Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. major Isolate
2.2. Plant Material
2.3. Pomegranate Juice Preparation
2.4. Pomegranate Juice Stability
2.5. Anti-Promastigote Assay Using MTT (In Vitro Assay)
2.6. Experimental Protocol
- Group l:
- Normal non-infected negative control group.
- Group 2:
- Infected un-treated positive control group: Mice were subcutaneously inoculated with 1 × 107 promastigotes in a shaved area above the tail.
- Group 3:
- Infected mice treated with Pentostam (Pen; 120 mg/kg subcutaneously) for four weeks starting with the first appearance of an ulcerative lesion.
- Group 4:
- Infected mice treated with 0.8 µg/mL pomegranate (Pom; P. granatum) juice for four weeks starting with the first appearance of an ulcerative lesion.
- Group 5:
- Infected mice treated with 0.8 µg/mL pomegranate (P. granatum) juice concurrently with the antibiotic ciprofloxacin (CIP, 10 mg/mL) for four weeks, starting with the first appearance of an ulcerative lesion.
- Group 6:
- Mice pretreated with 0.8 µg/mL pomegranate (P. granatum) juice for four weeks before infection.
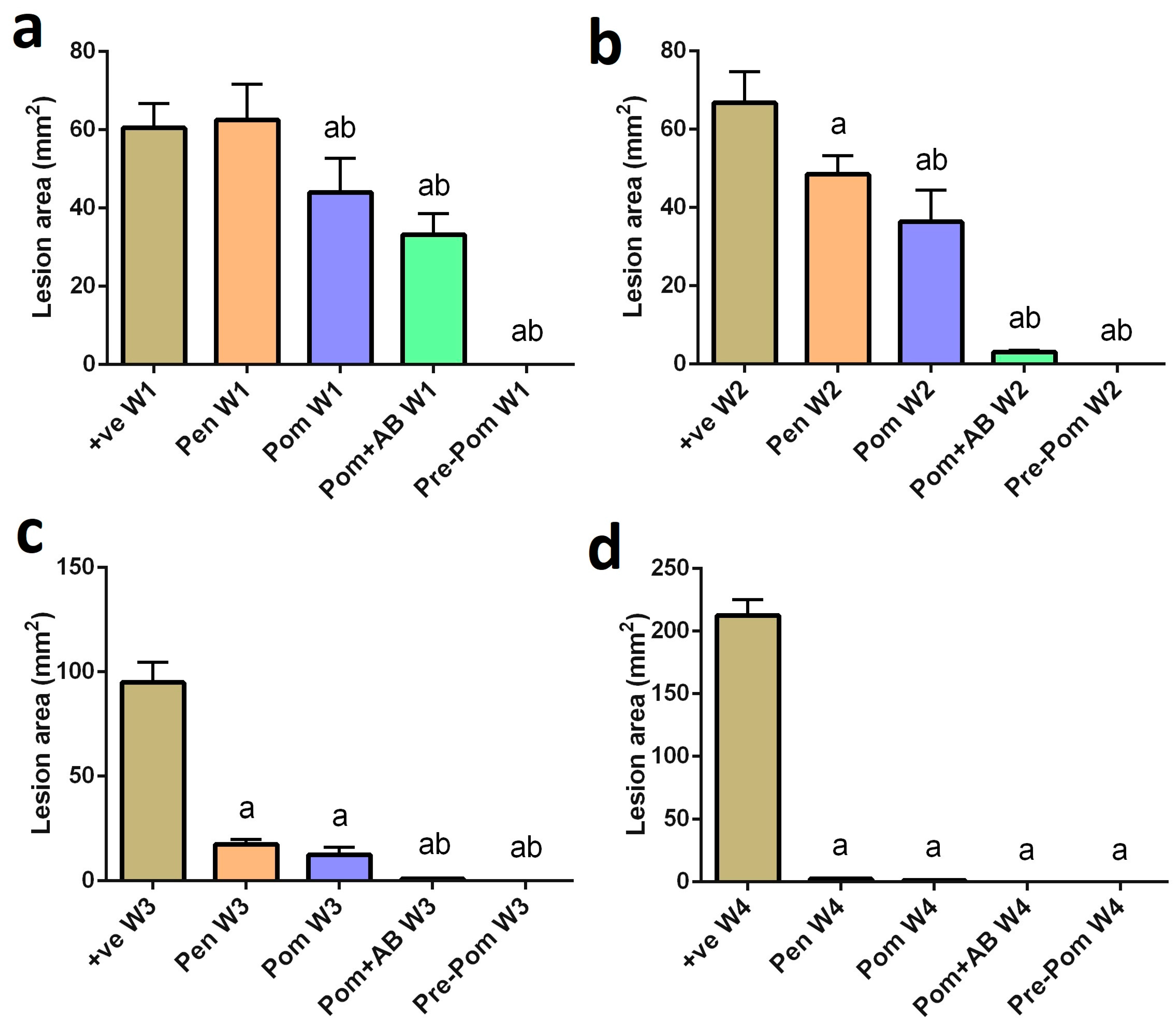
2.7. Measurement of Lesion Size
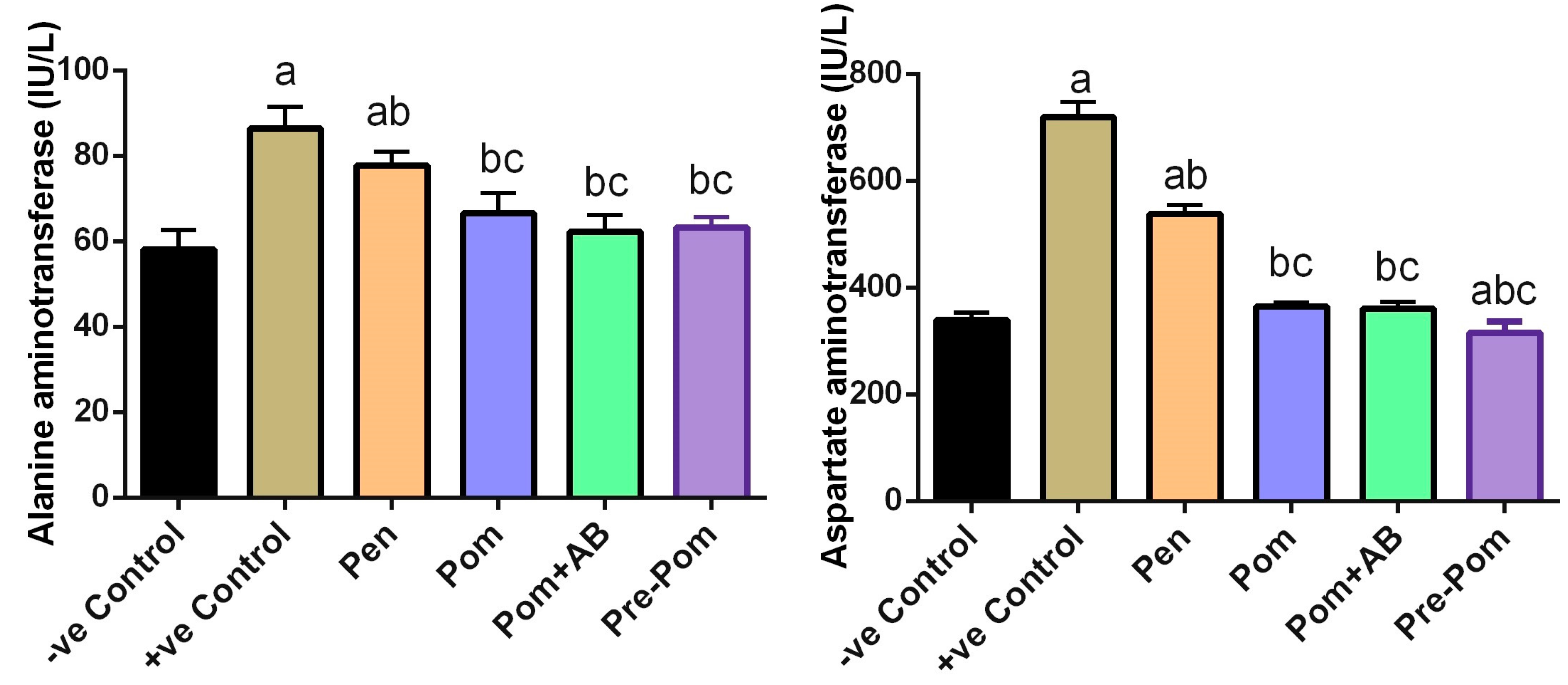
2.8. Liver Function Test
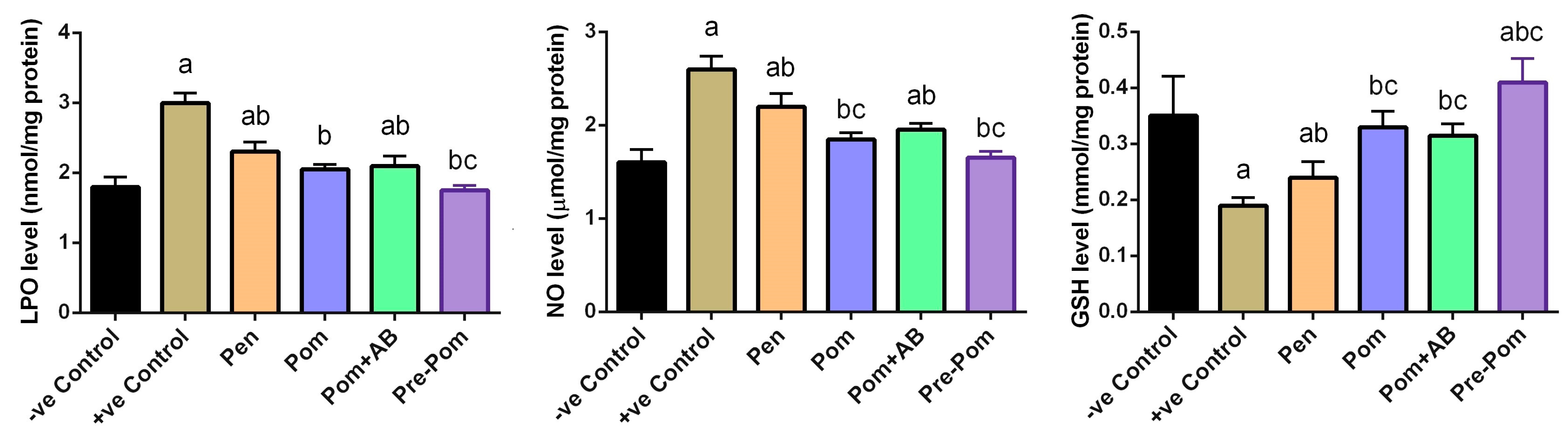
2.9. Oxidative Stress Markers
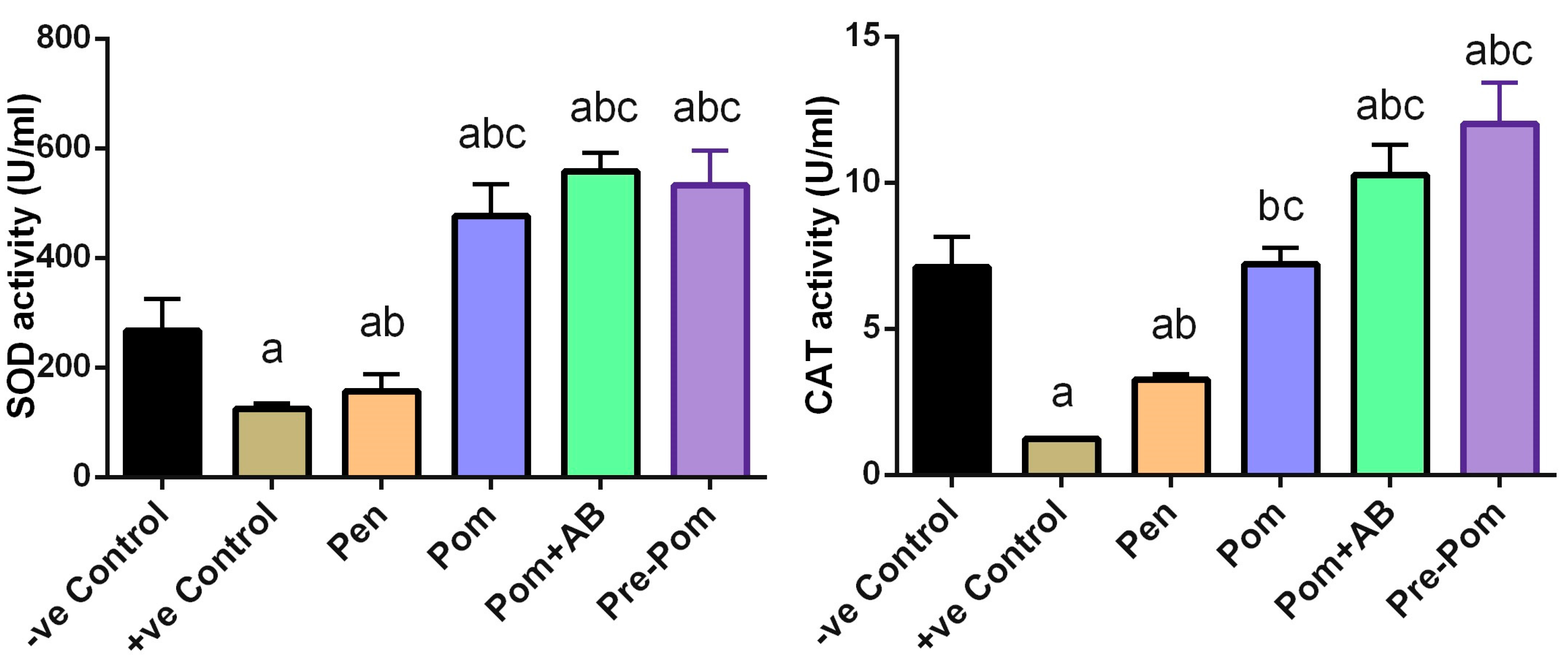
2.10. Enzymatic Antioxidant Activities
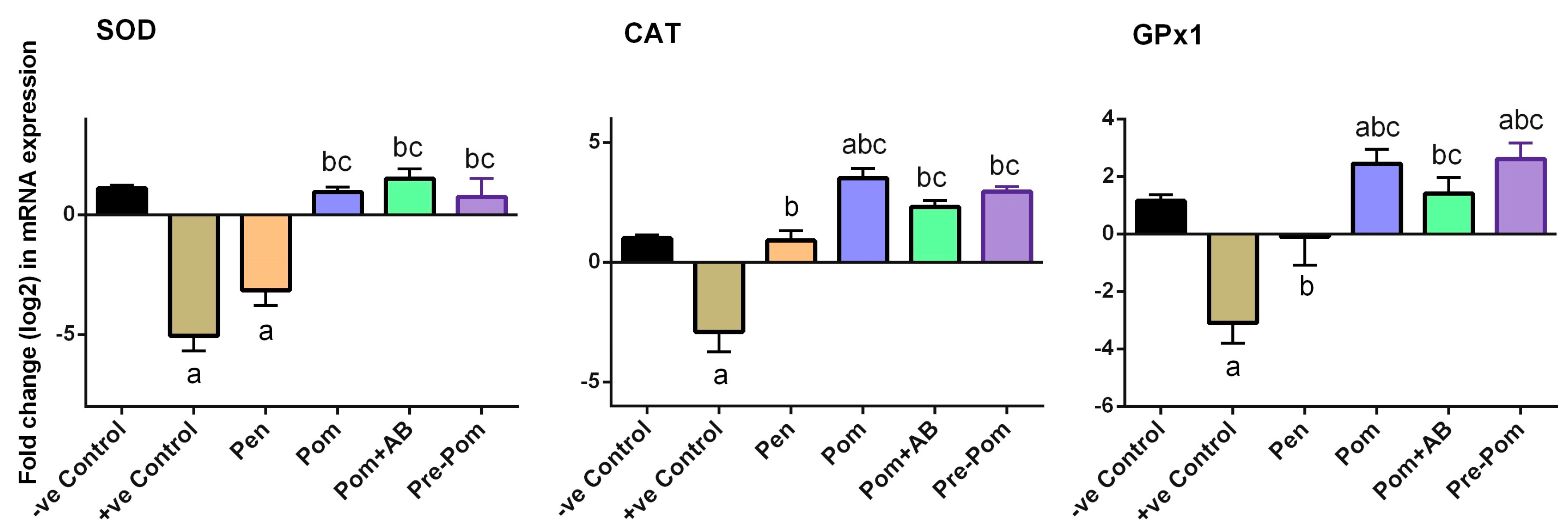
2.11. Gene Expression Profile by RT-PCR (Real-Time Polymerase Chain Reaction) in Skin
