Biomethylation and Volatilization of Arsenic by Model Protozoan Tetrahymena pyriformis under Different Phosphate Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing T. pyriformis
2.2. Growth of T. pyriformis Exposed to As at Different Phosphate Concentrations
2.3. Total As Concentrations and Speciation in the Cells and Media
2.4. Chemotrapping of Volatile Arsenic
2.5. Scanning Electron Microscopy (SEM) Analysis
3. Results and Discussion
3.1. Toxic Effects of As in T. pyriformis at Different Phosphate Concentrations
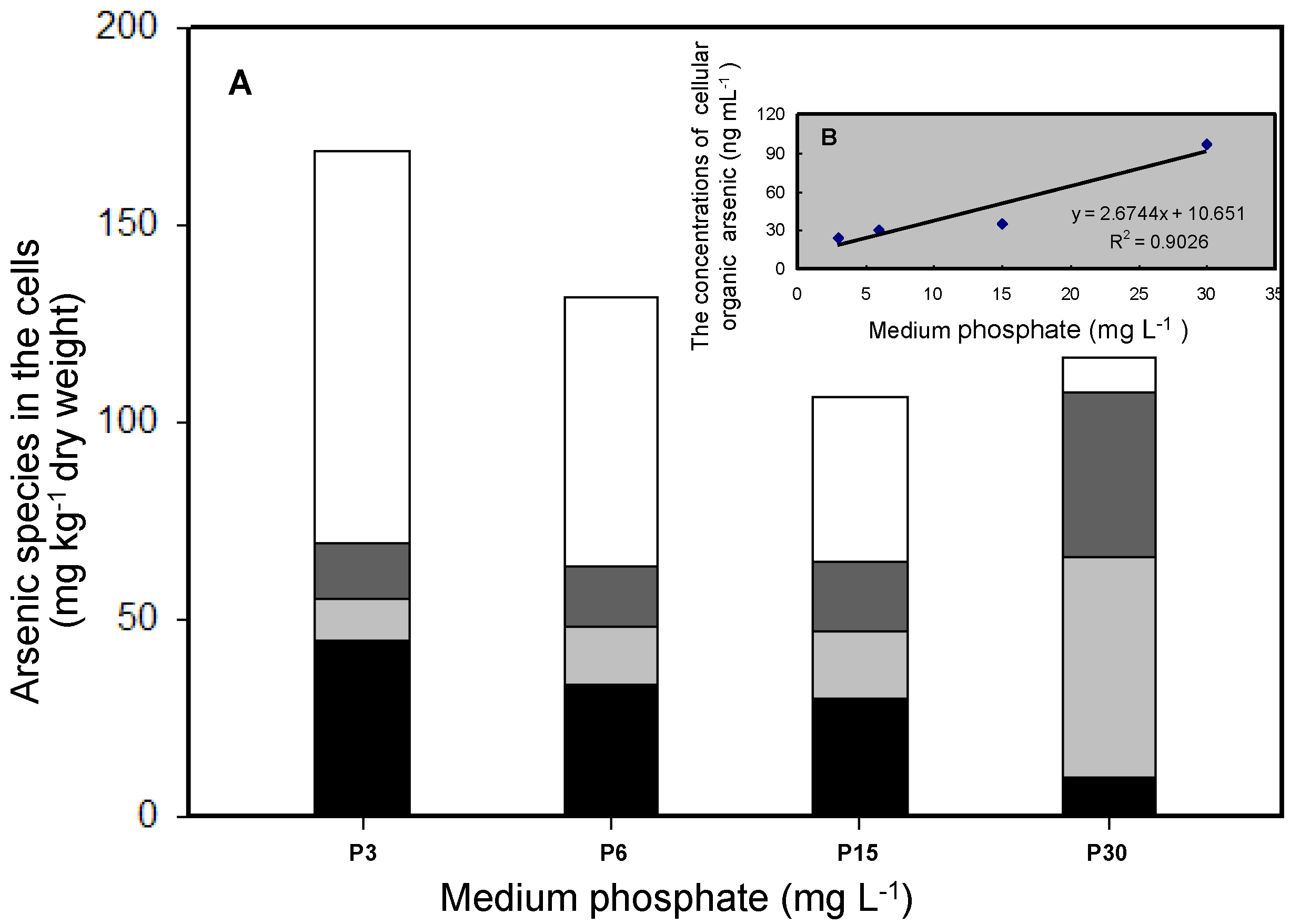
3.2. Effects of Phosphate on As Accumulation and Biotransformation in T. pyriformis
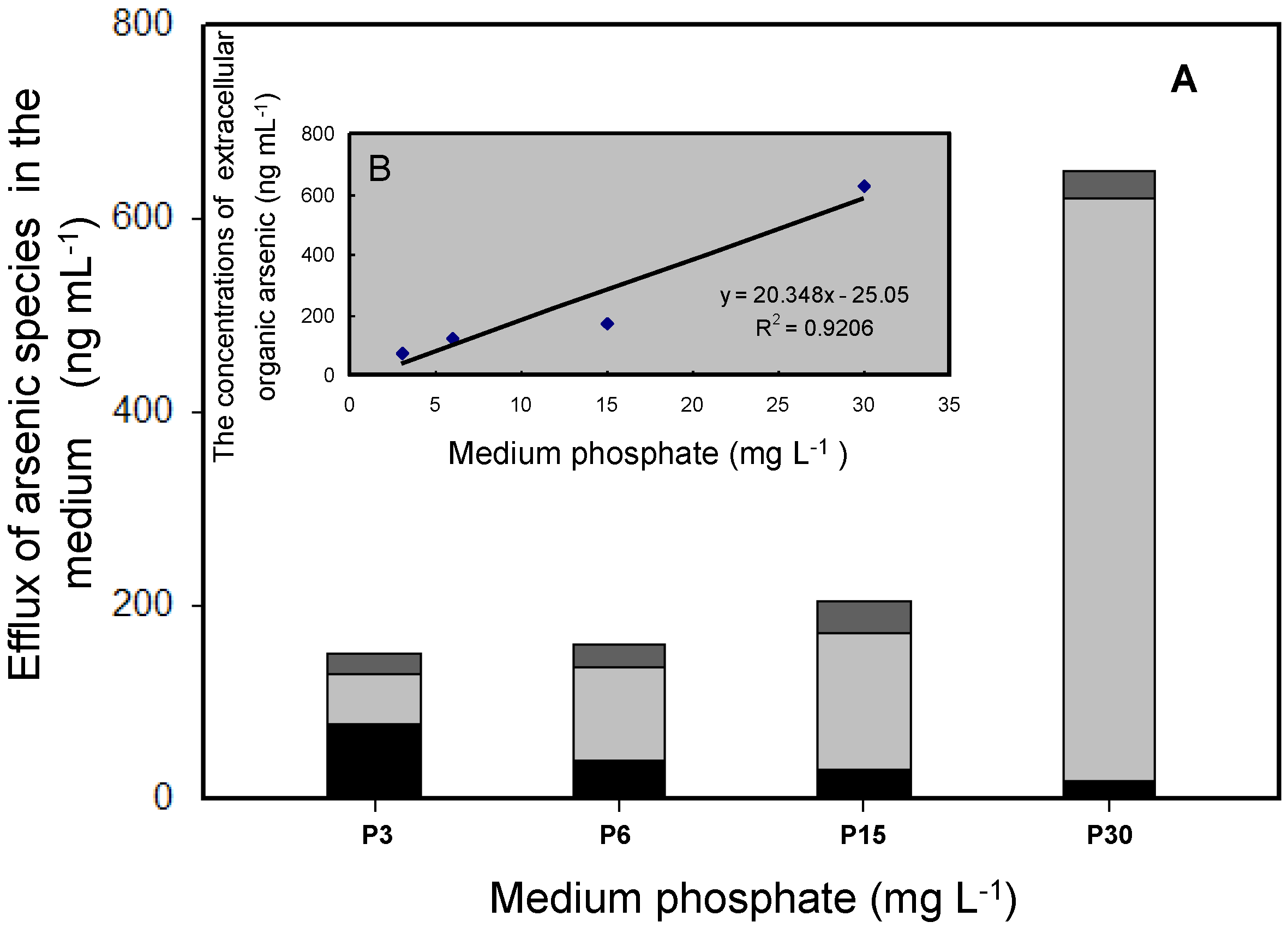
3.3. Volatilization of As by T. pyriformis at Different Phosphate Concentrations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in naturalwaters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar]
- Yang, C.Y.; Chang, C.C.; Tsai, S.S.; Chuang, Y.H.; Ho, K.C.; Wu, N.T. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ. Res. 2003, 91, 29–34. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Sun, G.X.; Lei, M.; Teng, M.; Liu, Y.X.; Chen, N.C.; Wang, L.H.; Carey, A.; Deacon, C.; Raab, A. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ. Sci. Technol. 2008, 42, 5008–5013. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.P.; Liu, Z. Transport pathways for arsenic and selenium: A mini review. Environ. Int. 2009, 35, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.X.; Chen, J.; Qin, J.; Sun, G.X.; Rosen, B.P.; Zhu, Y.G. Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol. 2011, 156, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Sun, G.X.; Zhu, Y.G. Identification and characterization of arsenite methyltransferase from an archaeon, Methanosarcina acetivorans C2A. Environ. Sci. Technol. 2014, 48, 12706–12713. