A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Procedure
2.2. Participants
2.3. Interventions
2.4. Measurements
2.5. Statistical Analyses
3. Results
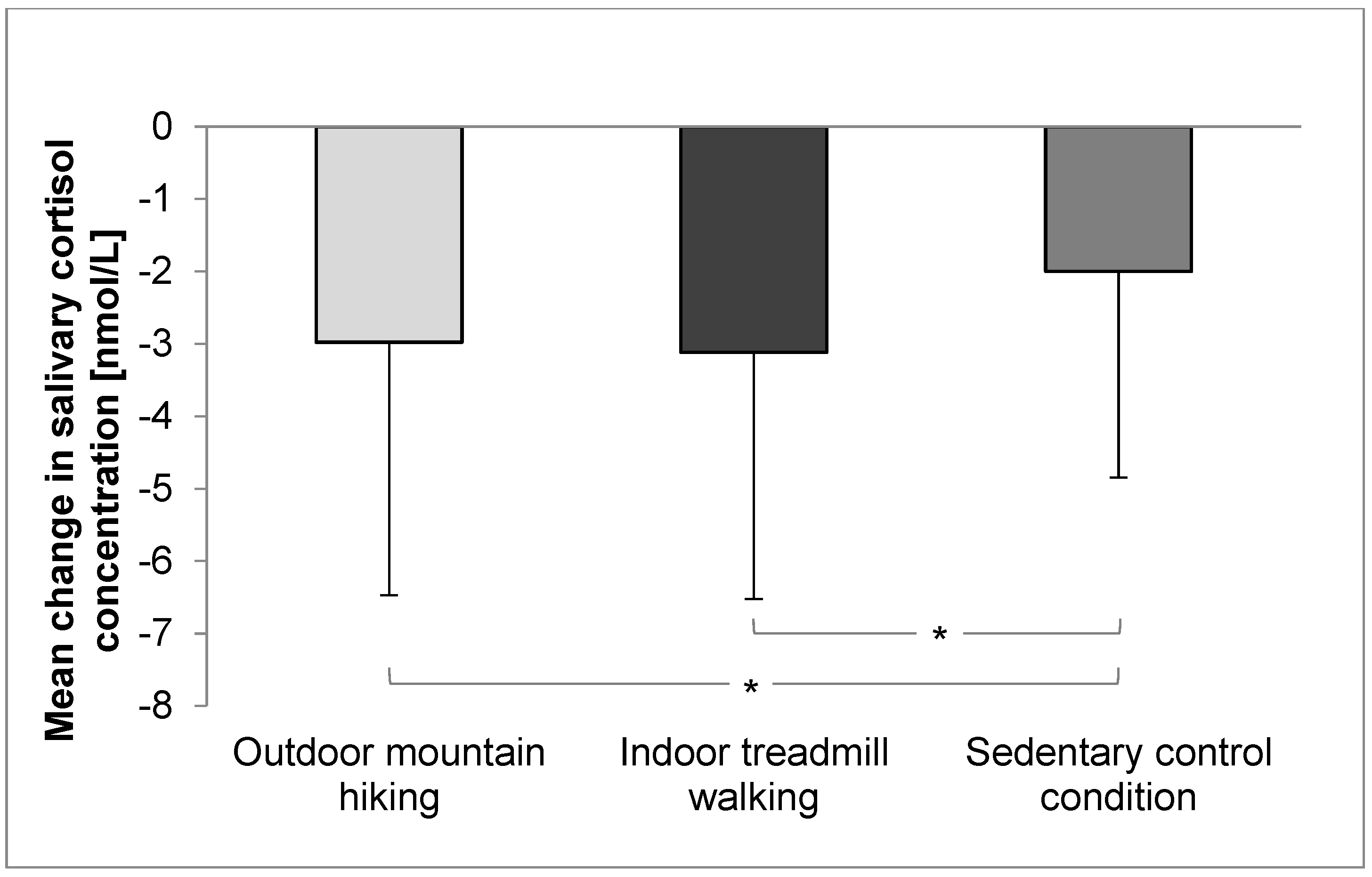
3.1. Salivary Cortisol Concentration and Cardiovascular Parameters
3.2. Influence of Sex and Blood Pressure on Salivary Cortisol Concentration and Cardiovascular Parameters
3.3. Walking Intensity
4. Discussion
4.1. Main Results
4.2. Effects of Physical Activity on Stress-Related Physiological Parameters
4.3. Environmental Effects on Stress-Related Physiological Parameters
4.4. Other Sources of Influence on Stress-Related Physiological Parameters
4.5. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, R.B.; Burke, T.F. Urbanization—An Emerging Humanitarian Disaster. N. Engl. J. Med. 2009, 361, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, R.; Julien, M. Urbanisation and health. Clin. Med. (Northfield Il.) 2005, 5, 137–141. [Google Scholar] [CrossRef]
- Harpham, T. Urbanisation and health in transition. The Lancet 1997, 349, S11–S13. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Hartig, T.; Mang, M.; Evans, G.W. Restorative effects of natural environment experiences. Environ. Behav. 1991, 23, 3–26. [Google Scholar] [CrossRef]
- Gerber, M.; Pühse, U. Review article: Do exercise and fitness protect against stress-induced health complaints? A review of the literature. Scand. J. Public Health 2009, 37, 801–819. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Taylor, A.; Steptoe, A. The effect of acute aerobic exercise on stress related blood pressure responses: A systematic review and meta-analysis. Biol. Psychol. 2006, 71, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.; Pretty, J. What is the best dose of nature and green exercise for improving mental health? A multi-study analysis. Environ. Sci. Technol. 2010, 44, 3947–3955. [Google Scholar] [CrossRef] [PubMed]
- Thompson Coon, J.; Boddy, K.; Stein, K.; Whear, R.; Barton, J.; Depledge, M.H. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ. Sci. Technol. 2011, 45, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bowler, D.E.; Buyung-Ali, L.M.; Knight, T.M.; Pullin, A.S. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health 2010, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Haluza, D.; Schonbauer, R.; Cervinka, R. Green perspectives for public health: A narrative review on the physiological effects of experiencing outdoor nature. Int. J. Environ. Res. Public Health 2014, 11, 5445–5461. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, M.; Berkhof, H.; Nicolson, N.; Sulon, J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 1996, 58, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.M.; Bryan, A. In-task and post-task affective response to exercise: Translating exercise intentions into behaviour. Br. J. Health Psychol. 2010, 15, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.E.; Kates, A. Can the affective response to exercise predict future motives and physical activity behavior? A systematic review of published evidence. Ann. Behav. Med. 2015, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Dunsiger, S.; Ciccolo, J.T.; Lewis, B.A.; Albrecht, A.E.; Marcus, B.H. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychol. Sport Exerc. 