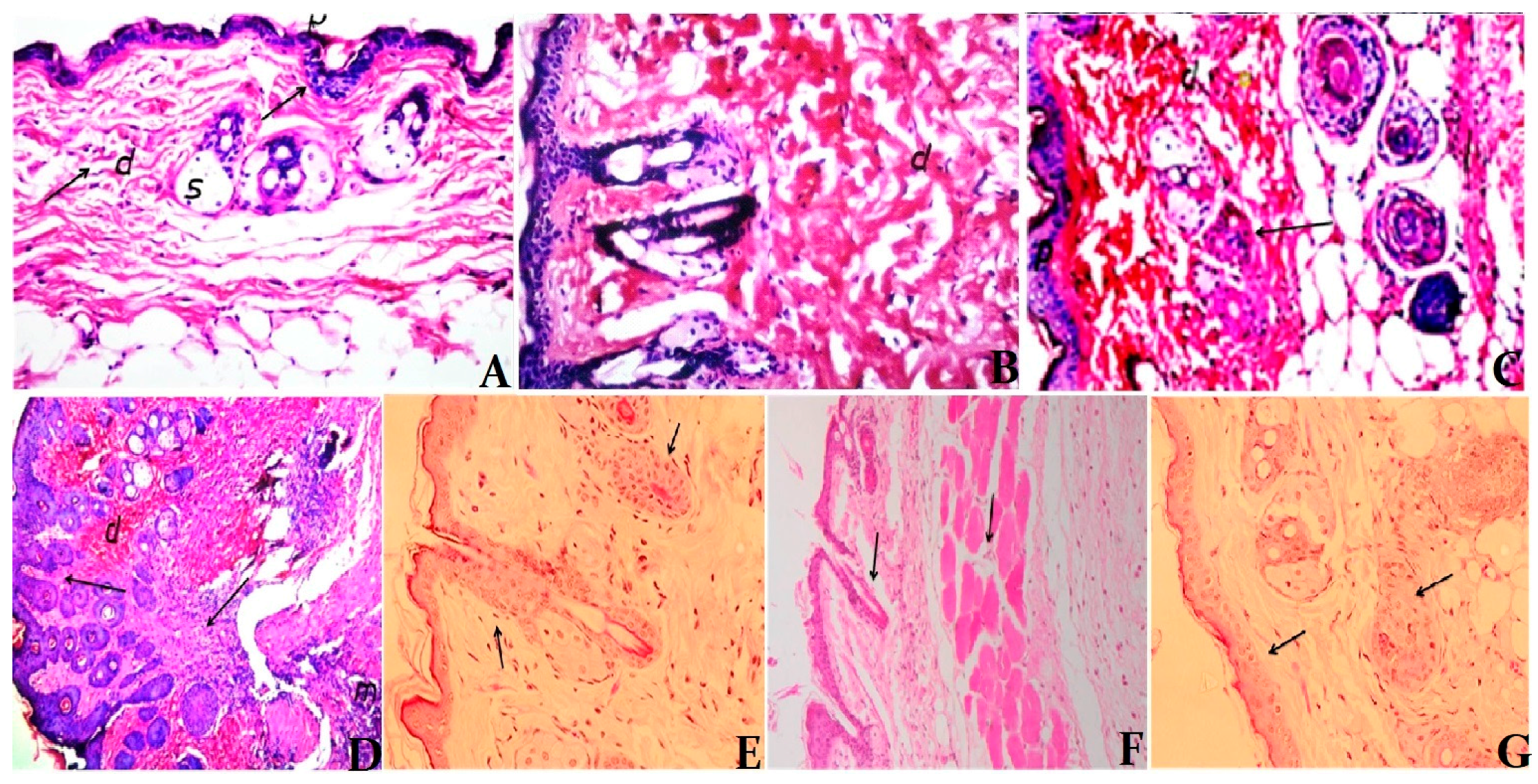
2.12. Histological Changes
2.13. Statistical Analysis
3. Results
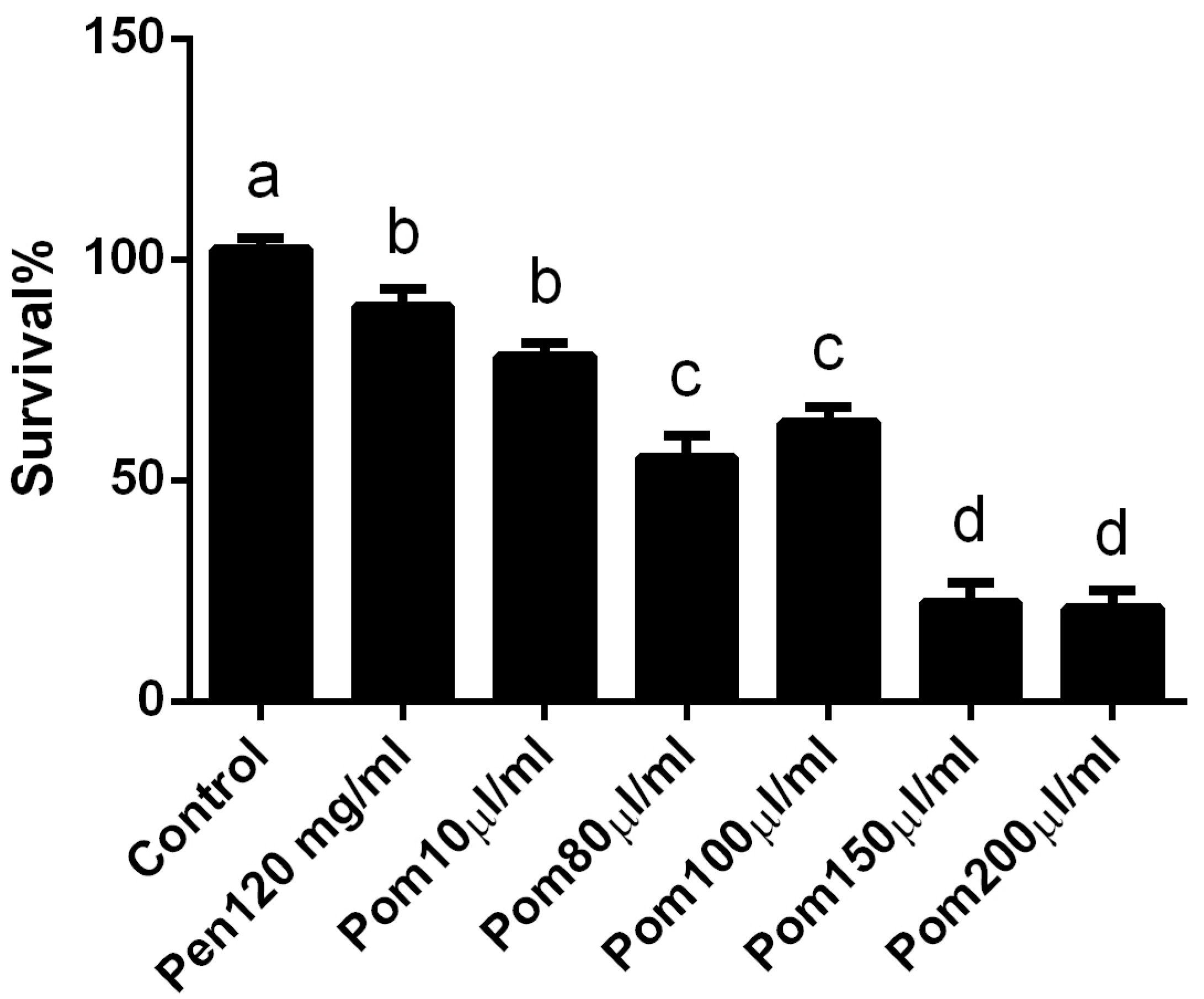
3.1. In Vivo Cytotoxicity of Pomegranate Juice
3.2. Antileishmanial Activity of Pomegranate Juice
3.3. Effect on Liver Function after Pomegranate Juice Treatment
3.4. Antioxidant Activity of Pomegranate Juice
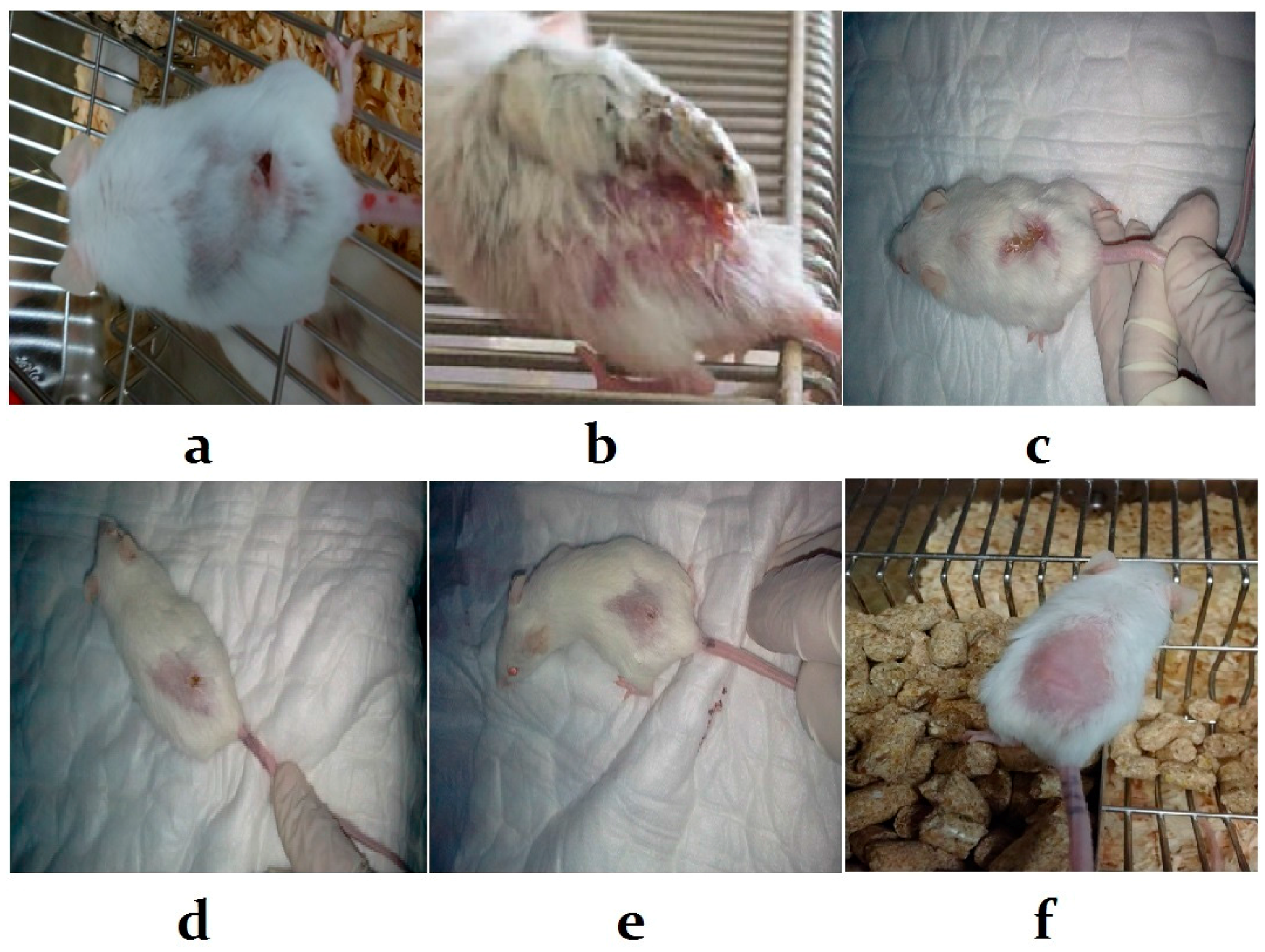
3.5. Histological Examination
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khademvatan, S.; Gharavi, M.J.; Rahim, F.; Saki, J. Miltefosine-induced apoptotic cell death on Leishmania major and L. tropica strains. Korean J. Parasitol. 2011, 49, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Ziaie, H.; Sadeghian, G. Isolation of bacteria causing secondary bacterial infection in the lesions of cutaneous leishmaniasis. Indian J. Dermatol. 2008, 53, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.M.; Amer, E.I.; Mossallam, S.F.; Gomaa, M.M.; Baddour, N.M. Miltefosine for old world cutaneous leishmaniasis: An experimental study on Leishmania major infected mice. Alexandria J. Med. 2012, 48, 261–271. [Google Scholar] [CrossRef]
- Kedzierski, L. Leishmaniasis vaccine: Where are we today? J. Glob. Infect. Dis. 2010, 2, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shirbazou, S.; Norozi, M. Induction of oxidative stress in skin and lung of infected BALB/c mice with iranian strain of Leishmania major (MRHO/IR/75/ER). Iran. J. Parasitol. 2014, 9, 60–69. [Google Scholar] [PubMed]
