; Schlosser, S. The Iowa follow-up of chemically sensitive persons. Ann. N. Y. Acad. Sci. 2001, 933, 48–56. [Google Scholar] [CrossRef] [PubMed]
| Item | Question and Answer Options |
|---|---|
| Annoyance | Are you feeling ill or annoyed by the following types of environmental exposures or situations? |
| Chemicals (1)–(6) | (1) Vehicle exhaust (2) Paint or paint thinner (3) Perfumes, air fresheners or other fragrances (4) New furnishings such as new carpeting, flooring, shower curtain, or the interior of a new car (5) Fresh ink on newspapers (6) Tobacco smoke |
| Indoor molds (7) | (7) Indoor molds in moisture-damaged buildings |
| EMFs (8) | (8) Electromagnetic fields |
| Other environmental factors (9)–(12) | (9) Beauty salons or hair salons (10) Detergent departments in shops (11) Moldy odors (12) Dust |
| Sensitivity | Are you exceptionally/unusually sensitive to the environmental exposures or situations above? |
| Symptoms | Have you ever had the following symptoms from the environmental exposures or situations listed above? - Neurological symptoms (e.g., headache, numbness, tingling) - Cognitive symptoms (e.g., memory deterioration, concentration impaired) - Pulmonary symptoms (e.g., dyspnea, coughing, wheezing) - Dermal symptoms (e.g., erythema, rash) - Muscles or joint pain - Gastrointestinal symptoms (e.g., flatulence, stomach ache) - Cardiac symptoms (e.g., palpitations) - General symptoms (e.g., fever, night sweats, fatigue, weight loss, increase in weight) |
| Behavioral changes | Have you made any behavioral changes to avoid the symptoms above? - Behavior or lifestyle change to minimize exposure - Changed interior decorations or furnishings at home - Moved to another apartment - Changed workplace, resigned from workplace or occupation - Taken vitamins, nutritional supplements, or changed diet - Eliminated the cause using antifungal agents, or chemicals - Used protective equipment (e.g., respirator, gauntlet, clothing) |
| Disability | If you recognize the problems mentioned above, how difficult have these problems made it for you to do your work, take care of things at home or get along with other people? |
| Environmental Factor | Degree of Annoyance * | |||
|---|---|---|---|---|
| Not at All | Some | Rather Much | Very Much | |
| Chemicals | ||||
| Vehicle exhaust | 263 (38.7) | 324 (47.7) | 71 (10.5) | 21 (3.1) |
| Paint or paint thinner | 211 (31.4) | 324 (48.1) | 110 (16.3) | 28 (4.2) |
| Perfumes, air fresheners, or other fragrance | 273 (40.3) | 258 (38.0) | 117 (17.3) | 30 (4.4) |
| New furnishings such as new carpeting, flooring, shower curtain, or the interior of a new car | 441 (65.7) | 190 (28.3) | 33 (4.9) | 7 (1.1) |
| Fresh ink of newspapers | 544 (80.0) | 110 (16.2) | 23 (3.4) | 3 (0.4) |
| Tobacco smoke | 133 (19.6) | 211 (31.1) | 175 (25.8) | 159 (23.5) |
| Indoor molds | 200 (29.7) | 252 (37.4) | 148 (21.9) | 74 (11.0) |
| Electromagnetic fields | 568 (84.7) | 83 (12.4) | 15 (2.2) | 5 (0.7) |
| Other factors | ||||
| Beauty salons or hair salons | 468 (68.8) | 175 (25.8) | 28 (4.1) | 9 (1.3) |
| Shop detergent departments | 487 (71.7) | 146 (21.5) | 39 (5.8) | 7 (1.0) |
| Moldy odors | 217 (32.1) | 300 (44.3) | 114 (16.8) | 46 (6.8) |
| Dust | 178 (26.4) | 310 (45.9) | 147 (21.8) | 40 (5.9) |
| Definitions of EI | EI Attributed to | ||||
|---|---|---|---|---|---|
| Any of the 12 Environmental Factors | Chemicals **, Molds, or EMFs (Any of the Three) | Chemicals ** | Molds | EMFs | |
| A | 457 (67.2) | 310 (45.6) | 198 (29.1) | 222 (32.6) | 20 (2.9) |
| B | 302 (44.4) | 226 (33.2) | 155 (22.8) | 166 (24.4) | 16 (2.4) |
| C | 145 (21.3) | 119 (17.5) | 80 (11.8) | 93 (13.7) | 9 (1.3) |
| D | 122 (17.9) | 102 (15.0) | 67 (9.9) | 83 (12.2) | 9 (1.3) |
| E | 68 (10.0) | 57 (8.4) | 39 (5.7) | 52 (7.6) | 5 (0.7) |
| F | 15 (2.2) | 15 (2.2) | 10 (1.5) | 15 (2.2) | 2 (0.3) |
| Number and Type of EI | Degree of Disability (Lifestyle or Functional Impairments) *** | ||||
|---|---|---|---|---|---|
| Not Difficult at All | Somewhat Difficult | Very Difficult | Extremely Difficult | Total | |
| Only one EI | 30 (56.6) | 18 (33.9) | 3 (5.7) | 2 (3.8) | 53 (100) |
| Chemicals only | 13 (72.2) | 5 (27.8) | - | - | 18 (100) |
| Indoor molds only | 16 (47.1) | 13 (38.2) | 3 (8.8) | 2 (5.9) | 34 (100) |
| EMFs only | 1 (100) | - | - | - | 1 (100) |
| Two different types of EI | 12 (29.3) | 21 (51.2) | 5 (12.2) | 3 (7.3) | 41 (100) |
| Chemicals and molds | 12 (29.3) | 21 (51.2) | 5 (12.2) | 3 (7.3) | 41 (100) |
| Chemicals and EMFs | - | - | - | - | - |
| Molds and EMFs | - | - | - | - | - |
| All three (chemicals, molds, EMFs) EIs | 3 (37.5) | 3 (37.5) | 1 (12.5) | 1 (12.5) | 8 (100) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuokko, A.; Karvala, K.; Lampi, J.; Keski-Nisula, L.; Pasanen, M.; Voutilainen, R.; Pekkanen, J.; Sainio, M. Environmental Intolerance, Symptoms and Disability Among Fertile-Aged Women. Int. J. Environ. Res. Public Health 2018, 15, 293. https://doi.org/10.3390/ijerph15020293
Vuokko A, Karvala K, Lampi J, Keski-Nisula L, Pasanen M, Voutilainen R, Pekkanen J, Sainio M. Environmental Intolerance, Symptoms and Disability Among Fertile-Aged Women. International Journal of Environmental Research and Public Health. 2018; 15(2):293. https://doi.org/10.3390/ijerph15020293
Chicago/Turabian StyleVuokko, Aki, Kirsi Karvala, Jussi Lampi, Leea Keski-Nisula, Markku Pasanen, Raimo Voutilainen, Juha Pekkanen, and Markku Sainio. 2018. "Environmental Intolerance, Symptoms and Disability Among Fertile-Aged Women" International Journal of Environmental Research and Public Health 15, no. 2: 293. https://doi.org/10.3390/ijerph15020293
APA StyleVuokko, A., Karvala, K., Lampi, J., Keski-Nisula, L., Pasanen, M., Voutilainen, R., Pekkanen, J., & Sainio, M. (2018). Environmental Intolerance, Symptoms and Disability Among Fertile-Aged Women. International Journal of Environmental Research and Public Health, 15(2), 293. https://doi.org/10.3390/ijerph15020293




