Seroprevalence and Determinants Associated with Mumps Antibodies after 20 Years of MMR Vaccination in Urban Area of Shanghai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Serological Survey
2.3. Laboratory Testing
2.4. Statistical Analysis
3. Results
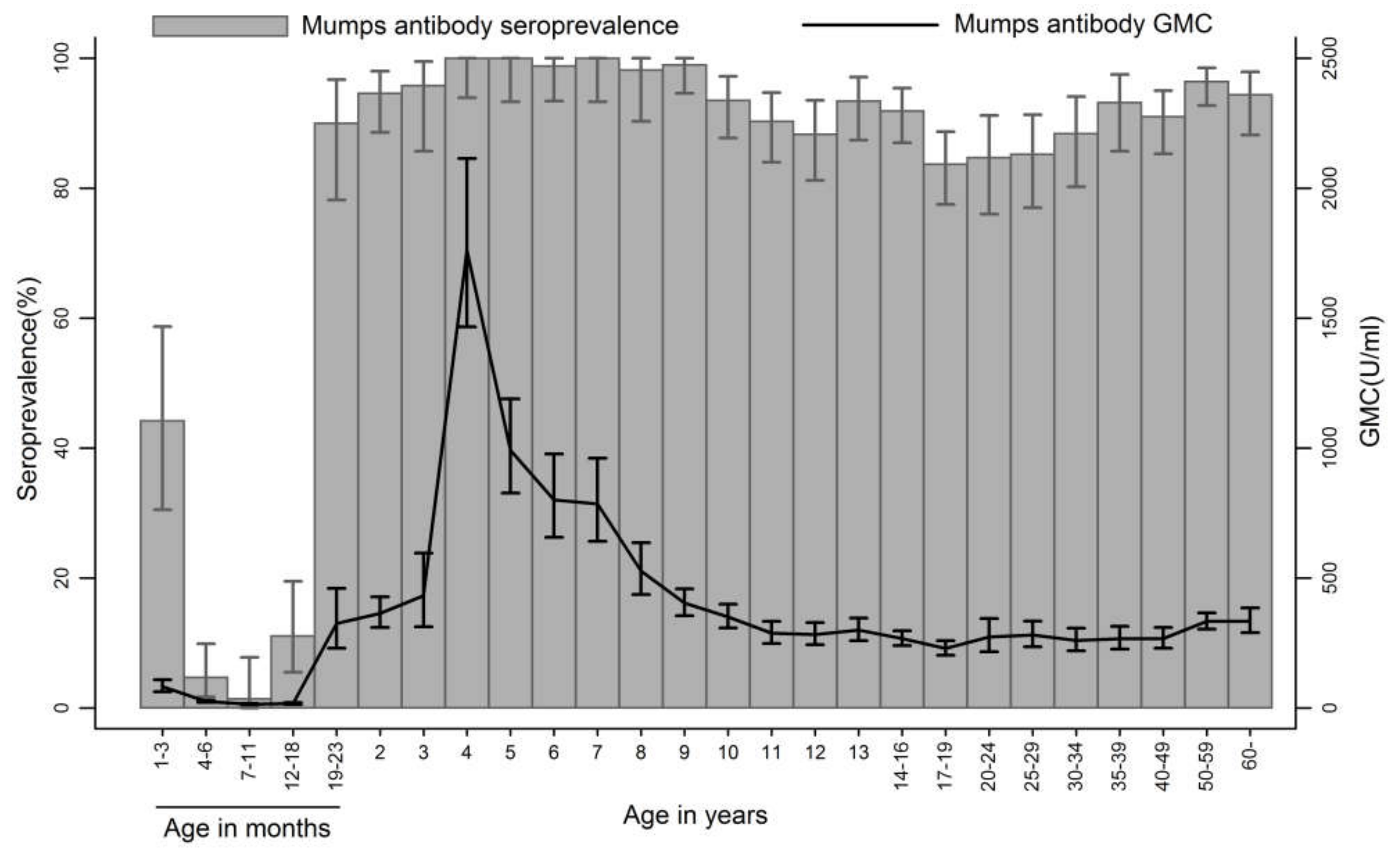
3.1. Seroprevalence and GMCs of Mumps Antibodies
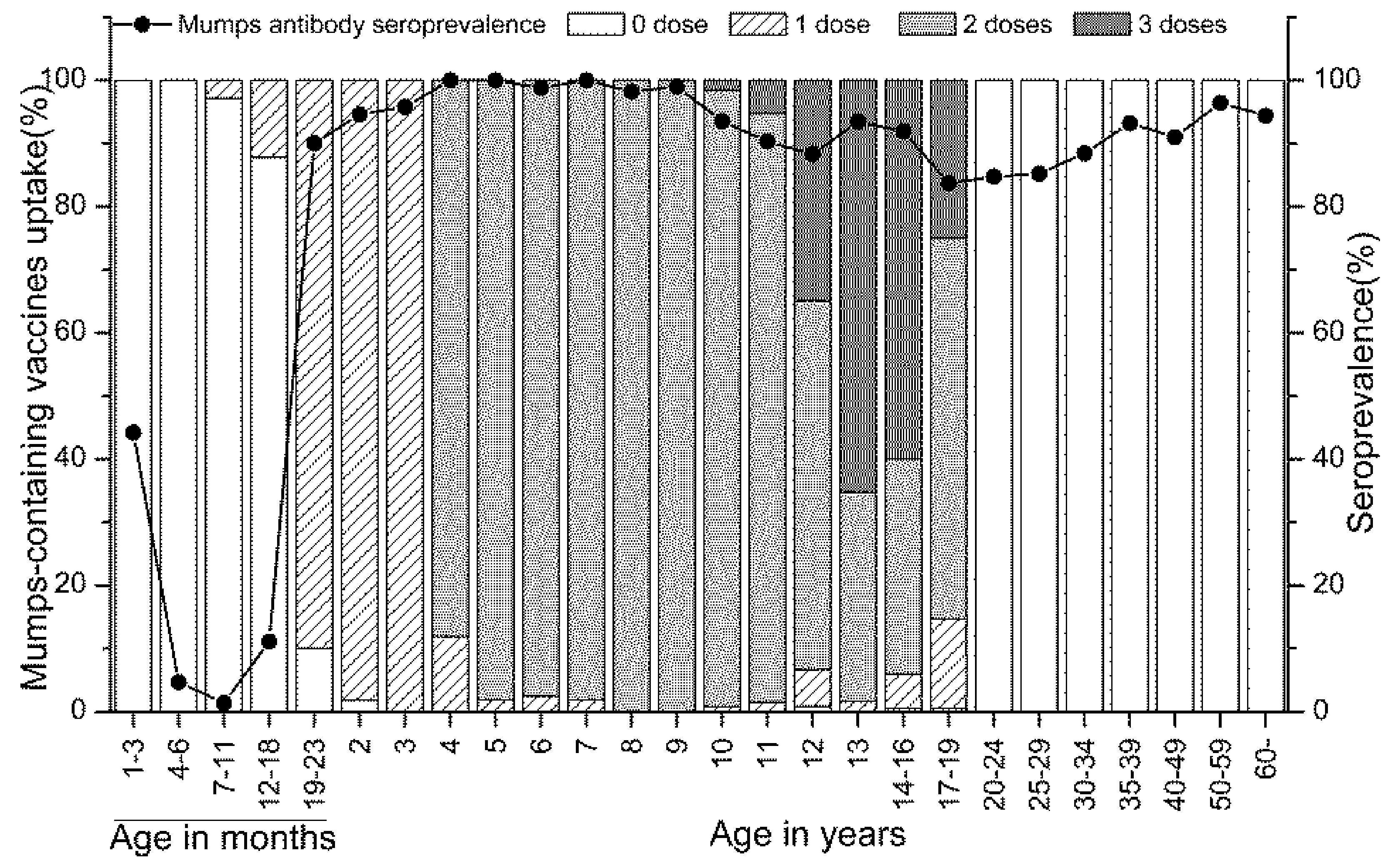
3.2. Vaccination Coverage and Mumps Seroprevalence
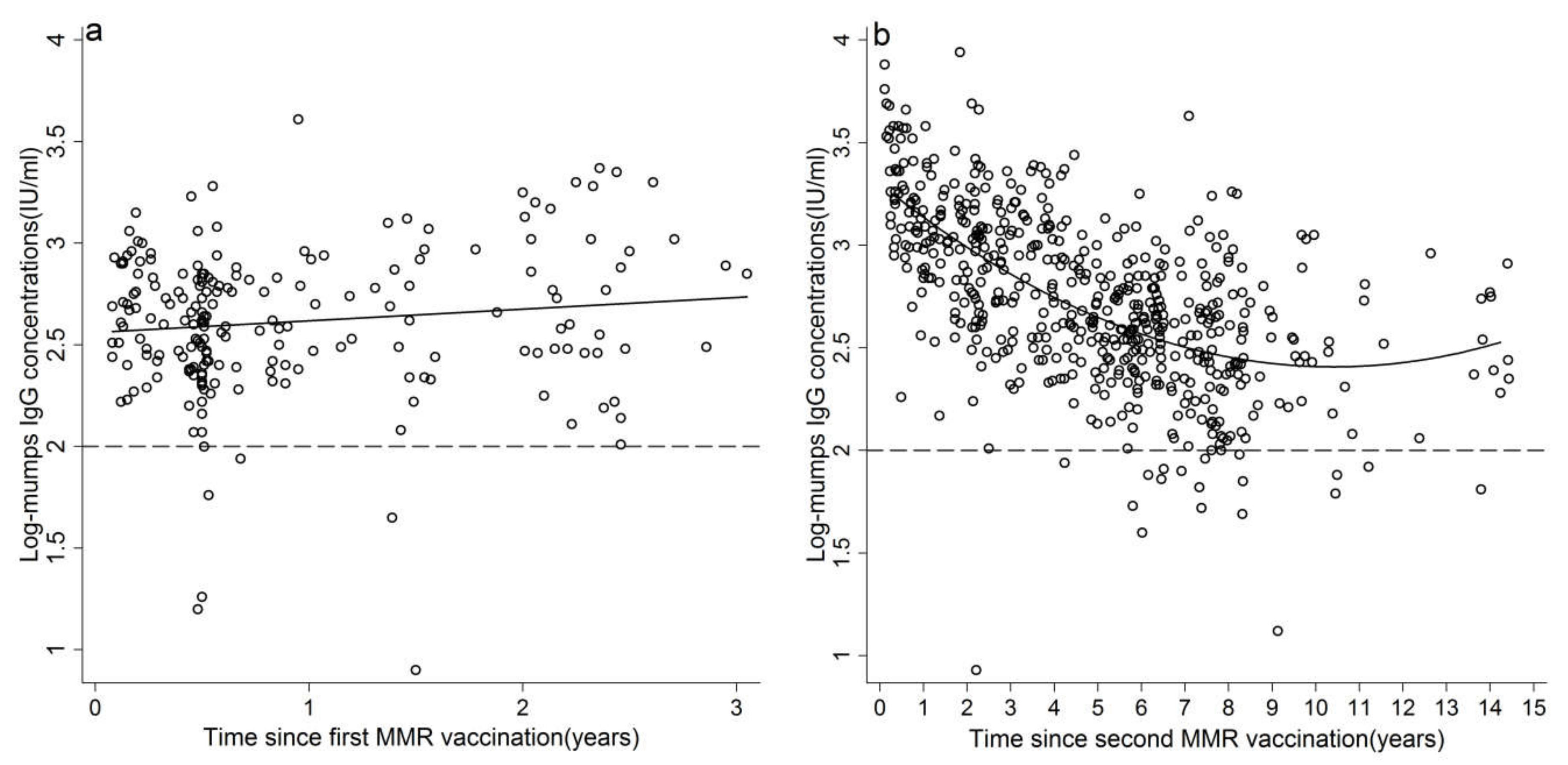
3.3. Waning Immunity of Mumps
3.4. Determinants for Seronegativity of Mumps Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, A.; Zhu, Z.; Hu, Y.; Deng, X.; Sun, Z.; Zhang, Y.; Mao, N.; Xu, S.; Fang, X.; Gao, H.; et al. Mumps epidemiology and mumps virus genotypes circulating in mainland China during 2013–2015. PLoS ONE 2017, 12, e0169561. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhu, Z.; Chen, M.; Zheng, H.; Liu, L.; Wang, Y.; Ma, Y.; Wang, C.; Fang, X.; Li, P.; et al. Epidemiologic and genetic characteristics of mumps viruses isolated in China from 1995 to 2010. Infect. Genet. Evol. 2014, 21, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Rubin, S.; Mühlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Li, Z.; Yang, J.; Sun, X. Epidemiology of mumps in Shanghai, 1990–2015. Chin. J. Vaccines Immun. 2018, 24, 43–47. [Google Scholar]
- Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases. Update on Mumps Epidemiology in the United States, 2017 and Review of Studies of 3rd Dose of MMR Vaccine. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-06/mumps-02-marin.pdf (accessed on 3 March 2018).