- Aguiar, M.G.; Pereira, A.M.M.; Fernandes, A.P.; Ferreira, L.A.M. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with leishmania (Leishmania) amazonensis. Antimicrob. Agents Chemother. 2010, 54, 4699–4704. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Moneim, A.E.; Al-Quraishy, S. Indigofera oblongifolia ameliorates lead acetate-induced testicular oxidative damage and apoptosis in a rat model. Biol. Trace Elem. Res. 2016, 173, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fadavi, A.; Barzegar, M.; Hossein Azizi, M. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in iran. J. Food Compos. Anal. 2006, 19, 676–680. [Google Scholar] [CrossRef]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Abdel Moneim, A.E. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement. Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Metwally, D.M.; Al-Olayan, E.M.; El-Khadragy, M.F.; Alkathiri, B. Anti-leishmanial activity (in vitro and in vivo) of allicin and allicin cream using Leishmania major (Sub-strain Zymowme LON4) and BALB/c mice. PLoS ONE 2016, 11, e0161296. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nahrevanian, H.; Farahmand, M.; Aghighi, Z.; Assmar, M.; Amirkhani, A. Pharmacological evaluation of anti-leishmanial activity by in vivo nitric oxide modulation in balb/c mice infected with Leishmania major MRHO/IR/75/ER: An iranian strain of cutaneous leishmaniasis. Exp. Parasitol. 