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Bao, P.; Sun, G.X. Identification and catalytic residues of the arsenite methyltransferase from a sulfate-reducing bacterium, Clostridium sp. BXM. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Yoshinaga, M.; Zhao, F.J.; Rosen, B.P. Earth abides arsenic biotransformations. Annu. Rev. Earth Planet. Sci. 2014, 42, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Qin, B.Q.; Zhu, G.W.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Xiao, K.Q.; Wang, L.H.; Bai, R.; Zhang, S.Y.; Yin, X.X. Arsenic biotransformation in cyanobacterium Synechocystis sp. PCC 6803 under different phosphate regimes. Fresenius Environ. Bull. 2012, 9, 2551–2557. [Google Scholar]
- Wang, L.H.; Duan, G.L. Effect of external and internal phosphate status on arsenic toxicity andaccumulation in rice seedlings. J. Environ. Sci.-China 2009, 21, 346–351. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Sun, G.X.; Yin, X.X.; Rensing, C.; Zhu, Y.G. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 2013, 93, 47–53. [Google Scholar] [CrossRef]
- Catarecha, P.; Segura, M.D.; Franco-Zorrilla, J.M.; GarciaPonce, B.; Lanza, M. A mutant of the Arabidopsis phosphate transporter PHT1; 1 displays enhanced arsenic accumulation. Plant Cell 2007, 19, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Sauvant, N.P.; Pepin, D.; Piccinni, E. Thtrahymena pyriformis: A tool for toxicological studies. Chemosphere 1999, 38, 1631–1669. [Google Scholar] [CrossRef]
- Yin, X.X.; Zhang, Y.Y.; Yang, J.; Zhu, Y.G. Rapid biotransformation of arsenic by a model protozoan Tetrahymena pyriformis. Environ. Pollut. 2011, 159, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Stolz, J.F.; Hollibaugh, J.T. The microbial arsenic cycle in Mono Lake, California. FEMS Microbiol. Ecol. 2004, 48, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Williams, P.N.; Carey, A.M.; Zhu, Y.G.; Deacon, C.; Raab, A.; Feldmann, J.; Islam, R.M.; Meharg, A.A. Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ. Sci. Technol. 2008, 42, 7542–7546. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Schat, H.; Vooijs, R.; Verkleij, J.A.C.; Ernst, W.H. Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol. 2003, 157, 33–38. [Google Scholar] [CrossRef]