- European Centre for Disease Prevention and Control. Mumps-Annual Epidemiological Report for 2015. Available online: https://ecdc.europa.eu/en/publications-data/mumps-annual-epidemiological-report-2015 (accessed on 3 March 2018).
- Vygen, S.; Fischer, A.; Meurice, L.; Mounchetrou Njoya, I.; Gregoris, M.; Ndiaye, B.; Ghenassia, A.; Poujol, I.; Stahl, J.; Antona, D.; et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill. 2016, 21, 30156. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Grad, Y.H. Vaccine waning and mumps re-emergence in the United States. Sci. Transl. Med. 2018, 10, eaao5945. [Google Scholar] [CrossRef] [PubMed]
- Hamami, D.; Cameron, R.; Pollock, K.G.; Shankland, C. Waning immunity is associated with periodic large outbreaks of mumps: A mathematical modeling study of Scottish data. Front. Physiol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.J.; Gay, N.J.; Kretzschmar, M.; Pebody, R.G.; Wachmann, H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: Implications for modeling studies. Epidemiol. Infect. 2000, 125, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Crombie, J.A.; Grenfell, B.T. The epidemiology of mumps in the UK: A preliminary study of virus transmission, herd immunity and the potential impact of immunization. Epidemiol. Infect. 1987, 99, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Vaccination and herd immunity to infectious diseases. Nature 1985, 318, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Tsai, C.; Tsi, Y.; Wang, Y.; Lin, T.; Lee, D.; Chen, C. Humoral immunity to mumps in a highly vaccinated population in Taiwan. J. Microbiol. Immunol. Infect. 2017. [Google Scholar] [CrossRef] [PubMed]
- Smits, G.; Mollema, L.; Hahné, S.; de Melker, H.; Tcherniaeva, I.; Waaijenborg, S.; van Binnendijk, R.; van der Klis, F.; Berbers, G. Seroprevalence of mumps in the Netherlands: Dynamics over a decade with high vaccination coverage and recent outbreaks. PLoS ONE 2013, 8, e58234. [Google Scholar] [CrossRef] [PubMed]
- Kontio, M.; Jokinen, S.; Paunio, M.; Petola, H.; Davidkin, I. Waning antibody levels and avidity: Implications for MMR vaccine-induced protection. J. Infect. Dis. 2012, 206, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, C.W.; Forghani, B.; Beck, C.; Brown, C.; Bi, D.; Cossen, C.; Sullivan, B.J. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J. Infect. Dis. 2009, 199, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Beleni, A.I.; Borgmann, S. Mumps in the vaccination age: Global epidemiology and the situation in Germany. Int. J. Environ. Res. Public Health 2018, 15, 1618. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Committee on Infectious Diseases. Age for routine administration of the second dose of measles-mumps-rubella vaccine. Pediatrics 1998, 101, 129–133. [Google Scholar] [CrossRef]
