Geographical Accessibility of the Sarcoma Referral Networks in France. Intermediate Results from the IGéAS Research Program
Abstract
1. Introduction
- -
- RRePS: Pathological referral network for soft tissue and visceral sarcomas
- -
- ResOs: Pathological and clinical referral network for bone sarcomas
- -
- Netsarc: Clinical referral network for soft tissue and visceral sarcomas
2. Materials and Methods
2.1. National Network Databases
2.2. IGéAS Cohort Extraction
- -
- Patient living in France at time of diagnosis;
- -
- Diagnoses of sarcoma/GIST/desmoid tumor/intermediate malignity tumor between the 1 January 2011 and the 31 December 2014;
- -
- Patient who benefits from a pathological review or a sarcoma MTB discussion in an expert center belonging to the sarcoma referral networks.
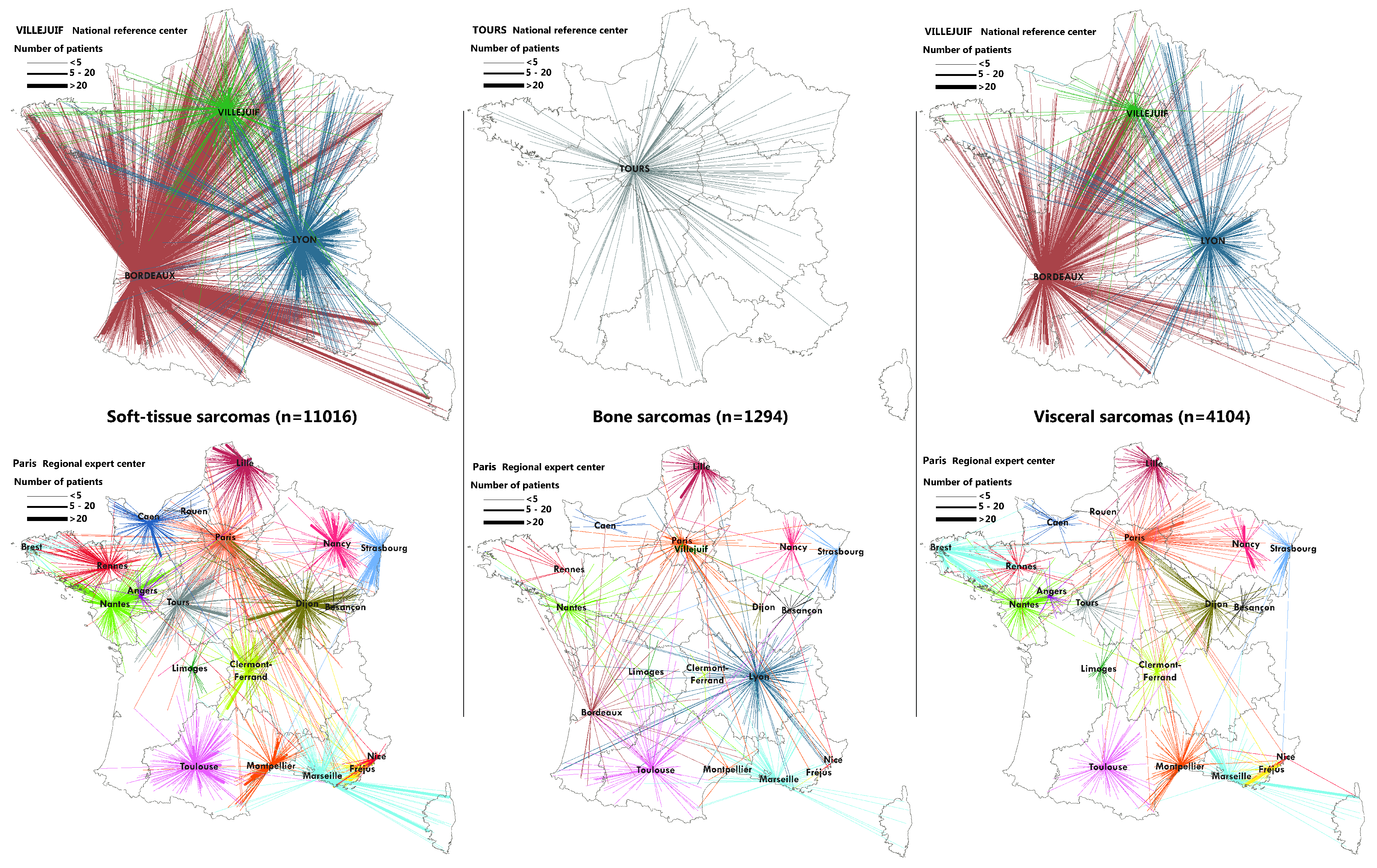
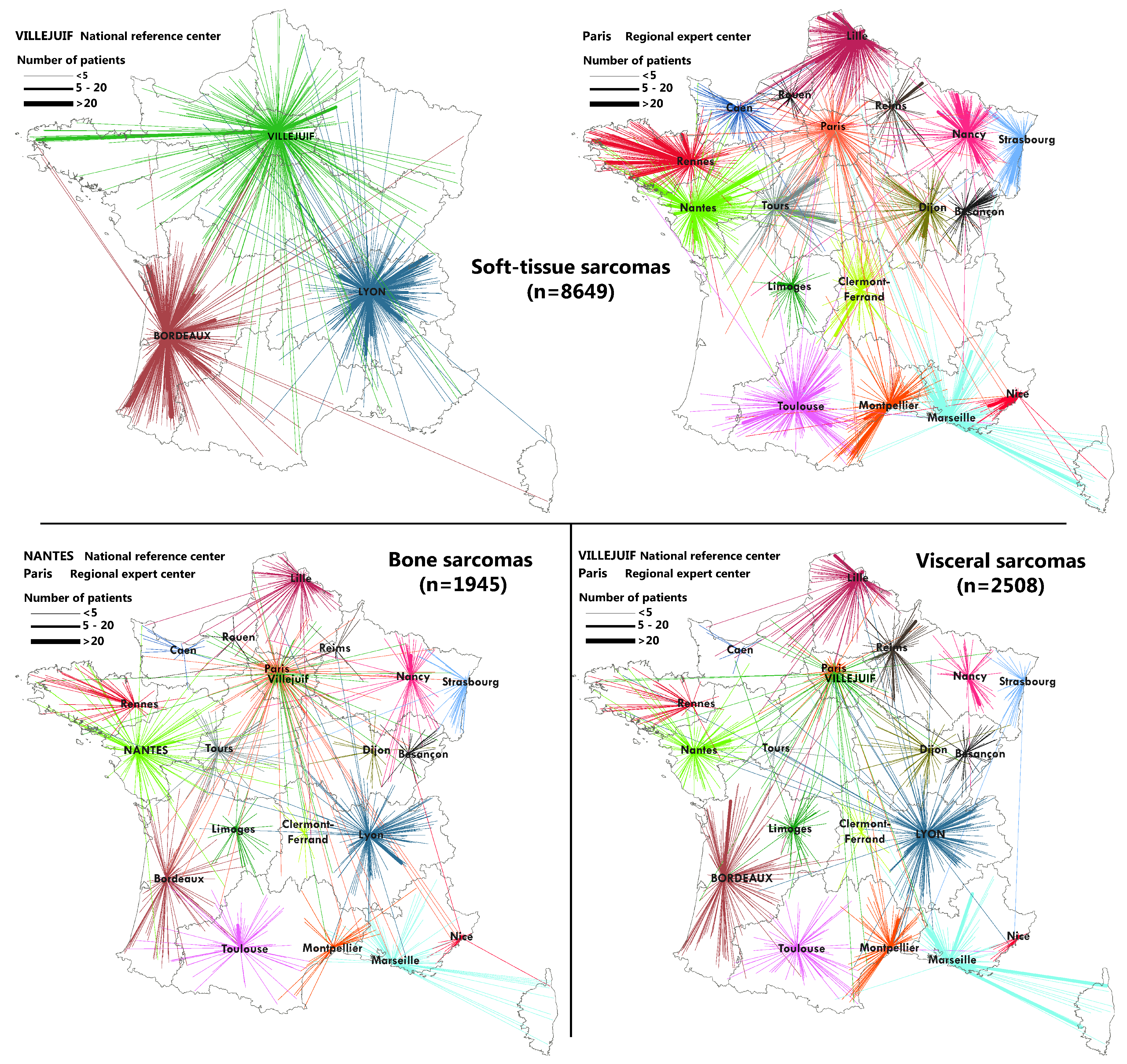
2.3. Flow Maps
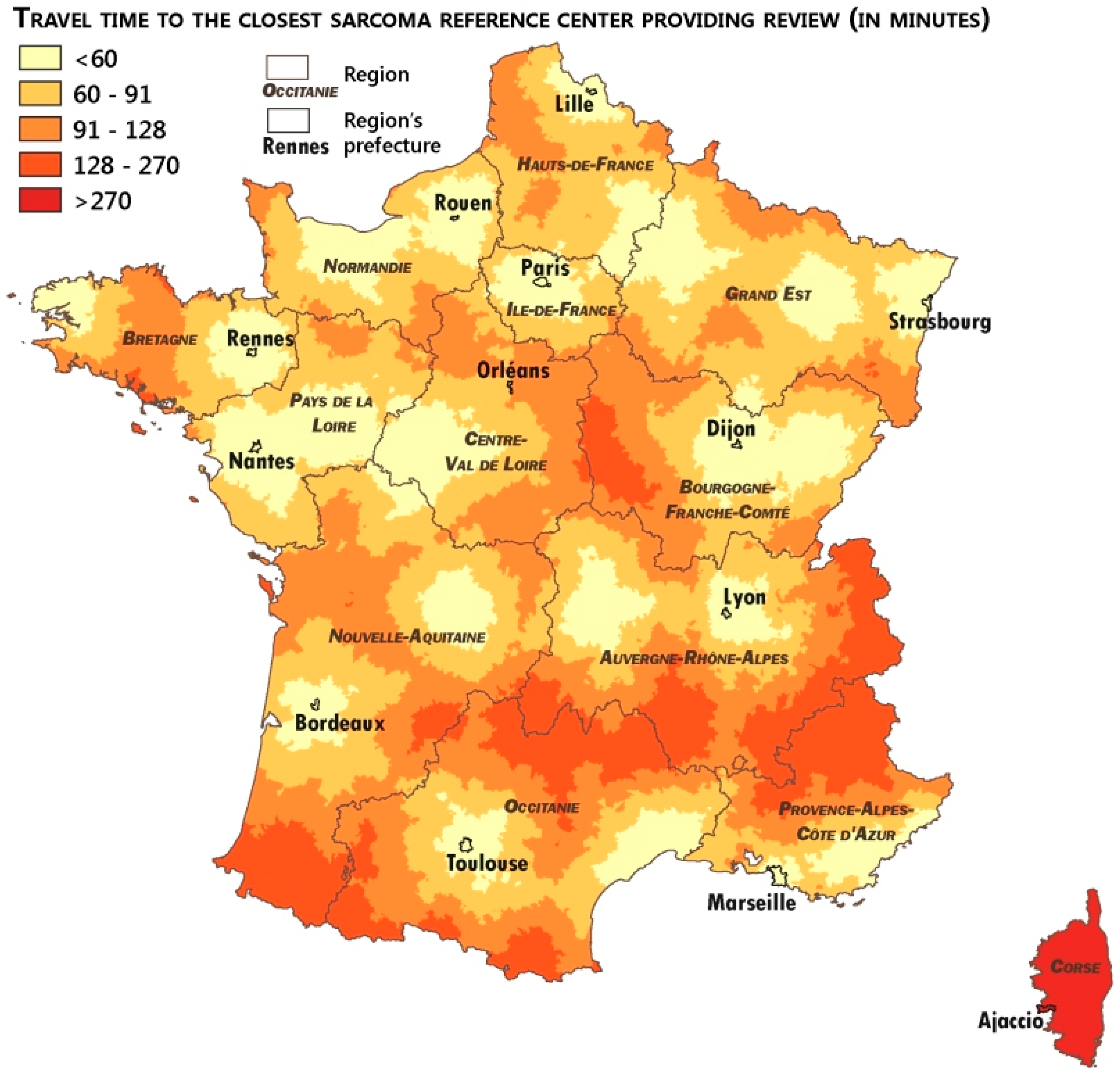
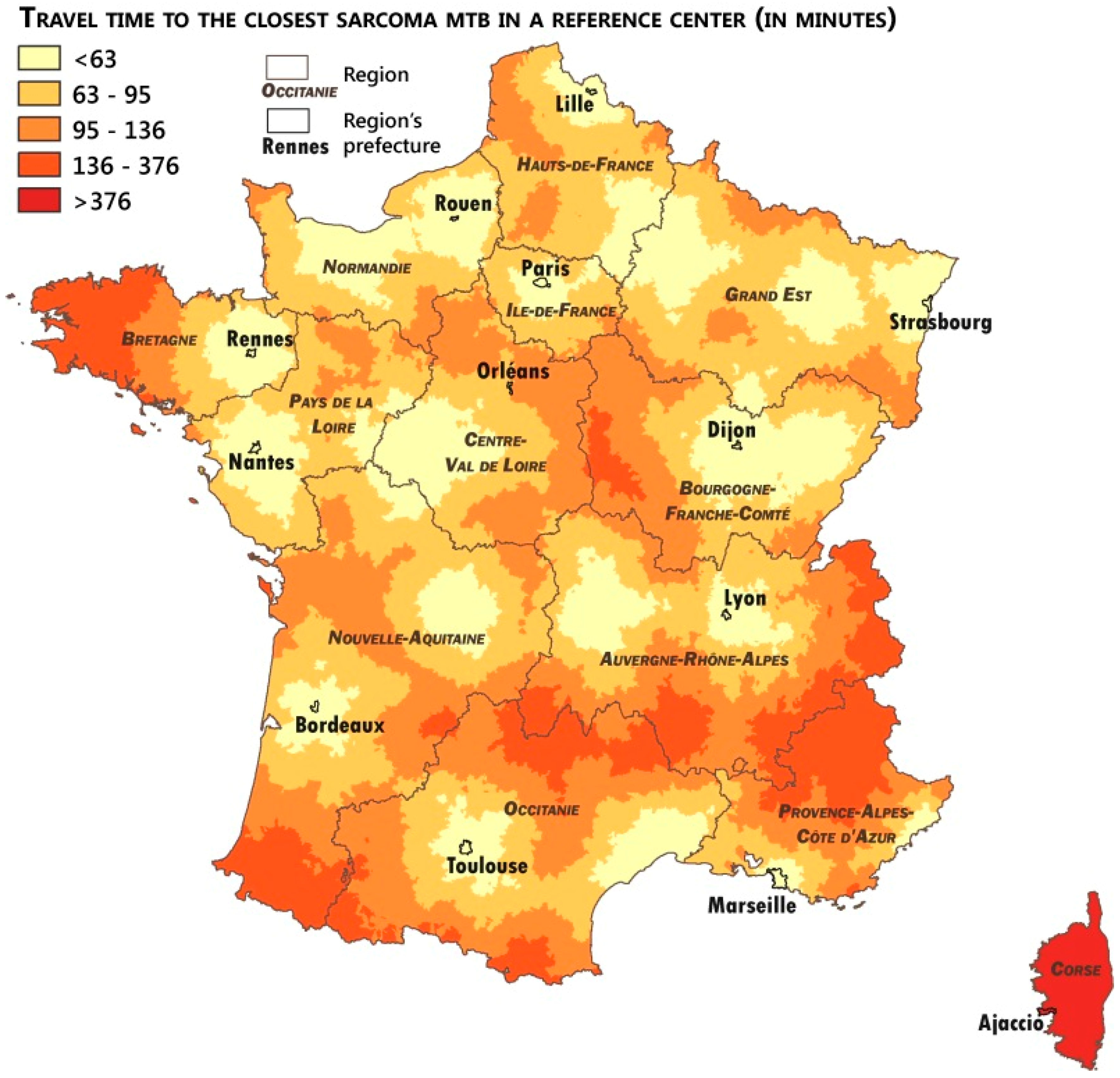
2.4. Measuring the Accessibility of the Networks
3. Results
3.1. IGéAS Cohort Description
3.2. Geographical Coverage of the Sarcoma Referral Networks
3.3. Analysis of the Patients’ Remoteness to Sarcoma Expertise
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gatta, G.; Trama, A.; Capocaccia, R. Epidemiology of Rare Cancers and Inequalities in Oncologic Outcomes. Eur. J. Surg. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Derbel, O.; Heudel, P.E.; Cropet, C.; Meeus, P.; Vaz, G.; Biron, P. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS ONE 2017, 12, e0158406. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Thiesse, P.; Ranchère-Vince, D.; Chauvin, F.; Bobin, J.-Y.; Sunyach, M.-P. Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann. Oncol. 2004, 15, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Montesco, M.C.; Coindre, J.M.; Dei Tos, A.P.; Lurkin, A.; Ranchère-Vince, D. Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann. Oncol. 2012, 23, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Raut, C.P.; Pollock, R.E.; Rutkowski, P.; Strauss, D.C.; Hayes, A.J. Technical considerations in surgery for retroperitoneal sarcomas: Position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann. Surg. Oncol. 2012, 19, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, Y.A.; Huddy, J.R.; Miller, J.D.; Strauss, D.C.; Thomas, J.M.; Hayes, A.J. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann. Surg. Oncol. 2012, 19, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Bonvalot, S.; Tzanis, D.; Casali, P.G.; Trama, A.; Gronchi, A. Treatment challenges in and outside a network setting: Soft tissue sarcomas. Eur. J. Surg. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Coindre, J.-M.; Ducimetière, F.; Ray-Coquard, I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016, 17, e62–e69. [Google Scholar] [CrossRef]
- Perrier, L.; Rascle, P.; Ray-Coquard, I.; Nguyen, B.B.; Morelle, M.; Vince, D.R. Cost of Discordant Diagnoses in Sarcoma, Gist, and Desmoid Tumors in France: Results from the RREPS (Reseau De Reference En Pathologie Des Sarcomes) Network. Value Health 2014, 17, A95–A96. [Google Scholar] [CrossRef]
- Penel, N.; Coindre, J.-M.; Bonvalot, S.; Italiano, A.; Neuville, A.; Le Cesne, A. Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France. Eur. J. Cancer 2016, 58, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.; Judson, I. Role of expert centres in the management of sarcomas—A UK perspective. Eur. J. Cancer 2014, 50, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Rööser, B.; Rydholm, A.; Alvegård, T. Centralization of soft tissue sarcoma. Status in Sweden in 1982. Acta Orthop. Scand. 1987, 58, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Trugeon, A.; Thomas, N.; Michelot, F.; Fédération Nationale des OBSERVATOIRES Régionaux de Santé (France). Inégalités Socio-Sanitaires en France: De la Région Au Canton; Elsevier Masson: Issy-les-Moulineaux, France, 2010. [Google Scholar]
- Chen, C.-S.; Liu, T.-C.; Lin, H.-C.; Lien, Y.-C. Does high surgeon and hospital surgical volume raise the five-year survival rate for breast cancer? A population-based study. Breast Cancer Res. Treat 2008, 110, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Eppsteiner, R.W.; Csikesz, N.G.; McPhee, J.T.; Tseng, J.F.; Shah, S.A. Surgeon Volume Impacts Hospital Mortality for Pancreatic Resection. Ann. Surg. 2009, 249, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.; Flynn, R.; Baxter, G.; Donnelly, M.; Lawrence, J. Travel time and cancer care: An example of the inverse care law? Rural Remote Health 2008, 8, 1003. [Google Scholar] [PubMed]
- Onega, T.; Duell, E.J.; Shi, X.; Wang, D.; Demidenko, E.; Goodman, D. Geographic access to cancer care in the U.S. Cancer 2008, 112, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Blais, S.; Dejardin, O.; Boutreux, S.; Launoy, G. Social determinants of access to reference care centres for patients with colorectal cancer—A multilevel analysis. Eur. J. Cancer 2006, 42, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Fayet, Y.; Chasles, V.; Ducimetiere, F.; Ray-Coquard, I. Modeling the leading process to geographical inequalities of cancers. A solution to understand and tackle this public health issue. Soc. Sci. Med. 2018. submitted. [Google Scholar]
- Pornet, C.; Delpierre, C.; Dejardin, O.; Grosclaude, P.; Launay, L.; Guittet, L. Construction of an adaptable European transnational ecological deprivation index: The French version. J. Epidemiol. Commun. Health 2012, 66, 982–989. [Google Scholar] [CrossRef] [PubMed]
| IGéAS Cohort | 20,590 | |
| Sex | ||
| Male | 10,475 | (50.9%) |
| Female | 10,115 | (49.1%) |
| Age at Diagnosis | ||
| Mean (Std) | 57.2 (20.5) | |
| Median (min; max) | 60.5 (0.0; 105.2) | |
| Geographic Origin | ||
| Mainland France | 20,102 | (97.6%) |
| Overseas territories | 488 | (2.4%) |