2007, 116, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Alshabanah, O.A.; Hafez, M.M.; Al-Harbi, M.M.; Hassan, Z.K.; Al Rejaie, S.S.; Asiri, Y.A.; Sayed-Ahmed, M.M. Doxorubicin toxicity can be ameliorated during antioxidant l-carnitine supplementation. Oxid Med. Cell. Longev. 2010, 3, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, J.; Nasereddin, A.; Niederwieser, I.; Jaffe, C.L.; Beck, H.P.; Felger, I. Identification and differentiation of leishmania species in clinical samples by pcr amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J. Clin. Microbiol. 2003, 41, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Nabors, G.S.; Afonso, L.C.; Farrell, J.P.; Scott, P. Switch from a type 2 to a type 1 t helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc. Natl. Acad. Sci. USA 1995, 92, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a cosmeceutical source: Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Venkataram, M.; Moosa, M.; Devi, L. Histopathological spectrum in cutaneous leishmaniasis: A study in oman. Indian J. Dermatol. Venereol. Leprol. 2001, 67, 294–298. [Google Scholar] [PubMed]
- Souza, L.W.; Souza, S.V.; Botelho, A.C. Comparative analysis of the geographic distribution of the histopathological spectrum and leishmania species of american cutaneous leishmaniasis in brazil. Anais Bras. Dermatol. 2012, 87, 369–374. [Google Scholar] [CrossRef]
- Inacio, J.D.; Gervazoni, L.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. The effect of (−)-epigallocatechin 3-o-gallate in vitro and in vivo in leishmania braziliensis: Involvement of reactive oxygen species as a mechanism of action. PLoS Negl. Trop. Dis. 2014, 8, e3093. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Kayser, O.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L.Y. Antileishmanial activity of hydrolyzable tannins and their modulatory effects on nitric oxide and tumour necrosis factor-alpha release in macrophages in vitro. Planta. Med. 2001, 67, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Iqbal, J.; Staerk, D.; Kongstad, K.T. Characterization of antileishmanial compounds from Lawsonia inermis L. Leaves using semi-high resolution antileishmanial profiling combined with HPLC-HRMS-SPE-NMR. Front. Pharmacol. 2017, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, M.; Gorji-Bandpy, M.; Ganji, D.D.; Soleimani, S. Heat flux boundary condition for nanofluid filled enclosure in presence of magnetic field. J. Mol. Liq. 2014, 193, 174–184. [Google Scholar] [CrossRef]
- Sakthianandeswaren, A.; Elso, C.M.; Simpson, K.; Curtis, J.M.; Kumar, B.; Speed, T.P.; Handman, E.; Foote, S.J. The wound repair response controls outcome to cutaneous leishmaniasis. Proc. Natl. Acad. Sci. USA 2005, 102, 15551–15556. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int J. Infect. Dis. 2011, 15, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Al-Quraishy, S.; Abdel Moneim, A.E.; Delic, D. Protective effect of azadirachta indica extract against eimeria papillata-induced coccidiosis. Parasitol. Res. 2013, 112, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E.; El-Khadragy, M.F. The potential effects of pomegranate (Punica granatum) juice on carbon tetrachloride-induced nephrotoxicity in rats. J. Physiol. Biochem. 