Efficacy and Safety Evaluation of a Chlorine Dioxide Solution
Abstract
:1. Introduction
2. Materials and Methods
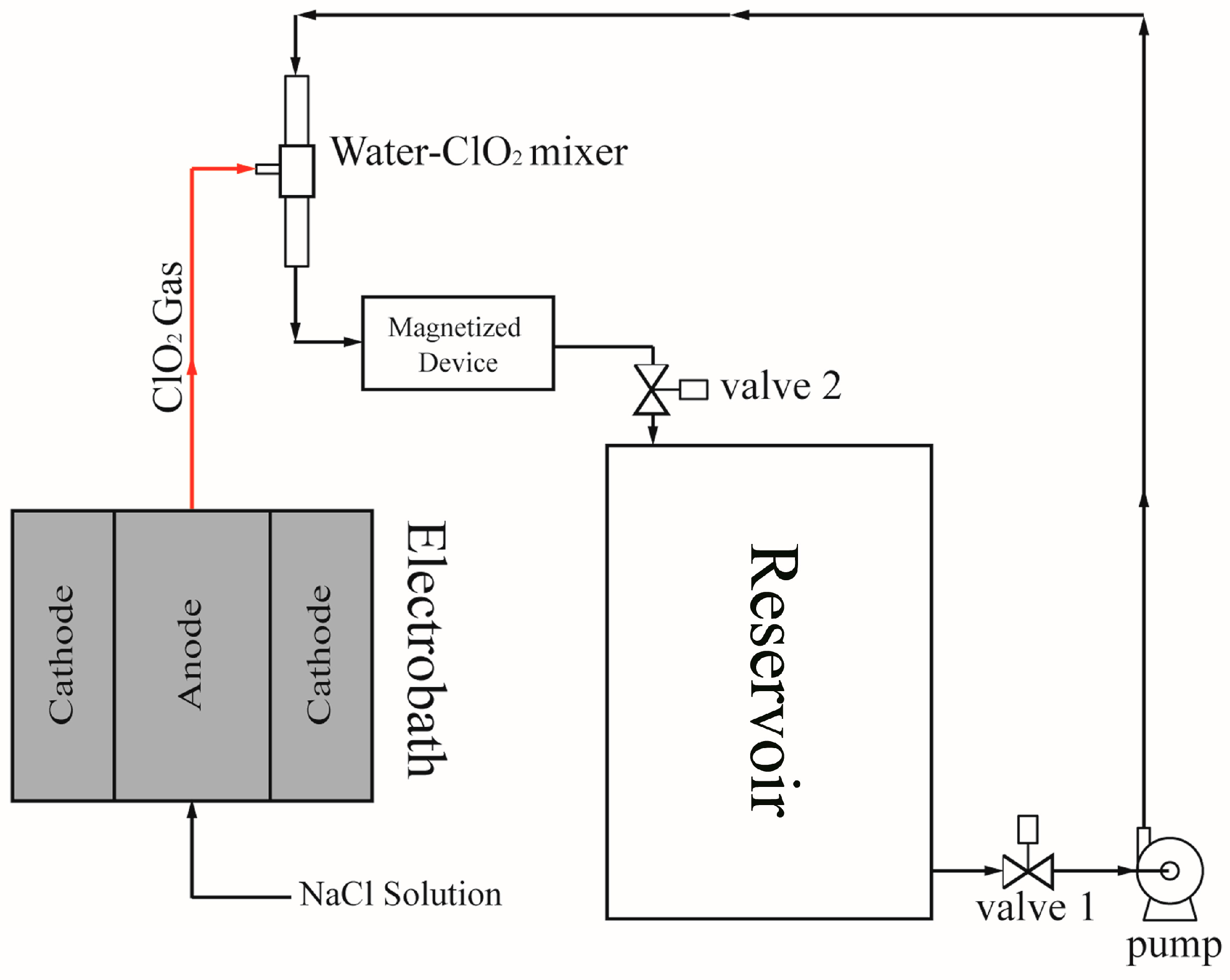
2.1. Electrolytic Method for Gas Chlorine Dioxide Production
2.2. Antimicrobial Efficacy Test
2.3. Antiviral Assay
2.4. In Vitro Cytotoxicity Test (MTT Assay)
2.5. White Rabbit Ocular Irritation Test
2.6. Inhalation Toxicity Test
2.6.1. Evaluation of the Organ Weight
2.6.2. Hematoxylin and Eosin Staining of Mouse Lung Sections
2.7. Subchronic Oral Toxicity Test
2.7.1. Evaluation of the Clinical Symptoms
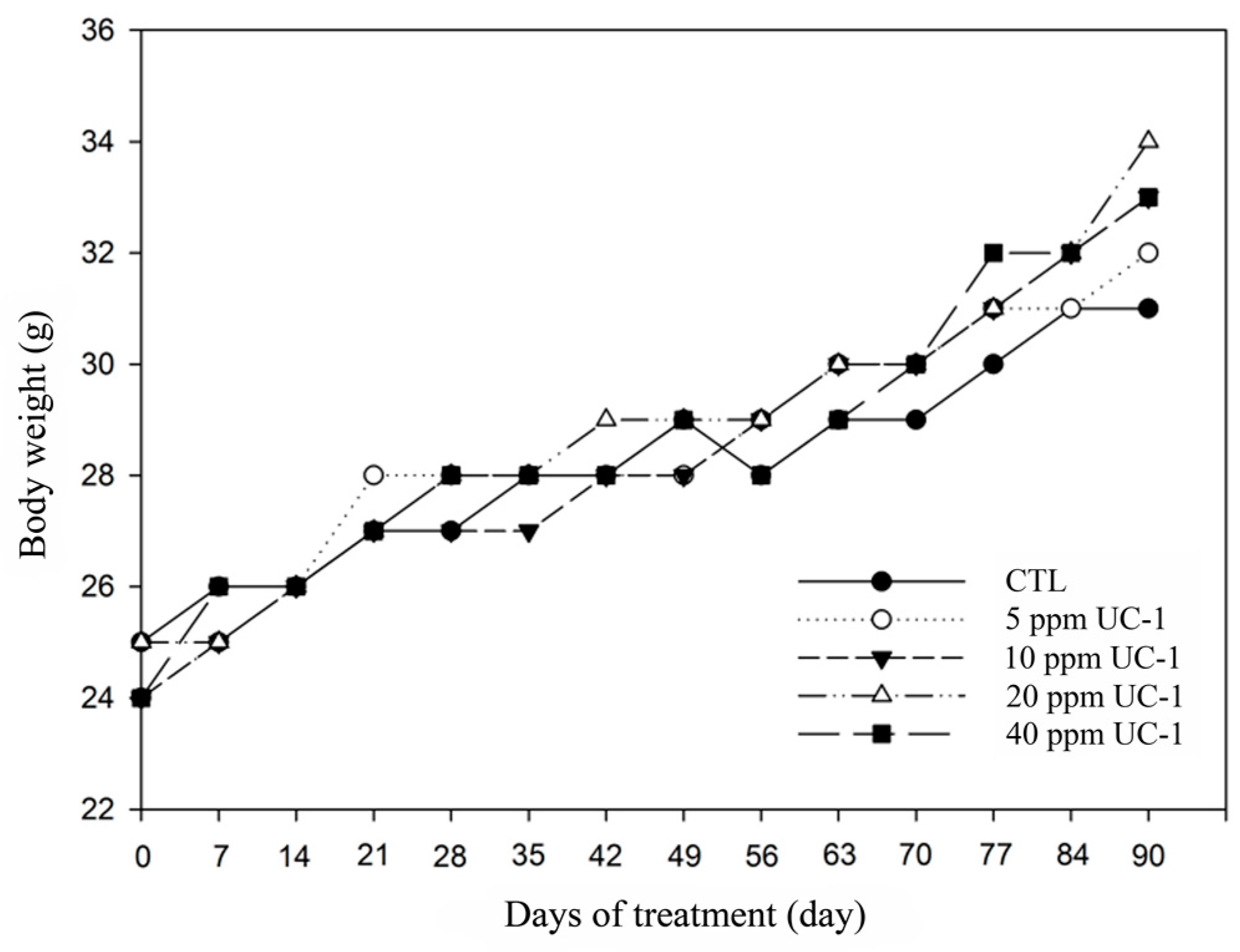
2.7.2. Body Weight
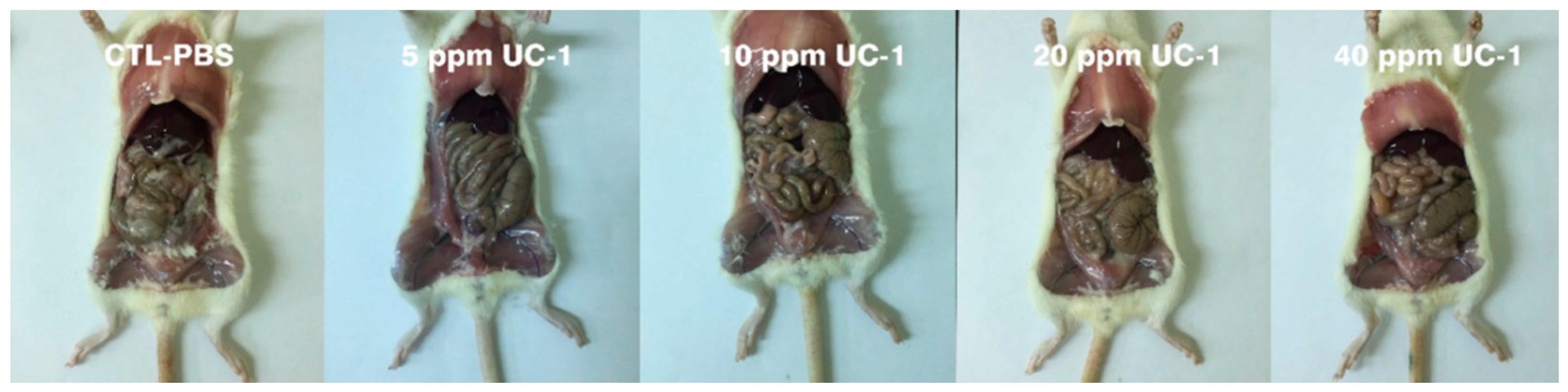
2.7.3. Necropsy, Gross Examination, and Organ Weighing
2.8. Statistical Analysis
2.9. Ethical Statement
3. Results
3.1. Antimicrobial Efficacy Test
3.2. Antiviral Assay
3.3. In Vitro Cytotoxicity Test (MTT Assay)
3.4. Ocular Irritation Test
3.5. Inhalation Toxicity Test
3.6. Subchronic Oral Toxicity Test
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanekata, T.; Fukuda, T.; Miura, T.; Morino, H.; Lee, C.; Maeda, K.; Araki, K.; Otake, T.; Kawahata, T.; Shibata, T. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci. 2010, 15, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Shibata, T. Protective effect of low-concentration chlorine dioxide gas against influenza a virus infection. J. Gen. Virol. 