| Type of Tumor | ||
| Soft tissue | 13,081 | (63.5%) |
| Viscera | 5170 | (25.1%) |
| Bone | 2339 | (11.4%) |
| Histotype | ||
| Sarcoma | 12,562 | (61.0%) |
| Leiomyosarcoma | 2249 | (10.9%) |
| Undifferentiated sarcoma | 2116 | (10.3%) |
| Liposarcoma | 1986 | (9.6%) |
| Miscellaneous sarcomas | 1794 | (8.7%) |
| Other sarcomas | 801 | (3.9%) |
| Chondrosarcoma | 715 | (3.5%) |
| Osteosarcoma | 620 | (3.0%) |
| Myxofibrosarcoma | 568 | (2.8%) |
| Rhabdomyosarcoma | 484 | (2.4%) |
| Synovial sarcoma | 386 | (1.9%) |
| Malignant peripheral nerve sheath tumor | 258 | (1.3%) |
| Non classified sarcoma | 585 | (2.8%) |
| Tumor of intermediate malignancy | 5343 | (25.9%) |
| Intermediate fibroblastic/myofibroblastic tumors | 2722 | (13.2%) |
| Atypical lipomatous tumor | 796 | (3.9%) |
| Intermediate tumors of uncertain differentiation | 635 | (3.1%) |
| Intermediate vascular tumors | 612 | (3.0%) |
| Intermediate fibrohistiocytic tumors | 334 | (1.6%) |
| Other tumors of intermediate malignancy | 124 | (0.6%) |
| Intermediate chondrogenic tumors | 66 | (0.3%) |
| Intermediate osteogenic tumors | 23 | (0.1%) |
| Non classified tumor of intermediate malignancy | 31 | (0.1%) |
| GIST | 2685 | (13.0%) |
| Year of Diagnosis | ||
| 2011 | 4594 | (22.3%) |
| 2012 | 5041 | (24.5%) |
| 2013 | 5372 | (26.1%) |
| 2014 | 5583 | (27.1%) |
| Type of Reviewed Sampling | ||
| Tumor resection | 9376 | (45.5%) |
| Microbiopsy | 4489 | (21.8%) |