2008, 9, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Peacock, J.; Sellens, M.; Griffin, M. The mental and physical health outcomes of green exercise. Int. J. Environ. Health Res. 2005, 15, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Schobersberger, W.; Leichtfried, V.; Mueck-Weymann, M.; Humpeler, E. Austrian moderate altitude studies (amas): Benefits of exposure to moderate altitudes (1,500–2,500 m). Sleep Breath 2010, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sturm, J.; Plöderl, M.; Fartacek, C.; Kralovec, K.; Neunhäuserer, D.; Niederseer, D.; Hitzl, W.; Niebauer, J.; Schiepek, G.; Fartacek, R. Physical exercise through mountain hiking in high-risk suicide patients. A randomized crossover trial. Acta Psychiatr. Scand. 2012, 126, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, M.; Einwanger, J.; Hartl, A.; Kopp, M. Affective responses in mountain hiking—A randomized crossover trial focusing on differences between indoor and outdoor activity. PLoS ONE 2017, 12, e0177719. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J.; Thomas, S.; Weller, I. The canadian home fitness test. 1991 update. Sports Med. 1991, 11, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, A.E.; Custers, M.H. Gardening promotes neuroendocrine and affective restoration from stress. J. Health Psychol. 2011, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Garatachea, N.; Almeida, R.; Casajus, J.A.; Bailon, R. Validation of heart rate monitor polar rs800 for heart rate variability analysis during exercise. J. Strength Cond. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Donovan, G.; Jakovljevic, D.; Hodges, L.; Sandercock, G.; Brodie, D. Validity and reliability of short-term heart-rate variability from the polar s810. Med. Sci. Sports Exerc. 2009, 41, 243. [Google Scholar] [CrossRef] [PubMed]
- Task Force of The European Society of Cardiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the european society of cardiology and the north american society of pacing and electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J., 3rd; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Burr, R.L. Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep 2007, 30, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 377–381. [Google Scholar] [CrossRef]
- Noble, B.J.; Robertson, R.J. Perceived Exertion; Human Kinetics: Mitcham, Australia, 1996. [Google Scholar]
- Field, A. Discovering Statistics Using Spss, 3rd ed.; SAGE: London, UK, 2009. [Google Scholar]
- Liu, S.Y.; Wrosch, C.; Miller, G.E.; Pruessner, J.C. Self-esteem change and diurnal cortisol secretion in older adulthood. Psychoneuroendocrinology 2014, 41, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, ON, Canada, 1988. [Google Scholar]
- Heaney, J.L.J.; Carroll, D.; Phillips, A.C. DHEA, DHEA-s and cortisol responses to acute exercise in older adults in relation to exercise training status and sex. Age 2013, 35, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Ida, I.; Wada, N.; Sohmiya, M.; Tazawa, M.; Shirakura, K. A clinical study of the efficacy of a single session of individual exercise for depressive patients, assessed by the change in saliva free cortisol level. Biopsychosoc. Med. 2013, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Kuratorium für Verkehrssicherheit. Freizeitunfallstatistik 2005. Available online: http://unfallstatistik.kfv.at/uploads/media/Freizeitunfallstatistik_2005.pdf (accessed on 24 December 2015).
