An Eco-Safety Assessment of Glyoxal-Containing Cellulose Ether on Freeze-Dried Microbial Strain, Cyanobacteria, Daphnia, and Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
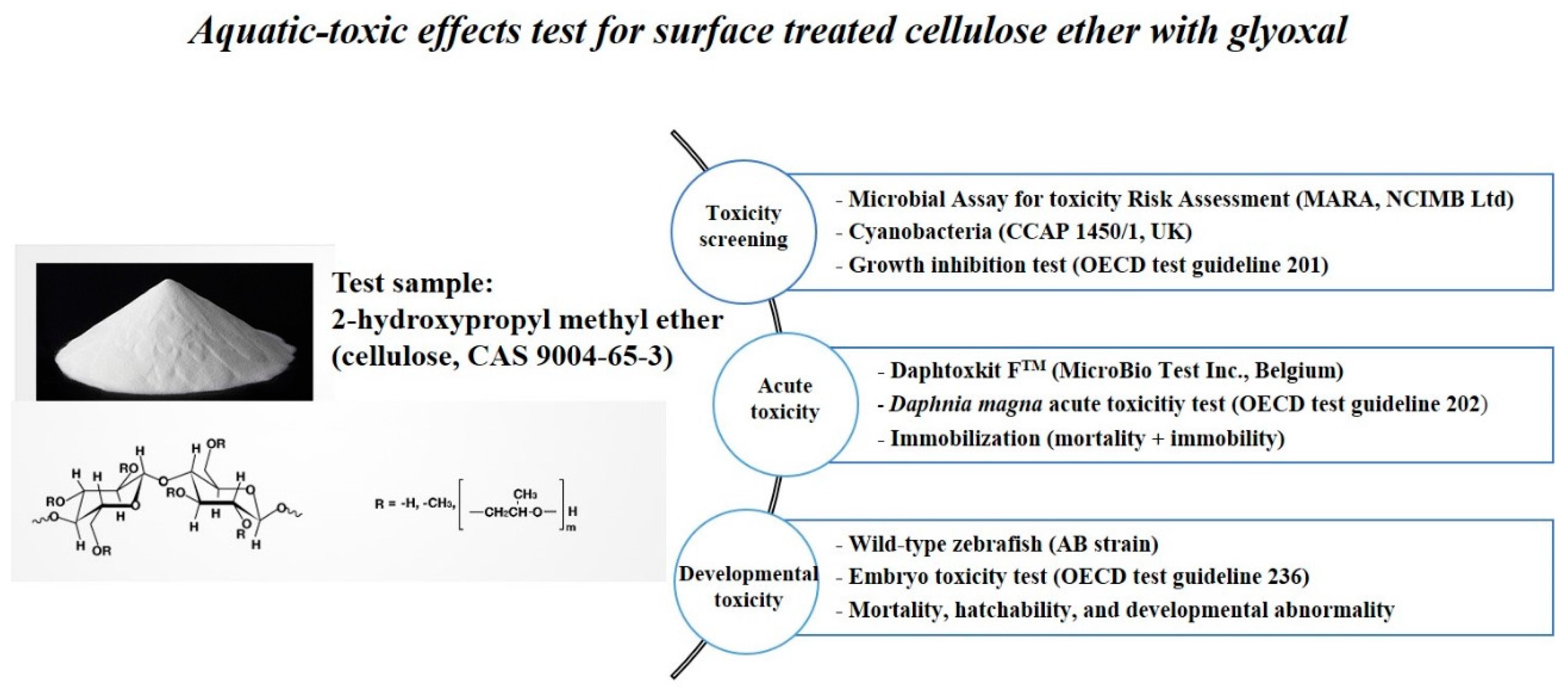
2.1. Test Samples
2.1.1. The Aquatic-Toxic Effects on Aquatic Organisms
2.1.2. Microbial Array for Toxicity Risk Assessment (MARA)
2.1.3. Algal Growth Inhibition Test
2.1.4. Daphnia Magna Acute Toxicity Test
2.1.5. Zebrafish Embryo Toxicity Test
2.1.6. Statiscal Analysis
2.1.7. Ethical Statement
3. Results and Discussion
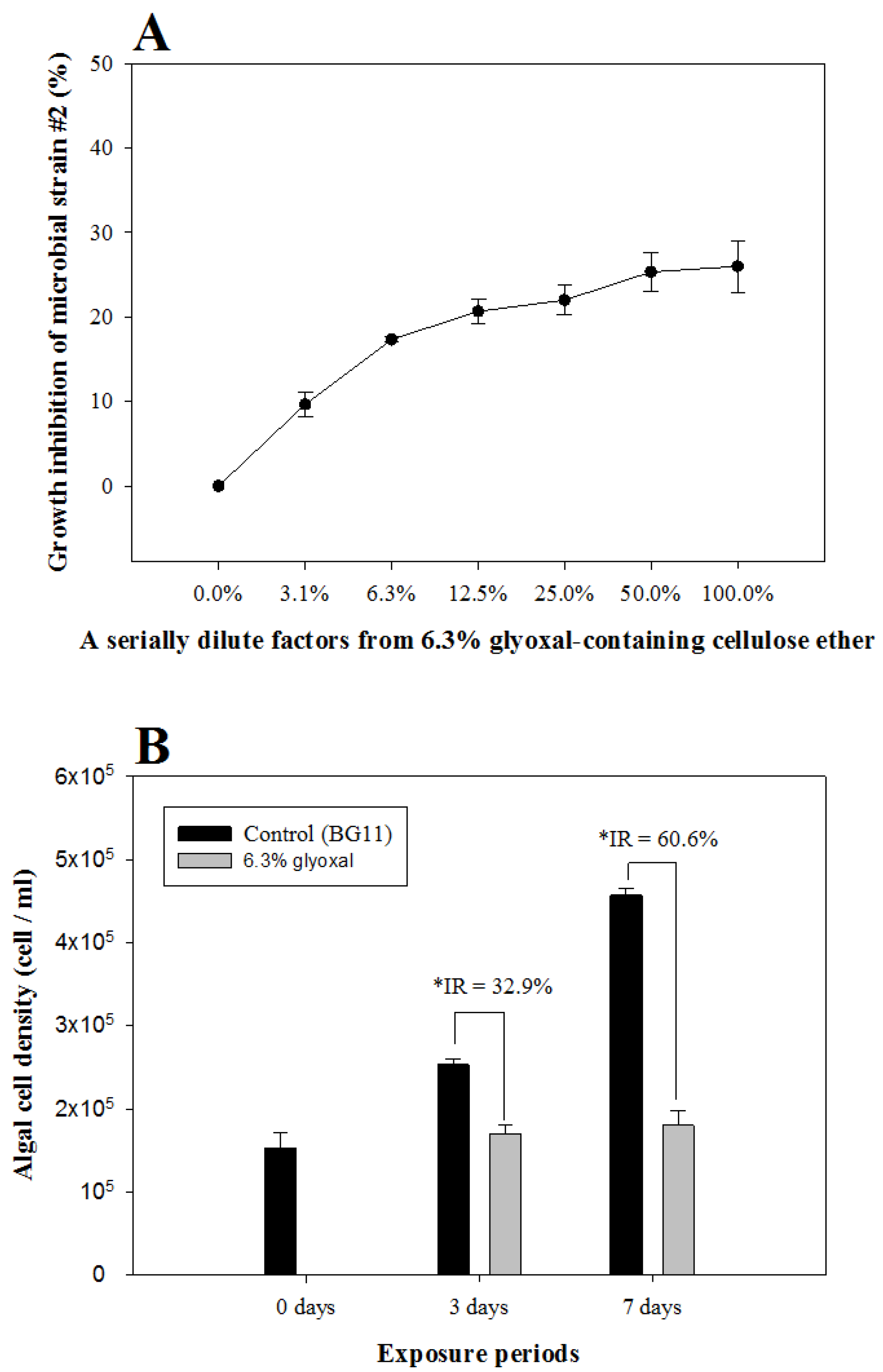
3.1. MARA and Cyanobacteria Algal Growth Inhibition
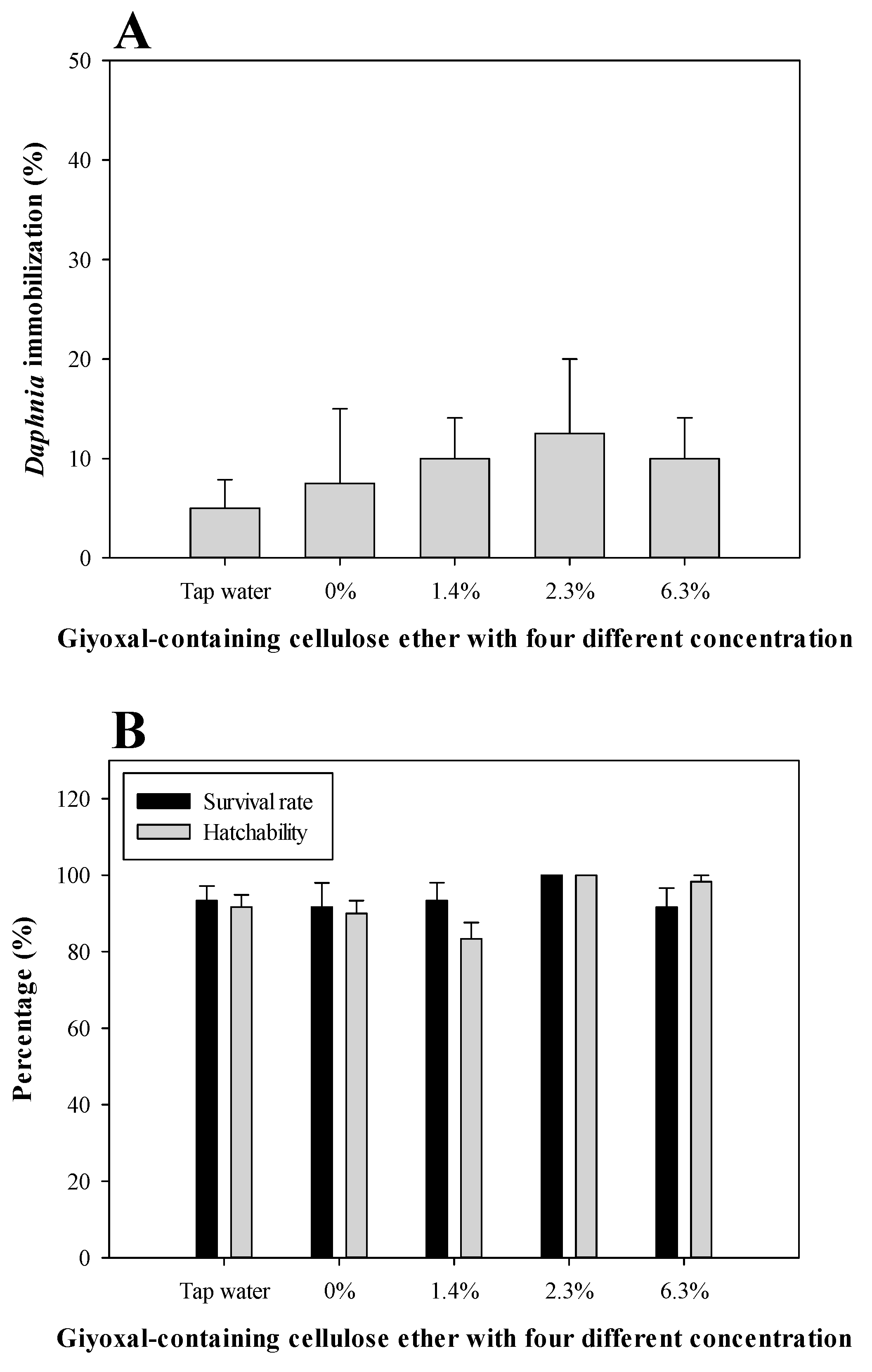
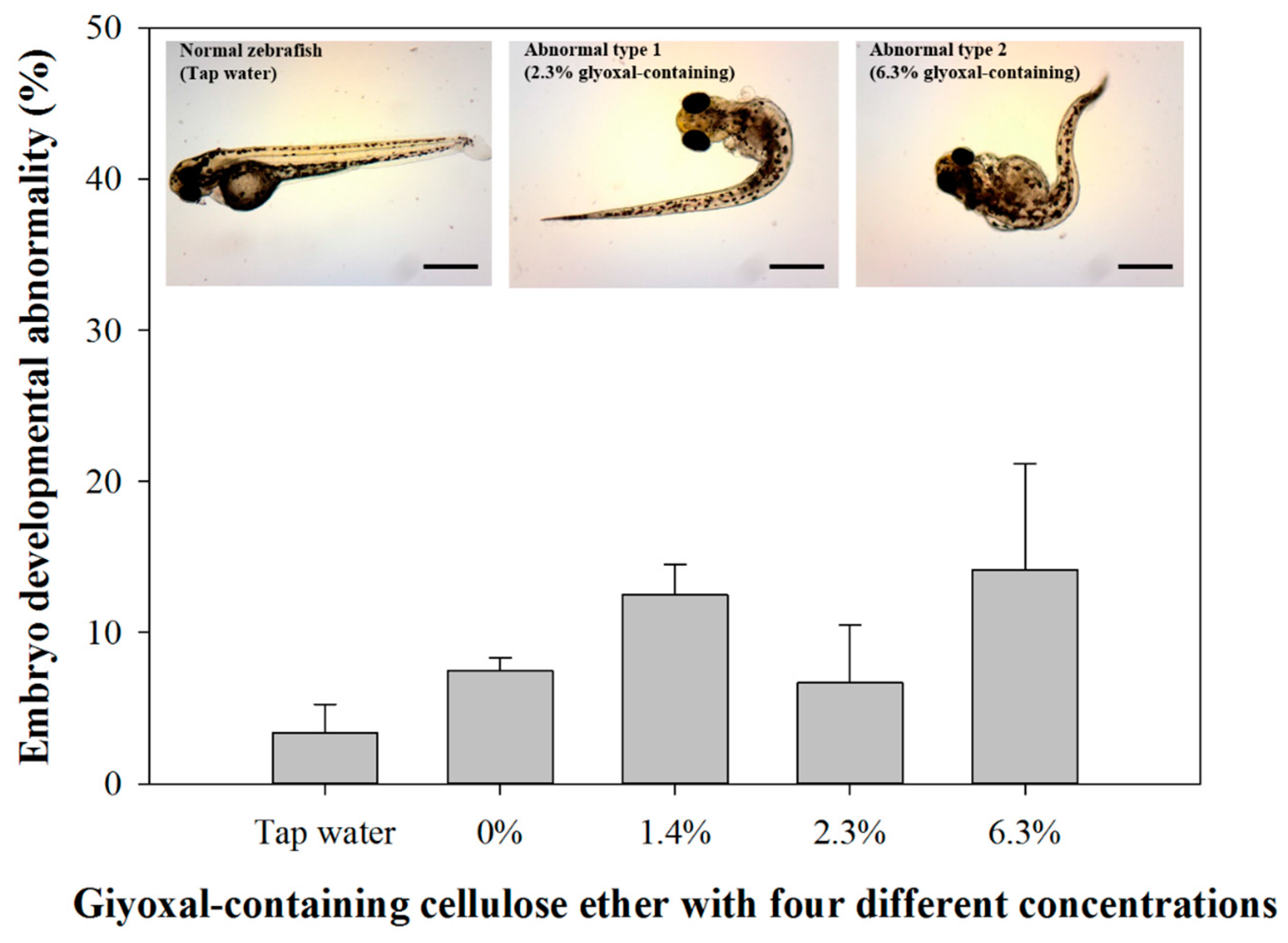
3.2. Daphnia Magna Acute Toxicity and Zebrafish Embryo Toxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carlsson, A. Nonionic Cellulose Ethers Interactions with Surfactans, Solubility and Other Aspects; Lund University: Lund, Sweden, 1989. [Google Scholar]