| No expert review | 3777 | (18.3%) |
| Open biopsy | 2945 | (14.3%) |
| Curettage | 3 | (0.0%) |
| Days between Date of Sampling and Date of Review | ||
| Mean (Std) | 43.4 (125.9) | |
| Access to Pathological Expertise | ||
| Optimal access | 11,841 | (57.5%) |
| Late access | 4972 | (24.1%) |
| No access | 3777 | (18.3%) |
| Clinical Situation at First MTB | ||
| No expert MTB discussion | 7227 | (35.1%) |
| Complementary treatment | 4710 | (22.9%) |
| Before surgery | 3794 | (18.4%) |
| After treatment | 1300 | (6.3%) |
| Before neo-adjuvant treatment | 1283 | (6.2%) |
| Before biopsy | 1240 | (6.0%) |
| After progression | 1031 | (5.0%) |
| Unknown | 5 | (0.0%) |
| Access to Clinical Expertise | ||
| Optimal access | 6318 | (30.7%) |
| Late access | 7045 | (34.2%) |
| No access | 7227 | (35.1%) |
| Travel Time Distance to Expert Centers | Pathological Review | MTB Discussion | |
|---|---|---|---|
| Number of Communes with expert centers | 38 | 21 | |
| Median travel time (in minutes) | 80.5 | 82.5 | |
| Average travel time (in minutes) | 83.0 | 85.9 | |
| Average travel time (in minutes) by quintiles | Q1 | 41.2 | 43.1 |
| Q2 | 65.7 | 67.9 | |
| Q3 | 81.4 | 83.7 | |
| Q4 | 97.2 | 99.6 | |
| Q5 | 131.6 | 137.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayet, Y.; Coindre, J.-M.; Dalban, C.; Gouin, F.; De Pinieux, G.; Farsi, F.; Ducimetière, F.; Chemin-Airiau, C.; Jean-Denis, M.; Chabaud, S.; et al. Geographical Accessibility of the Sarcoma Referral Networks in France. Intermediate Results from the IGéAS Research Program. Int. J. Environ. Res. Public Health 2018, 15, 2204. https://doi.org/10.3390/ijerph15102204