- Statistik Austria. Population statistics. Available online: http://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/bevoelkerung/bevoelkerungsstruktur/bevoelkerung_nach_alter_geschlecht/index.html (accessed on 3 March 2016).
- Burtscher, M. High altitude headache: Epidemiology, pathophysiology, therapy and prophylaxis. Wien. Klin. Wochenschr. 1999, 111, 830–836. [Google Scholar] [PubMed]
- Powell, J.; DiLeo, T.; Roberge, R.; Coca, A.; Kim, J.H. Salivary and serum cortisol levels during recovery from intense exercise and prolonged, moderate exercise. Biol. Sport 2015, 32, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.; De Palo, E.F. An update: Salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 2011, 21, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Lackner, H.K.; Weiss, E.M.; Hinghofer-Szalkay, H.; Papousek, I. Cardiovascular effects of acute positive emotional arousal. Appl. Psychophysiol. Biofeedback 2014, 39, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lakin, R.; Notarius, C.; Thomas, S.; Goodman, J. Effects of moderate-intensity aerobic cycling and swim exercise on post-exertional blood pressure in healthy young untrained and triathlon-trained men and women. Clin. Sci. (Lond.) 2013, 125, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Rezk, C.C.; Marrache, R.C.B.; Tinucci, T.; Mion, D.; Forjaz, C.L.M. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: Influence of exercise intensity. Eur. J. Appl. Physiol. 2006, 98, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American college of sports medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Gidlow, C.J.; Jones, M.V.; Hurst, G.; Masterson, D.; Clark-Carter, D.; Tarvainen, M.P.; Smith, G.; Nieuwenhuijsen, M. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J. Environ. Psychol. 2016, 45, 22–29. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrvainen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complement. Alternat. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef] [PubMed]
- Tyrväinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The influence of urban green environments on stress relief measures: A field experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N.; Schlotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.J.; Charlamb, M.; Sherman, H.B. Circadian patterns of heart rate variability in normals, chronic stable angina and diabetes mellitus. Int. J. Cardiol. 1999, 71, 41–48. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Kudielka, B.M.; Gaab, J.; Schommer, N.C.; Hellhammer, D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Patel, V.; Saxena, S.; Maj, M.; Maselko, J.; Phillips, M.R.; Rahman, A. No health without mental health. Lancet 2007, 370, 859–877. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Philippe, M.; Junker, G.; Gatterer, H.; Melmer, A.; Burtscher, M. Acute effects of concentric and eccentric exercise matched for energy expenditure on glucose metabolism in healthy females: A randomized crossover trial. Springerplus 2016, 5, 1455. [Google Scholar] [CrossRef] [PubMed]

| Total Group | Female | Male | |||
|---|---|---|---|---|---|
| Mean (SD 1) | Minimum | Maximum | Mean (SD 1) | Mean (SD 1) | |
| Age (years) | 32.0 (12.0) | 19.0 | 66.0 | 35.9 (15.7) | 28.5 (7.3) |