- Feller, R.L.; Wilt, M. Research in Conservation: Evaluation of Cellulose Ethers for Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 1990. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Boh, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3359–3393. [Google Scholar] [CrossRef] [PubMed]
- Updergraff, D.M. Semimicro determination of cellulose in biological materials. Aanl. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Wikström, L. Surface Treatment of Cellulose Ethers Ytmodifiering av Cellulosaetrar; University of Borås/School of Engineering, Borås Academic Digital Archive (BADA): Borås, Sweden, 2014. [Google Scholar]
- European Chemical Industry Council. Cellulose Ethers Treated with Glyoxal (Additives); Cellulose Ethers Sector Group: Brussels, Belgium, 2008. [Google Scholar]
- Rojas, J.; Azebede, E. Functionalization and crosslinking of microcrystalline cellulose in aqueous media: A safe and economic approach. Int. J. Pharma. Sci. Rev. Res. 2011, 8, 28–36. [Google Scholar]
- Patural, L.; Marcha, P.; Govin, A.; Grosseau, P.; Ruot, B.; Devès, O. Cellulose ethers influence on water retention and consistency in cement-based mortars. Cem. Concr. Res. 2011, 41, 46–55. [Google Scholar] [CrossRef]
- Pourchez, J.; Govin, A.; Grosseau, P.; Guyonnet, R.; Guilhot, B.; Ruot, B. Alkaline stability of cellulose ethers and impact of their degradation products on cement hydration. Cem. Concr. Res. 2006, 36, 1252–1256. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006; Official Journal of the European Union: Luxembourg, 2008. [Google Scholar]