- Dayan, G.H.; Quinlisk, M.P.; Parker, A.A.; Barskey, A.E.; Harris, M.L.; Hill Schwartz, J.M.; Hunt, K.; Finley, C.G.; Leschinsky, D.P.; O’Keefe, A.L.; et al. Recent resurgence of mumps in the United States. N. Engl. J. Med. 2008, 358, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Ogbuanu, I.U.; Kutty, P.K.; Hudson, J.M.; Blog, D.; Abedi, G.R.; Goodell, S.; Lawler, J.; Mclean, H.Q.; Pollock, L.; Rausch-Phung, E.; et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012, 130, e1567-74. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.E.; Aguon, A.; Valencia, E.; Oliva, R.; Guerrero, M.L.; Reyes, R.; Lizama, A.; Diras, D.; Mathew, A.; Camacho, E.J.; et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control-Guam 2009 to 2010. Pediatr. Infect. Dis. J. 2013, 32, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Quinlisk, P.; Weigel, A.; Riley, J.; James, L.; Patterson, J.; Hickman, C.; Rota, P.A.; Steward, R.; Clemmons, N.; et al. Mumps outbreak in a highly vaccinated university-affiliated setting before and after a measles-mumps-rubella (MMR) vaccination campaign-Iowa, July 2015-May 2016. Clin. Infect. Dis. 2018, 66, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fiebelkorn, A.P.; Coleman, L.A.; Belongia, E.A.; Freeman, S.K.; York, D.; Bi, D.; Zhang, C.; Ngo, L.; Rubin, S. Mumps antibody response in young adults after a third dose of measles-mumps-rubella vaccine. Open Forum Infect. Dis. 2014, 1, ofu094. [Google Scholar] [CrossRef] [PubMed]
- Latner, D.R.; Fiebelkorn, A.P.; McGrew, M.; Williams, N.J.; Coleman, L.A.; Mclean, H.Q.; Rubin, S.; Hickman, C.J. Mumps virus nucleoprotein and hemagglutinin-specific antibody response following a third dose of measles mumps rubella vaccine. Open Forum Infect. Dis. 2017, 4, ofx263. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Marlow, M.; Moore, K.L.; Patel, M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. Morb. Mortal. Wkly. Rep. 2018, 67, 33–38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Mumps virus vaccines. Wkly. Epidemiol. Rec. 2007, 82, 51–60. [Google Scholar]
- Scepanovic, P.; Alanio, C.; Hammer, C.; Hodel, F.; Bergstedt, J.; Patin, E.; Thorball, C.W.; Chaturvedi, N.; Charbit, B.; Abel, L.; et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, J.; Ma, C.; Fan, C.; Wen, N.; Luo, H.; Wang, H.; Li, L.; Hao, L. Epidemic profile of mumps in China during 2004–2013. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 611–614. [Google Scholar] [PubMed]
- Corinne, V.; Mathieu, R.; Geert, L.R.; Van Damme, P.; Hoppenbrouwers, K. Long-term persistence of antibodies after one or two doses of MMR-vaccine. Vaccine 2007, 25, 6672–6676. [Google Scholar] [CrossRef]
| Age | Total | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | U/mL (95% CI) | n | % (95% CI) | U/mL (95% CI) | n | % (95% CI) | U/mL (95% CI) | |
| 1–3 months | 52 | 44 (30–59) | 82 (62–109) | 23 | 35 (16–57) | 79 (50–126) | 29 | 52 (33–71) | 85 (59–121) |
| 4–6 months | 128 | 5 (2–10) | 25 (21–29) | 65 | 6 (2–15) | 26 (21–32) | 63 | 3 (0–11) | 24 (19–30) |