| Height (m) | 1.74 (0.10) | 1.52 | 1.95 | 1.66 (0.08) | 1.82 (0.06) |
| Weight (kg) | 69.0 (11.0) | 46.0 | 92.0 | 60.9 (6.7) | 77.0 (7.7) |
| Body mass index (kg/m2) | 23.0 (2.0) | 18.1 | 26.6 | 22.1 (2.2) | 23.3 (1.5) |
| Physical activity (h/week) | 8.0 (5.0) | 0.0 | 25.0 | 7.0 (3.9) | 9.2 (6.3) |
| Mountain tours (n/year) | 27.2 (26.2) | 0.0 | 100.0 | 36.7 (27.0) | 18.6 (22.7) |
| Outdoor Mountain Hiking | Indoor Treadmill Walking | Sedentary Control Condition | p-Value | η2p 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL 2 | FU 3 | BL 2 | FU 3 | BL 2 | FU 3 | Condition | Time | Interaction | M-C 4 | M-T 5 | |
| Cortisol [nmol/L] | 4.7 (3.7) | 1.8 (1.2) | 5.0 (3.5) | 1.8 (1.1) | 4.3 (3.2) | 2.3 (1.8) | 0.616 | <0.001 * | 0.032 * | 0.10 | 0.00 |
| SDNN 6 [ms] | 76.3 (40.9) | 85.4 (47.5) | 70.7 (40.6) | 77.9 (41.6) | 74.2 (51.3) | 86.5 (32.4) | 0.518 | 0.009 * | 0.833 | 0.00 | 0.00 |
| RMSSD 7 [ms] | 58.4 (44.5) | 68.6 (56.6) | 51.5 (43.9) | 57.3 (41.0) | 46.7 (27.0) | 61.8 (27.7) | 0.402 | <0.001 * | 0.502 | 0.02 | 0.00 |
| Total power [ms2] | 9497 (10,715) | 10,504 (10,254) | 7906 (10,552) | 10,055 (10,574) | 10,443 (26,532) | 11,568 (9456) | 0.127 | 0.005 * | 0.030 | 0.12 | 0.01 |
| LF 8 [ms2] | 2331 (2399) | 2967 (2729) | 1973 (2934) | 2614 (2505) | 2084 (2005) | 2622 (1836) | 0.083 | 0.068 | 0.030 | 0.13 | 0.00 |
| LFn 9 | 63.8 (17.7) | 66.4 (16.4) | 66.5 (14.7) | 71.1 (13.1) | 69.6 (14.5) | 65.0 (14.7) | 0.205 | 0.516 | 0.024 | 0.09 | 0.01 |
| HF 10 [ms2] | 1785 (2538) | 2409 (3568) | 1489 (2659) | 1548 (1996) | 946 (928) | 1581 (1388) | 0.096 | 0.012 | 0.498 | 0.03 | 0.00 |
| HFn 11 | 36.2 (17.7) | 33.6 (16.4) | 33.5 (14.7) | 28.9 (13.1) | 30.4 (14.5) | 35.0 (14.7) | 0.205 | 0.515 | 0.024 | 0.09 | 0.01 |
| LF/HF 12 | 292.3 (314.9) | 313.7 (316.9) | 293.1 (275.0) | 356.8 (361.1) | 327.6 (254.6) | 276.7 (268.8) | 0.323 | 0.563 | 0.027 | 0.09 | 0.01 |
| Systolic BP 13 [mmHg] | 127.2 (11.6) | 121.3 (11.2) | 123.5 (13.2) | 119.0 (11.0) | 121.6 (13.7) | 119.8 (14.7) | 0.006* | <0.001 * | 0.155 | 0.07 | 0.01 |
| Diastolic BP [mmHg] | 77.7 (7.6) | 78.3 (7.8) | 75.8 (7.4) | 72.6 (8.1) | 73.0 (10.2) | 73.5 (8.1) | <0.001* | 0.371 | 0.040 | 0.00 | 0.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedermeier, M.; Grafetstätter, C.; Hartl, A.; Kopp, M. A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking. Int. J. Environ. Res. Public Health 2017, 14, 905. https://doi.org/10.3390/ijerph14080905
Niedermeier M, Grafetstätter C, Hartl A, Kopp M. A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking. International Journal of Environmental Research and Public Health. 2017; 14(8):905. https://doi.org/10.3390/ijerph14080905
Chicago/Turabian StyleNiedermeier, Martin, Carina Grafetstätter, Arnulf Hartl, and Martin Kopp. 2017. "A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking" International Journal of Environmental Research and Public Health 14, no. 8: 905. https://doi.org/10.3390/ijerph14080905
APA StyleNiedermeier, M., Grafetstätter, C., Hartl, A., & Kopp, M. (2017). A Randomized Crossover Trial on Acute Stress-Related Physiological Responses to Mountain Hiking. International Journal of Environmental Research and Public Health, 14(8), 905. https://doi.org/10.3390/ijerph14080905