2013, 69, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Ozbilge, H.; Aksoy, N.; Kilic, E.; Saraymen, R.; Yazar, S.; Vural, H. Evaluation of oxidative stress in cutaneous leishmaniasis. J. Dermatol. 2005, 32, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.; Mukhopadhyay, R.; Ghosal, J.; Biswas, T. Oxidative damage of erythrocytes: A possible mechanism for premature hemolysis in experimental visceral leishmaniasis in hamsters. Ann. Hematol. 2001, 80, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D.P.; Majhi, S.; Chandra, L.; Rijal, S.; Baral, N. Erythrocyte glutathione status in human visceral leishmaniasis. Indian J. Clin. Biochem. 2008, 23, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Soltani, S.; Mohri, M.; Khoshnegah, J. Canine visceral leishmaniasis: Relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 2012, 111, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Aksoy, N.; Ozbilge, H. Alterations of oxidative-antioxidative status in human cutaneous leishmaniasis. Cell. Biochem. Funct. 2004, 22, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Serarslan, G.; Yilmaz, H.R.; Sogut, S. Serum antioxidant activities, malondialdehyde and nitric oxide levels in human cutaneous leishmaniasis. Clin. Exp. Dermatol. 2005, 30, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Azadirachta indica attenuates cisplatin-induced neurotoxicity in rats. Indian J. Pharmacol. 