| 7–11 months | 69 | 1 (0–8) | 14 (12–17) | 38 | 0 (0-9) | 14 (12–16) | 31 | 3 (0–17) | 15 (12–20) |
| 12–18 months | 90 | 11 (5–19) | 17 (14–21) | 47 | 13 (5–26) | 19 (14–26) | 43 | 9 (3–22) | 16 (12–21) |
| 19–23 months | 50 | 90 (78–97) | 325 (230–460) | 31 | 90 (74–98) | 364 (236–562) | 19 | 89 (67–99) | 271 (145–505) |
| 2 years | 111 | 95 (89–98) | 364 (310–427) | 52 | 92 (81–98) | 329 (262–414) | 59 | 97 (88–100) | 398 (317–499) |
| 3 years | 48 | 96 (86–99) | 432 (313–596) | 23 | 96 (78–100) | 368 (218–621) | 25 | 96 (80–100) | 500 (328–761) |
| 4 years | 59 | 100 (94–100) | 1761 (1467–2114) | 26 | 100 (87–100) | 1731 (1344–2229) | 33 | 100 (89-100) | 1785 (1361–2341) |
| 5 years | 53 | 100 (93–100) | 992 (827–1190) | 29 | 100 (88–100) | 1057 (805–1388) | 24 | 100 (86-100) | 918 (716–1178) |
| 6 years | 82 | 99 (93–100) | 801 (656–978) | 52 | 100 (93–100) | 772 (634–939) | 30 | 97 (83–100) | 855 (549–1333) |
| 7 years | 53 | 100 (93–100) | 785 (641–961) | 33 | 100 (89–100) | 748 (580–965) | 20 | 100 (83–100) | 849 (591–1220) |
| 8 years | 55 | 98 (90–100) | 527 (437–636) | 29 | 100 (88–100) | 480 (375–614) | 26 | 96 (80–100) | 586 (434–790) |
| 9 years | 100 | 99 (95–100) | 404 (355–459) | 58 | 98 (91–100) | 387 (317–472) | 42 | 100 (92–100) | 428 (374–491) |
| 10 years | 124 | 94 (88–97) | 351 (308–399) | 66 | 91 (81–97) | 321 (269–382) | 58 | 97 (88–100) | 388 (320–471) |
| 11 years | 134 | 90 (84–95) | 288 (249–333) | 83 | 90 (82–96) | 271 (232–317) | 51 | 90 (79–97) | 316 (235–426) |
| 12 years | 120 | 88 (81–93) | 283 (244–329) | 68 | 85 (75–93) | 260 (211–321) | 52 | 92 (81–98) | 316 (256–389) |
| 13 years | 121 | 93 (87–97) | 300 (259–346) | 53 | 89 (77–96) | 295 (224–388) | 68 | 97 (90–100) | 303 (261–353) |
| 14-16 years | 185 | 92 (87–95) | 267 (240–297) | 95 | 88 (80–94) | 247 (212–288) | 90 | 96 (89–99) | 289 (250–335) |
| 17–19 years | 184 | 84 (78–89) | 229 (202–259) | 101 | 83 (74–90) | 217 (186–254) | 83 | 84 (75–91) | 244 (199–299) |
| 20–24 years | 98 | 85 (76–91) | 273 (217–344) | 56 | 84 (72–92) | 250 (181–346) | 42 | 85 (71–95) | 308 (220–430) |
| 25–29 years | 108 | 85 (77–91) | 281 (236–334) | 55 | 84 (71–92) | 248 (199–311) | 53 | 87 (75–95) | 319 (243–419) |
| 30–34 years | 95 | 88 (80–94) | 260 (220–307) | 50 | 88 (76–95) | 231 (185–288) | 45 | 89 (76–96) | 296 (230–382) |
| 35–39 years | 88 | 93 (86–97) | 266 (226–314) | 45 | 91 (79–96) | 243 (190–312) | 43 | 95 (84–99) | 293 (235–366) |
| 40–49 years | 155 | 91 (85–95) | 267 (230–310) | 50 | 94 (83–99) | 289 (229–364) | 105 | 90 (82–95) | 257 (212–312) |
| 50–59 years | 193 | 96 (93–99) | 333 (304–366) | 91 | 97 (91–99) | 321 (280-369) | 102 | 96 (90–99) | 345 (303–392) |
| 60 and above | 107 | 94 (88–98) | 334 (290–385) | 45 | 91 (79–98) | 292 (224–381) | 62 | 97 (89–100) | 368 (316–429) |
| Total | 2662 | 82 (81–83) | 245 (233–257) | 1233 | 81 (79–83) | 234 (219–251) | 1199 | 83 (81–85) | 257 (239–275) |