- Panucio, M.R.; Logoteta, B.; Beone, M.G.; Cognin, M.; Caco, G. Arsenic uptake and speciation and the effects of phosphate nutrition in hydroponically grown kikuyu grass (Pennisetum. clandestinum Hochst.). Environ. Sci. Pollut. Res. 2012, 19, 3046–3053. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.R.; Gong, Y.; Wang, C.; Liu, X.; Liu, J.T. Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes. Environ. Toxicol. Chem. 2011, 30, 1754–1759. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.T.; Silver, S. Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Sun, G.X.; Jia, Y.; Meharg, A.A.; Zhu, Y.G. A review on completing arsenic biogeochemical cycle: Microbial volatilization of arsines in environment. J. Environ. Sci.-China 2014, 21, 371–381. [Google Scholar] [CrossRef]
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.G. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012, 17, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.M.; Megharaj, M.; Naidu, R. Influence of phosphate on toxicity and bioaccumulation of arsenic in a soil isolate of microalga Chlorella sp. Environ. Sci. Pollut. Res. 2016, 23, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.X.; Wang, L.H.; Bai, R.; Huang, H.; Sun, G.X. Accumulation and transformation of arsenic in the blue-green alga Synechocysis sp. PCC6803. Water Air Soil Pollut. 2012, 223, 1183–1190. [Google Scholar] [CrossRef]
- Bhattacharjee, H.; Rosen, B.P. Arsenic Metabolism in Prokaryotic and Eukaryotic Microbes. Molecular Microbiology of Heavy Metals. In Microbiology Monographs; Springer: Heidelberg, Germany, 2007; Volume 6, pp. 371–406. [Google Scholar]