Fayet Y, Coindre J-M, Dalban C, Gouin F, De Pinieux G, Farsi F, Ducimetière F, Chemin-Airiau C, Jean-Denis M, Chabaud S, et al. Geographical Accessibility of the Sarcoma Referral Networks in France. Intermediate Results from the IGéAS Research Program. International Journal of Environmental Research and Public Health. 2018; 15(10):2204. https://doi.org/10.3390/ijerph15102204
Chicago/Turabian StyleFayet, Yohan, Jean-Michel Coindre, Cécile Dalban, François Gouin, Gonzague De Pinieux, Fadila Farsi, Françoise Ducimetière, Claire Chemin-Airiau, Myriam Jean-Denis, Sylvie Chabaud, and et al. 2018. "Geographical Accessibility of the Sarcoma Referral Networks in France. Intermediate Results from the IGéAS Research Program" International Journal of Environmental Research and Public Health 15, no. 10: 2204. https://doi.org/10.3390/ijerph15102204
APA StyleFayet, Y., Coindre, J.-M., Dalban, C., Gouin, F., De Pinieux, G., Farsi, F., Ducimetière, F., Chemin-Airiau, C., Jean-Denis, M., Chabaud, S., Blay, J.-Y., & Ray-Coquard, I. (2018). Geographical Accessibility of the Sarcoma Referral Networks in France. Intermediate Results from the IGéAS Research Program. International Journal of Environmental Research and Public Health, 15(10), 2204. https://doi.org/10.3390/ijerph15102204