- European Commission (EC). Commission Regulation (EU) 2015/1221 of 24 July 2015 Amending Regulation (EC) No 1272/2008 of the European Parliament and of the Council on Classification, Labelling and Packaging of Substances and Mixtures, for the Purposes of Its Adaptation to Technical and Scientific Progress; Official Journal of the European Union: Luxembourg, 2015. [Google Scholar]
- Hartung, T.; Rovida, C. Chemical regulators have overreached. Nature 2009, 460, 1080–1081. [Google Scholar] [CrossRef] [PubMed]
- Schöning, G. Classification & labelling inventory: Role of ECHA and notification requirements. Ann. Super. Sanità 2011, 47, 140–145. [Google Scholar]
- Risk Policy Analysts (RPA). Study on Specific Needs for Information on the Content of Dangerous Substances in Construction Products; Final Report Prepared for DG Enterprise & Industry; RPA: Norfolk, UK, 2013. [Google Scholar]
- Scientific Committee on Consumer Products (SCCP). Opinion on: Glyoxal (SCCP/0881/05) Adopted by the SCCP during the 4th Plenary of 21 June 2005; SCCP: Luxembourg, 2005. [Google Scholar]
- World Health Organization (WHO). Concise International Chemical Assessment Document 57: Glyoxal. International Programme on Chemical Safety II; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Organization for Economic Co-Operation and Development (OECD). Glyoxal, CAS 107-22-2. In Summary of Responses to the OECD Request for Available Data on High Production Volume Chemicals; OECD: Paris, France, 1992. [Google Scholar]
- Bollman, M.A.; Banne, W.K.; Smith, S.; DeWhitt, K.; Kapustka, L. Report on Algal Toxicity Tests on Selected Office of Toxic Substances (OTS) Chemicals; EPA/6003/3-90-41, PB-90-212606; Environmental Protection Agency: Corvallis, OR, USA, 1990.