2008, 89, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Morino, H.; Fukuda, T.; Miura, T.; Lee, C.; Shibata, T.; Sanekata, T. Inactivation of feline calicivirus, a norovirus surrogate, by chlorine dioxide gas. Biocontrol Sci. 2009, 14, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.S. Comparative testing and evaluation of hard-surface disinfectants. J. Ind. Microbiol. 1989, 4, 145–154. [Google Scholar] [CrossRef]
- Junli, H.; Li, W.; Nenqi, R.; Li, L.X.; Fun, S.R.; Guanle, Y. Disinfection effect of chlorine dioxide on viruses, algae and animal planktons in water. Water Res. 1997, 31, 455–460. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Du, W.X.; Otwell, W.S.; Wei, C.I. Determination of chlorate and chlorite and mutagenicity of seafood treated with aqueous chlorine dioxide. J. Agric. Food Chem. 1999, 47, 3586–3591. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, H.; Poppendieck, D.; Corsi, R.L. Chlorine dioxide reactions with indoor materials during building disinfection: Surface uptake. Environ. Sci. Technol. 2009, 43, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Huang, T.C.; Chung, C.C.; Huang, H.H.; Chen, H.H. Application of highly purified electrolyzed chlorine dioxide for Tilapia fillet disinfection. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, S.; Kim, Y.; Kim, B.S.; Beuchat, L.R.; Hoikyung, K.; Ryu, J.H. Reduction of Salmonella enterica on the surface of eggshells by sequential treatment with aqueous chlorine dioxide and drying. Int. J. Food Microbiol. 2015, 210, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Ernst, W.; Herges, G.R. Chloroxyanion residues in cantaloupe and tomatoes after chlorine dioxide gas sanitation. J. Agric. Food Chem. 2015, 63, 9640–9649. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.S.; Chen, I.M.; Liang, C.K.; Shih, C.H. Evaluation of disinfection efficiency in pet’s hospital by using chlorine dioxide. Sustain. Environ. Res. 2016, 26, 191–195. [Google Scholar] [CrossRef]
- Hsu, M.S.; Wu, M.Y.; Huang, Y.T.; Liao, C.H. Efficacy of chlorine dioxide disinfection to non-fermentative Gram-negative bacilli and non-tuberculous mycobacteria in a hospital water system. J. Hosp. Infect. 2016, 93, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Yeturu, S.K.; Acharya, S.; Urala, A.S.; Pentapati, K.C. Effect of Aloe vera, chlorine dioxide, and chlorhexidine mouth rinses on plaque and gingivitis: A randomized controlled trial. J. Oral Biol. Craniofac. Res. 2016, 6, 54–58. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Microbial and Disinfection Byproduct Rules Simultaneous Compliance Guidance Manual; U.S. Environmental Protection: Washington, DC, USA, 1999.