- Xu, X.Y.; McGrath, S.P.; Zhao, F.J. Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol. 2007, 176, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chang, Y.; Yan, Y.; Xiong, J.; Xue, X.M.; Yuan, D.; Sun, G.X.; Zhu, Y.G.; Miao, W. Identification and characterization of the arsenite methyltransferase from a protozoan, Tetrahymena pyriformis. Aquat. Toxicol. 2014, 149, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mestrot, A.; Uroic, K.; Plantevin, T.; Islam, M.R.; Krupp, E.; Feldmann, J.; Meharg, A.A. Quantitative and qualitative trapping of arsines deployed to assess loss of volatile arsenic from paddy soil. Environ. Sci. Technol. 2009, 43, 8270–8275. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Wang, L.; Zhang, Z.; Fan, G.; Liu, J.; Sun, K.; Sun, G.-X. Biomethylation and Volatilization of Arsenic by Model Protozoan Tetrahymena pyriformis under Different Phosphate Regimes. Int. J. Environ. Res. Public Health 2017, 14, 188. https://doi.org/10.3390/ijerph14020188
Yin X, Wang L, Zhang Z, Fan G, Liu J, Sun K, Sun G-X. Biomethylation and Volatilization of Arsenic by Model Protozoan Tetrahymena pyriformis under Different Phosphate Regimes. International Journal of Environmental Research and Public Health. 2017; 14(2):188. https://doi.org/10.3390/ijerph14020188
Chicago/Turabian StyleYin, Xixiang, Lihong Wang, Zhanchao Zhang, Guolan Fan, Jianjun Liu, Kaizhen Sun, and Guo-Xin Sun. 2017. "Biomethylation and Volatilization of Arsenic by Model Protozoan Tetrahymena pyriformis under Different Phosphate Regimes" International Journal of Environmental Research and Public Health 14, no. 2: 188. https://doi.org/10.3390/ijerph14020188
APA StyleYin, X., Wang, L., Zhang, Z., Fan, G., Liu, J., Sun, K., & Sun, G.-X. (2017). Biomethylation and Volatilization of Arsenic by Model Protozoan Tetrahymena pyriformis under Different Phosphate Regimes. International Journal of Environmental Research and Public Health, 14(2), 188. https://doi.org/10.3390/ijerph14020188