2014, 46, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Shirbazou, S.; Jafari, M. The multiple forms of Leishmania major in balb/c mice lung in Iran. Iran. J. Parasitol. 2012, 7, 99–102. [Google Scholar] [PubMed]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.C.; Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [PubMed]
- Jafari, M.; Shirbazou, S.; Sadraie, S.H.; Kaka, G.; Norozi, M. The role of apoptosis in the cellular response of liver and spleen of BALB/c mice in cutaneous leishmaniasis. Iran. J. Med. Sci. 2015, 40, 133–142. [Google Scholar] [PubMed]
- Oliveira, F.J.; Cecchini, R. Oxidative stress of liver in hamsters infected with Leishmania (L.) chagasi. J. Parasitol. 2000, 86, 1067–1072. [Google Scholar] [CrossRef]
- Biswas, T.; Ghosh, D.K.; Mukherjee, N.; Ghosal, J. Lipid peroxidation of erythrocytes in visceral leishmaniasis. J. Parasitol. 1997, 83, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Prevention of carbon tetrachloride (CCl4)-induced toxicity in testes of rats treated with Physalis peruviana L. fruit. Toxicol. Ind. Health 2014, 32, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Monteiro, R.; Mateus, N.; Azevedo, I.; Calhau, C. Effect of pomegranate (Punica granatum) juice intake on hepatic oxidative stress. Eur. J. Nutr. 2007, 46, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Nada, A.; Zaki, H.S.; Abdel Moneim, A.E. Effect of Physalis peruviana L. on cadmium-induced testicular toxicity in rats. Biol. Trace Elem. Res. 2014, 159, 278–287. [Google Scholar] [CrossRef] [PubMed]