- Couri, D.; Abdel-Rahman, M.S.; Bull, R.J. Toxicological effects of chlorine dioxide, chlorite and chlorate. Environ. Health Perspect. 1982, 46, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Alexandria, VA, USA, 1995.
- U.S. Pharmacopeia. 34 NF29 Microbiological Tests/<51> Antimicrobial Effectiveness Testing; U.S. Pharmacopeial Convention: Rockville, MD, USA.
- Trinetta, V.; Morgan, M.; Linton, R.; Dermirci, A.; Ngadi, M.O. Chlorine dioxide for microbial decontamination of food. In Microbial Decontamination in the Food Industry; Demirci, A., Ngadi, M.O., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 533–562. [Google Scholar]
- Sorlini, S.; Gialdini, F.; Biasibetti, M.; Collivignarelli, C. Influence of drinking water treatments on chlorine dioxide consumption and chlorite/chlorate formation. Water Res. 2014, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kang, D.H. Antimicrobial effect of chlorine dioxide gas against foodborne pathogens under differing conditions of relative humidity. LWT- Food Sci. Technol. 2015, 60, 186–191. [Google Scholar] [CrossRef]
- Arango, J.; Rubino, M.; Auras, R.; Gillett, J.; Schilder, A.; Grzesiak, A.L. Evaluation of chlorine dioxide as an antimicrobial against Botrytis cinerea in California strawberries. Food Packag. Shelf Life 2016, 9, 45–54. [Google Scholar] [CrossRef]
- Paulet, G.D.; Desbrousses, S. On the action of ClO2 at low concentrations on laboratory animals. Arch. Mal. Prof. 1970, 31, 97–106. [Google Scholar] [PubMed]
- Paulet, G.D.; Desbrousses, S. Actions of discontinuous exposure to chlorine dioxide (ClO2) on the rat. Arch. Mal. Prof. 1974, 35, 797–804. [Google Scholar]
- Daniel, F.B.; Condie, L.W.; Robinson, M.; Stober, J.A.; York, R.G.; Olson, G.R.; Wang, S.R. Comparative subchronic toxicity studies of three disinfectants. J. Am. Water Works Assn. 1990, 82, 61–69. [Google Scholar]
- Bercz, J.P.; Jones, L.; Garner, L.; Murray, D.; Ludwig, D.A.; Boston, J. Subchronic toxicity of chlorine dioxide and related compounds in drinking water in the nonhuman primate. Environ. Health Perspect. 1982, 46, 47–55. [Google Scholar] [CrossRef] [PubMed]



| Organisms | Original Inoculum (CFU/mL) | Counts of UC-1 at Contact Time a (CFU/mL) | Percent Reductions (R) b |
|---|---|---|---|
| Escherichia coli c,* | 2.55 × 105 | <1 | >99.9 |
| Staphylococcus aureus c,* | 3.15 × 105 | <1 | >99.9 |
| Pseudomonas aeruginosa c,* | 2.55 × 105 | <1 | >99.9 |
| Staphylococcus aureus subsp. Aureus c,* | 2.60 × 105 | <1 | >99.9 |
| Bacillus subtilis subspecies c,* | 3.75 × 105 | 1.35 × 103 | 99.6 |
| Listeria monocytogenes c,* | 8.20 × 105 | <1 | >99.9 |
| Acinetobacter baumannii c,* | 5.40 × 105 | <1 | >99.9 |
| Salmonella enterica subspecies c,* | 4.80 × 105 | <1 | >99.9 |
| Klebsiella pneumoniae c,* | 9.30 × 105 | <1 | >99.9 |
| Penicillium funiculosum ,cΔ | 3.70 × 105 | 6.70 × 103 | 98.2 |
| Candida albicans c,Δ | 3.20 × 105 | <1 | >99.9 |
| Applied Regions | Test Component | Animal No. | Items for Grading | Clinical Observation (Time point/h) | |||
|---|---|---|---|---|---|---|---|
| 1 | 24 | 48 | 72 | ||||
