Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines—Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Human Serum Samples
2.3. Instrumentation and Reagents
2.4. Statistical Analysis
3. Results
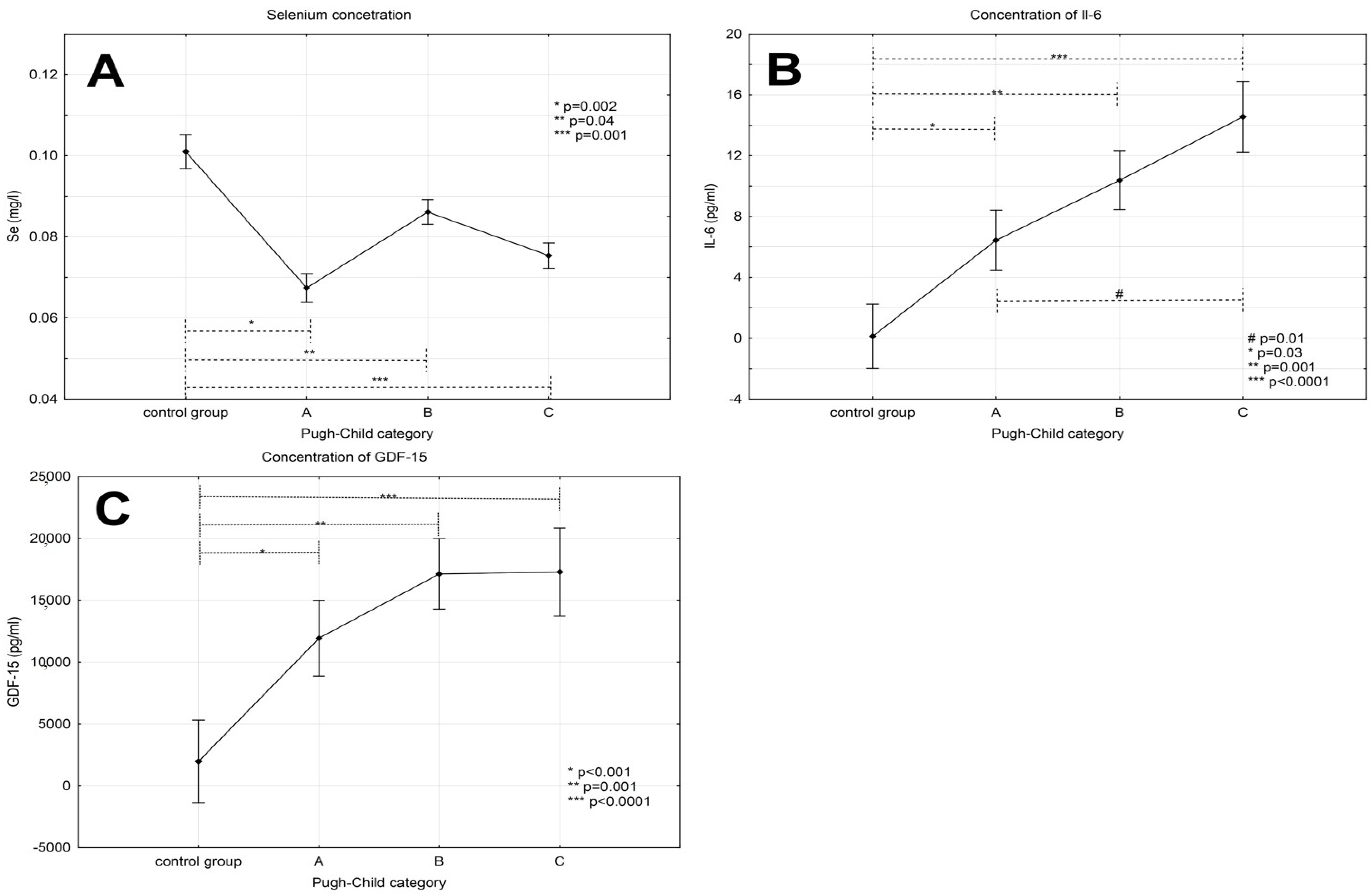
3.1. Serum Selenium Concentration in Patients with Alcoholic Liver Cirrhosis Compared to the Control Group
3.2. Levels of Interleukin-6 in Patients with Alcoholic Liver Cirrhosis
3.3. Levels of GDF-15 in Patients with Alcoholic Liver Cirrhosis
3.4. Correlations among Serum Selenium Concentration and GDF-15 and IL-6
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Shea, R.; Dasarthy, S.; McCullough, A. Alcoholic liver disease. Hepatology 2010, 51, 307–328. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Global Status Report on Alcohol; Department of Substance Abuse, World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Seth, D.; D’Souza El-Guindy, N.B.; Apte, M.; Mari, M.; Dooley, S.; Neuman, M.; Haber, P.S. Alcohol, signaling, and ECM turnover. Alcohol Clin. Exp. Res. 2010, 34, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, oxidative stress, and health aspects. Mol. Asp. Med. 2005, 26, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, B.É.; Pataki, V.; Jenei, T.; Adány, R.; Vokó, Z. Selenium levels in men with liver disease in Hungary. J. Trace Elem. Med. Biol. 2012, 26, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rua, R.M.; Ojeda, M.L.; Nogales, F.; Rubio, J.M.; Romero-Gómez, M.; Funuyet, J.; Murillo, M.L.; Carreras, O. Serum selenium levels and oxidative balance as differential markers in hepatic damage caused by alcohol. Life Sci. 2014, 94, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, A.; Klatka, M.; Astel, A.; Korona-Glowniak, I.; Dolliver, W.; Szwerc, W.; Kocjan, R. Serum and urinary selenium levels in obese children: A cross-sectional study. J. Trace Elem. Med. Biol. 2015, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Prystupa, A.; Błażewicz, A.; Kiciński, P.; Sak, J.J.; Niedziałek, J.; Załuska, W. Serum concentrations of selected heavy metals in patients with alcoholic liver cirrhosis from the Lublin Region in Eastern Poland. Int. J. Environ. Res. Public Health 2016, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol. Trace Elem. Res. 2015, 165, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.G.; Kolachi, N.F.; Afridi, H.I.; Kazi, N.G.; Arain, S.S. Effects of mineral supplementation on liver cirrhotic/cancer male patients. Biol. Trace Elem. Res. 2012, 150, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, M.; Nogueras-López, F.; Arcos-Cebrián, A.; Agil, A.; Navarro-Alarcón, M. Serum selenium levels in cirrhotic patients are not influenced by the disease severity index. Nutr. Res. 2010, 30, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Kaser, A.; Moschen, R. How to modulate inflammatory cytokines in liver diseases. Liver Int. 2006, 26, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Prystupa, A.; Kiciński, P.; Sak, J.; Boguszewska-Czubara, A.; Toruń-Jurkowska, A.; Załuska, W. Proinflammatory cytokines (IL-1α, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol. Res. Pract. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Yoneyama, H.; Kurokohchi, K.; Inukai, M.; Masugata, H.; Goda, F.; Haba, R.; Watababe, S.; Kubota, S.; Senda, S.; et al. Selenium deficiency is associated with insulin resistance in patients with hepatitis C virus-related chronic liver disease. Nutr. Res. 2011, 31, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Ye, G.; Khan, A.; Liu, J.; Gan, F.; Zhang, X.; Kumbhar, S.; Huang, K. Protective effects of Selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J. Agric. Food Chem. 2015, 63, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Potter, J.J.; Liu, X.; Torbenson, M.S.; Mezey, E. Selenium supplementation decreases hepatic fibrosis in mice after chronic carbon tetrachloride administration. Biol. Trace Elem. Res. 2010, 133, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.N.; Mahboob, T. Role of selenium in protection of liver cirrhosis. Pak. J. Pharm. Sci. 2013, 26, 1097–1102. [Google Scholar] [PubMed]
- He, Y.T.; Liu, D.W.; Ding, L.Y.; Li, Q.; Xiao, Y.H. Therapeutic effects and molecular mechanisms of anti-fibrosis herbs and selenium on rats with hepatic fibrosis. World J. Gastroenterol. 2004, 10, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.; Lowes, D.A.; Webster, N.R.; Talib, J.; Hall, L.; Davies, M.J.; Beattie, J.H.; Galley, H.F. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br. J. Anaesth. 2015, 114, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Li, H.; Guo, Y.; Jin, Y.; Lin, D. Sodium selenite inhibits the expression of VEGF, TGFbeta(1) and IL-6 induced by LPS in human PC3 cells via TLR4-NF-(K)B signaling blockage. Int. Immunopharmacol. 2010, 10, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Shilo, S.; Pardo, M.; Aharoni-Simon, M.; Glibter, S.; Tirosh, O. Selenium supplementation increases liver MnSOD expression: Molecular mechanism for hepato-protection. J. Inorg. Biochem. 2008, 102, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Byrne, D.W.; Norsworthy, B.K. Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Ho, C.T.; Hsu, H.S.; Lin, C.H.; Li, C.I.; Li, T.C.; Liu, C.S.; Lin, C.C.; Lin, W.Y. Selenium is inversely associated with interleukin-6 in the elderly. J. Nutr. Health Aging 2013, 17, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S. Effect of selenium on the levels of cytokines and trace elements in toxin-mediated oxidative stress in male rats. Biol. Trace Elem. Res. 2016, 169, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Daeian, N.; Radfar, M.; Jahangard-Rafsanjani, Z.; Hadjibabaie, M.; Ghavamzadeh, A. Selenium supplementation in patients undergoing hematopoietic stem cell transplantation: Effects on pro-inflammatory cytokines levels. Daru 2014, 17, 22–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS ONE 2015, 10, e0127518. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, S.H.; Kim, H.J.; Kim, K.H.; Lee, B.S.; Ku, B.J. Growth differentiation factor-15 predicts chronic liver disease severity. Gut Liver 2017, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Control Group (n = 20) | Alcoholic Liver Cirrhosis (n = 99) | ||
|---|---|---|---|---|
| P-Ch A (n = 29) | P-Ch B (n = 36) | P-Ch C (n = 34) | ||