- Conway, R.A.; Waggy, G.T.; Spiegel, M.H.; Berglund, R.L. Environmental fate and effects ethylene oxide. Environ. Sci. Technol. 1983, 17, 107–112. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Technical Guidance Document (TGD) in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances. Part II: Environmental Risk Assessment; European Commission: Luxembourg, 1996. [Google Scholar]
- Gabrielson, J. Assessing the Toxic Impact of Chemicals Using Bacteria, Microbiology and Tumorbiology Center; Karolinska Institute: Stockholm, Sweden, 2004. [Google Scholar]
- Organization for Economic Co-Operation and Development (OECD). OECD Guideline for the Testing of Chemicals 202, Daphnia sp., Acute Immobilisation Test; OECD: Paris, France, 2004. [Google Scholar]
- Organization for Economic Co-Operation and Development (OECD). OECD Guideline for the Testing of Chemicals 201, Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD: Paris, France, 2006. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). OECD Guideline for the Testing of Chemicals 236, Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Lotte Fine Chemical. Mecellose®: Total solution Provider. Available online: http://www.lotte-cellulose.com (accessed on 25 September 2016).
- Dando, T. Microbial Array for Toxicity Risk Assessment, NCIMB Reference: WI-NC-237; NCIMB Ltd.: Bucksburn, UK, 2008. [Google Scholar]
- Sangolkar, L.N.; Maske, S.S.; Muthal, P.L.; Kashyap, S.M.; Chakrabarti, T. Isolation and characterization of microcystin producing Microcystis from a central Indian water bloom. Harmful Algae 2009, 8, 674–684. [Google Scholar] [CrossRef]
- Jang, G.H.; Park, C.-B.; Kang, B.J.; Kim, Y.J.; Lee, K.H. Sequential assessment via daphnia and zebrafish for systematic toxicity screening of heterogeneous substances. Environ. Pollut. 2016, 216, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Weyers, A.; Sokull-Klüttgen, B.; Baraibar-Fentanes, J.; Vollmer, G. Acute toxicity data: A comprehensive comparison of results of fish, Daphnia and algae tests with new substances notified in the EU. Environ. Toxicol. Chem. 2000, 19, 1931–1933. [Google Scholar] [CrossRef]
| Classification | Glyoxal-Containing Cellulose Ethers with Four Different Concentrations | |||
|---|---|---|---|---|
| 0% | 1.4% | 2.3% | 6.3% | |
| Physical hazards | - | - | - | - |
| Health hazards | - | Skin sens. 1, Muta. 2 | Skin sens. 1, Muta. 2 | Skin sens. 1, Muta. 2 |
| Environmental hazards | - | - | - | - |
| Test Sample | Viscosity (mPa·s) | pH | Moisture (%) | NaCl (%) | Methoxyl (%) | Hydropropoxyl (%) | Total-Glyoxal (%) |
|---|---|---|---|---|---|---|---|
| A | 3.5 | 5.0–8.0 | 3 | 0.5 | 27–29 | 6–8 | 0 |
| B | 1.4 | ||||||
| C | 2.3 | ||||||
| D | 6.3 |
| MARA Number | Microbial Strain | |
|---|---|---|