| Left Eye | Test (50 ppm UC-1) | RB-160114-01 | Cornea | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 1 | 0 | 0 | |||
| RB-160114-03 | Cornea | 0 | 0 | 0 | 0 | ||
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 0 | 0 | 0 | |||
| RB-160114-06 | Cornea | 0 | 0 | 0 | 0 | ||
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 0 | 0 | 0 | |||
| Right Eye | Control (0.9% Saline) | RB-160114-01 | Cornea | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 0 | 0 | 0 | |||
| RB-160114-03 | Cornea | 0 | 0 | 0 | 0 | ||
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 0 | 0 | 0 | |||
| RB-160114-06 | Cornea | 0 | 0 | 0 | 0 | ||
| Iris | 0 | 0 | 0 | 0 | |||
| Conjunctivae | 0 | 0 | 0 | 0 | |||
| Test Component | Animal No. | Abnormality Rate (%) | Mortality (%) |
|---|---|---|---|
| Control: PBS | 5 | 0 | 0 |
| 10 ppm UC-1 | 5 | 0 | 0 |
| 20 ppm UC-1 | 5 | 0 | 0 |
| Test Component | Heart (g) | Liver (g) | Spleen (g) | Kidney (g) |
|---|---|---|---|---|
| Control: PBS | 0.3 ± 0.1 | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| 10 ppm UC-1 | 0.4 ± 0.1 | 0.7 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.2 |
| 20 ppm UC-1 | 0.3 ± 0.2 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| Test Component | Animal No. | Abnormality Rate (%) | Mortality (%) |
|---|---|---|---|
| Control: PBS | 5 | 0 | 0 |
| 5 ppm UC-1 | 5 | 0 | 0 |
| 10 ppm UC-1 | 5 | 0 | 0 |
| 20 ppm UC-1 | 5 | 0 | 0 |
| 40 ppm UC-1 | 5 | 0 | 0 |
| Test Component | Heart (g) | Liver (g) | Spleen (g) | Kidney (g) |
|---|---|---|---|---|
| Control: PBS | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| 5 ppm UC-1 | 0.5 ± 0.2 | 0.8 ± 0.3 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| 10 ppm UC-1 | 0.4 ± 0.2 | 0.9 ± 0.1 | 0.5 ± 0.3 | 0.7 ± 0.2 |
| 20 ppm UC-1 | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| 40 ppm UC-1 | 0.5 ± 0.1 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.-W.; Huang, B.-S.; Hsu, C.-W.; Peng, C.-W.; Cheng, M.-L.; Kao, J.-Y.; Way, T.-D.; Yin, H.-C.; Wang, S.-S. Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. Int. J. Environ. Res. Public Health 2017, 14, 329. https://doi.org/10.3390/ijerph14030329
Ma J-W, Huang B-S, Hsu C-W, Peng C-W, Cheng M-L, Kao J-Y, Way T-D, Yin H-C, Wang S-S. Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. International Journal of Environmental Research and Public Health. 2017; 14(3):329. https://doi.org/10.3390/ijerph14030329
Chicago/Turabian StyleMa, Jui-Wen, Bin-Syuan Huang, Chu-Wei Hsu, Chun-Wei Peng, Ming-Long Cheng, Jung-Yie Kao, Tzong-Der Way, Hao-Chang Yin, and Shan-Shue Wang. 2017. "Efficacy and Safety Evaluation of a Chlorine Dioxide Solution" International Journal of Environmental Research and Public Health 14, no. 3: 329. https://doi.org/10.3390/ijerph14030329
APA StyleMa, J.-W., Huang, B.-S., Hsu, C.-W., Peng, C.-W., Cheng, M.-L., Kao, J.-Y., Way, T.-D., Yin, H.-C., & Wang, S.-S. (2017). Efficacy and Safety Evaluation of a Chlorine Dioxide Solution. International Journal of Environmental Research and Public Health, 14(3), 329. https://doi.org/10.3390/ijerph14030329