| Age (years) | 48.9 ± 15.1 | 53.3 ± 12.3 | 54.6 ± 11.0 | 56.9 ± 7.7 |
| The percentage of men | 65% | 72.4% | 66.7% | 58.8% |
| Height (cm) | 170.4 ± 6.6 | 171.8 ± 7.8 | 175.3 ± 8.4 | 173.4 ± 6.9 |
| Body mass (kg) | 67.8 ± 7.8 | 68.1 ± 14.8 | 71.5 ± 13.2 | 70.2 ± 12.8 |
| Time of alcohol abuse (years) | - | 13.1 ± 4.8 | 14.1 ± 4.9 | 15.7 ± 5.4 |
| Ascites (%) | 0 | 32% | 61% | 85% |
| Esophageal varices (%) | 0 | 14% | 45% | 88% |
| Encephalopathy (%) | 0 | 28% | 51% | 91% |
| Caracteristic | Control Group (n = 20) | Alcoholic Liver Cirrhosis (n = 99) | ||
|---|---|---|---|---|
| P-Ch A (n = 29) | P-Ch B (n = 36) | P-Ch C (n = 34) | ||
| Bilirubin (mg/dL) | 0.68 ± 0.28 | 2.21 ± 1.4 | 4.12 ± 3.25 | 7.89 ± 7.94 |
| Albumin (g/dL) | - | 3.27 ± 0.76 | 2.78 ± 0.6 | 2.44 ± 0.46 |
| INR | - | 1.25 ± 0.27 | 1.44 ± 0.29 | 1.68 ± 0.41 |
| Blood platelets (g/L) | 226.8 ± 35.8 | 186.03 ± 76.9 | 123.6 ± 66.25 | 114.11 ± 61.6 |
| Mean cell volume (fL) | 94.65 ± 4.45 | 92.38 ± 6.25 | 91.9 ± 10.08 | 97.53 ± 8.02 |
| Urea (mg/dL) | - | 32.04 ± 20.1 | 23.49 ± 15.62 | 39.58 ± 16.1 |
| Sodium (mmol/L) | 139.82 ± 3.24 | 133.67 ± 5.3 | 135.38 ± 3.6 | 133.51 ± 6.63 |
| Potassium (mmol/L) | 4.41 ± 0.37 | 3.88 ± 0.6 | 3.94 ± 0.6 | 3.3 ± 0.66 |
| C-reactive protein (mg/L) | 2.11 ± 1.96 | 14.97 ± 12.62 | 19.21 ± 17.35 | 20.8 ± 19.92 |
| Variable | Correlation Coefficient p-Value | |
|---|---|---|
| Selenium | GDF-15 | r = −0.29 |
| p = 0.03 | ||
| IL-6 | r = −0.38 | |
| p = 0.03 | ||
| CRP | r = −0.17 | |
| NS | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prystupa, A.; Kiciński, P.; Luchowska-Kocot, D.; Błażewicz, A.; Niedziałek, J.; Mizerski, G.; Jojczuk, M.; Ochal, A.; Sak, J.J.; Załuska, W. Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines—Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis. Int. J. Environ. Res. Public Health 2017, 14, 437. https://doi.org/10.3390/ijerph14040437
Prystupa A, Kiciński P, Luchowska-Kocot D, Błażewicz A, Niedziałek J, Mizerski G, Jojczuk M, Ochal A, Sak JJ, Załuska W. Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines—Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis. International Journal of Environmental Research and Public Health. 2017; 14(4):437. https://doi.org/10.3390/ijerph14040437
Chicago/Turabian StylePrystupa, Andrzej, Paweł Kiciński, Dorota Luchowska-Kocot, Anna Błażewicz, Jarosław Niedziałek, Grzegorz Mizerski, Mariusz Jojczuk, Andrzej Ochal, Jarosław J. Sak, and Wojciech Załuska. 2017. "Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines—Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis" International Journal of Environmental Research and Public Health 14, no. 4: 437. https://doi.org/10.3390/ijerph14040437
APA StylePrystupa, A., Kiciński, P., Luchowska-Kocot, D., Błażewicz, A., Niedziałek, J., Mizerski, G., Jojczuk, M., Ochal, A., Sak, J. J., & Załuska, W. (2017). Association between Serum Selenium Concentrations and Levels of Proinflammatory and Profibrotic Cytokines—Interleukin-6 and Growth Differentiation Factor-15, in Patients with Alcoholic Liver Cirrhosis. International Journal of Environmental Research and Public Health, 14(4), 437. https://doi.org/10.3390/ijerph14040437