| #1 | Microbacterium sp. | (NCIMB 30255) |
| #2 | Brevundimonas diminuta | (NCIMB 30256) |
| #3 | Citrobacter freundii | (NCIMB 30257) |
| #4 | Comamonas testosteroni | (NCIMB 30258) |
| #5 | Enterococcus casseliflavus | (NCIMB 30259) |
| #6 | Delftia acidovorans | (NCIMB 30260) |
| #7 | Kurthia gibsonii | (NCIMB 30261) |
| #8 | Staphylococcus warneri | (NCIMB 30262) |
| #9 | Pseudsomonas chlororaphis | (NCIMB 30263) |
| #10 | Serratia rubra | (NCIMB 30264) |
| #11 | Pichia anomola | (NCIMB 30265) |
| MARA Number | Average Values of Growth Rate ± SEM (n = 4) | |||
|---|---|---|---|---|
| Four Different Glyoxal Concentrations | ||||
| 0% | 1.4% | 2.3% | 6.3% | |
| #1 | 100.0 ± 0.0 | 100.0 ± 0.0 | 99.3 ± 0.8 | 100.0 ± 0.0 |
| #2 | 86.3 ± 1.4 | 83.0 ± 0.8 | 77.5 ± 1.2 | 72.0 ± 3.1 † |
| #3 | 98.5 ± 1.2 | 96.5 ± 2.0 | 98.3 ± 0.9 | 92.3 ± 3.7 |
| #4 | 100.0 ± 0.0 | 99.5 ± 0.5 | 99.8 ± 0.3 | 98.0 ± 1.2 |
| #5 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 79.3 ± 1.2 |
| #6 | 98.8 ± 0.6 | 97.0 ± 0.8 | 87.8 ± 1.9 | 86.3 ± 1.9 |
| #7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 99.5 ± 0.5 | 100.0 ± 0.0 |
| #8 | 98.5 ± 1.0 | 99.0 ± 0.6 | 91.0 ± 1.8 | 91.3 ± 2.0 |
| #9 | 77.3 ± 1.9 | 77.5 ± 1.6 | 75.0 ± 2.0 | 86.7 ± 1.8 |
| #10 | 95.8 ± 2.5 | 94.0 ± 2.2 | 93.3 ± 1.9 | 87.3 ± 1.8 |
| #11 | 100.0 ± 0.0 | 100.0 ± 0.0 | 98.0 ± 2.0 | 98.7 ± 0.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-B.; Song, M.J.; Choi, N.W.; Kim, S.; Jeon, H.P.; Kim, S.; Kim, Y. An Eco-Safety Assessment of Glyoxal-Containing Cellulose Ether on Freeze-Dried Microbial Strain, Cyanobacteria, Daphnia, and Zebrafish. Int. J. Environ. Res. Public Health 2017, 14, 323. https://doi.org/10.3390/ijerph14030323
Park C-B, Song MJ, Choi NW, Kim S, Jeon HP, Kim S, Kim Y. An Eco-Safety Assessment of Glyoxal-Containing Cellulose Ether on Freeze-Dried Microbial Strain, Cyanobacteria, Daphnia, and Zebrafish. International Journal of Environmental Research and Public Health. 2017; 14(3):323. https://doi.org/10.3390/ijerph14030323
Chicago/Turabian StylePark, Chang-Beom, Min Ju Song, Nak Woon Choi, Sunghoon Kim, Hyun Pyo Jeon, Sanghun Kim, and Youngjun Kim. 2017. "An Eco-Safety Assessment of Glyoxal-Containing Cellulose Ether on Freeze-Dried Microbial Strain, Cyanobacteria, Daphnia, and Zebrafish" International Journal of Environmental Research and Public Health 14, no. 3: 323. https://doi.org/10.3390/ijerph14030323
APA StylePark, C.-B., Song, M. J., Choi, N. W., Kim, S., Jeon, H. P., Kim, S., & Kim, Y. (2017). An Eco-Safety Assessment of Glyoxal-Containing Cellulose Ether on Freeze-Dried Microbial Strain, Cyanobacteria, Daphnia, and Zebrafish. International Journal of Environmental Research and Public Health, 14(3), 323. https://doi.org/10.3390/ijerph14030323