| Factors | n | Seronegativity (%, 95% CI) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| Crude OR (95%CI) | p Value | Adjusted OR * (95% CI) | p Value | |||
| Sex | 0.128 | |||||
| Male | 1364 | 19.1 (17.0–21.2) | 1.2 (0.96–1.4) | 1.4 (1.0–1.8) | 0.026 | |
| Female | 1298 | 16.8 (14.8–18.9) | Reference | Reference | ||
| Age (years) | <0.0001 | |||||
| 0–18 months | 339 | 88.2 (84.3–91.4) | 165.0 (86.5–315.0) | 165.6 (86.7–316.4) | <0.0001 | |
| 19–36 months | 209 | 6.2 (3.4–10.4) | 1.5 (0.7–3.2) | 1.4 (0.7–3.2) | 0.366 | |
| 4–9 | 402 | 0.7 (0.2–2.2) | 0.2 (0.0–0.6) | 0.2 (0.0–0.6) | 0.005 | |
| 10–12 | 378 | 9.3 (6.5–12.4) | 2.3 (1.2–4.3) | 2.2 (1.1–4.2) | 0.021 | |
| 13–16 | 306 | 7.5 (4.8–11.1) | 1.8 (0.9–3.6) | 1.8 (0.9–3.6) | 0.107 | |
| 17–19 | 184 | 16.3 (11.3–22.5) | 4.3 (2.2–8.5) | 4.2 (2.1–8.3) | <0.0001 | |
| 20–29 | 206 | 15.0 (10.5–20.7) | 3.9 (2.0–7.7) | 3.8 (1.9–7.5) | <0.0001 | |
| 30–39 | 183 | 9.3 (5.5–14.5) | 2.3 (1.1–4.8) | 2.2 (1.0–4.7) | 0.037 | |
| 40–49 | 155 | 9.0 (5.0–14.7) | 2.2 (1.0–4.8) | 2.3 (1.0–5.0) | 0.038 | |
| ≥50 | 300 | 4.3 (2.3–7.3) | Reference | Reference | ||
| Household registration | 0.016 | |||||
| Local | 1449 | 19.6 (17.6–21.7) | 1.3 (1.0–1.6) | 1.1 (0.8–1.5) | 0.435 | |
| Migrant | 1213 | 16.0 (14.0–18.2) | Reference | Reference | ||
| Number of vaccinations | <0.0001 | |||||
| 0 dose | 1180 | 32.0 (29.4–34.8) | 7.0 (4.3–11.5) | 139.8 (38.3–510.9) | <0.0001 | |
| 1 dose | 274 | 8.8 (5.7–12.8) | 1.4 (0.8–2.7) | 3.9 (1.7–9.0) | 0.001 | |
| 2 doses | 921 | 6.3 (4.8–8.1) | 1.0 (0.6–1.7) | 1.7 (0.9–3.0) | 0.102 | |
| 3 doses | 287 | 6.3 (3.8–9.7) | Reference | Reference | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, H.; Zhou, Y.; Zhao, W.; Jiang, Q. Seroprevalence and Determinants Associated with Mumps Antibodies after 20 Years of MMR Vaccination in Urban Area of Shanghai, China. Int. J. Environ. Res. Public Health 2018, 15, 2089. https://doi.org/10.3390/ijerph15102089
Pang H, Zhou Y, Zhao W, Jiang Q. Seroprevalence and Determinants Associated with Mumps Antibodies after 20 Years of MMR Vaccination in Urban Area of Shanghai, China. International Journal of Environmental Research and Public Health. 2018; 15(10):2089. https://doi.org/10.3390/ijerph15102089
Chicago/Turabian StylePang, Hong, Yibiao Zhou, Wensui Zhao, and Qingwu Jiang. 2018. "Seroprevalence and Determinants Associated with Mumps Antibodies after 20 Years of MMR Vaccination in Urban Area of Shanghai, China" International Journal of Environmental Research and Public Health 15, no. 10: 2089. https://doi.org/10.3390/ijerph15102089
APA StylePang, H., Zhou, Y., Zhao, W., & Jiang, Q. (2018). Seroprevalence and Determinants Associated with Mumps Antibodies after 20 Years of MMR Vaccination in Urban Area of Shanghai, China. International Journal of Environmental Research and Public Health, 15(10), 2089. https://doi.org/10.3390/ijerph15102089