| Name | Accession Number | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|---|
| β-actin | NM_031144.3 | GGCATCCTGACCCTGAAGTA | GGGGTGTTGAAGGTCTCAAA |
| SOD2 | NM_001270850.1 | AGCTGCACCACAGCAAGCAC | TCCACCACCCTTAGGGCTCA |
| CAT | NM_012520.2 | TCCGGGATCTTTTTAACGCCATTG | TCGAGCACGGTAGGGACAGTTCAC |
| GPx1 | NM_017006.2 | CGGTTTCCCGTGCAATCAGT | ACACCGGGGACCAAATGATG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkathiri, B.; El-Khadragy, M.F.; Metwally, D.M.; Al-Olayan, E.M.; Bakhrebah, M.A.; Abdel Moneim, A.E. Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice. Int. J. Environ. Res. Public Health 2017, 14, 1592. https://doi.org/10.3390/ijerph14121592
Alkathiri B, El-Khadragy MF, Metwally DM, Al-Olayan EM, Bakhrebah MA, Abdel Moneim AE. Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice. International Journal of Environmental Research and Public Health. 2017; 14(12):1592. https://doi.org/10.3390/ijerph14121592
Chicago/Turabian StyleAlkathiri, Badriah, Manal F. El-Khadragy, Dina M. Metwally, Ebtesam M. Al-Olayan, Muhammed A. Bakhrebah, and Ahmed E. Abdel Moneim. 2017. "Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice" International Journal of Environmental Research and Public Health 14, no. 12: 1592. https://doi.org/10.3390/ijerph14121592
APA StyleAlkathiri, B., El-Khadragy, M. F., Metwally, D. M., Al-Olayan, E. M., Bakhrebah, M. A., & Abdel Moneim, A. E. (2017). Pomegranate (Punica granatum) Juice Shows Antioxidant Activity against Cutaneous Leishmaniasis-Induced Oxidative Stress in Female BALB/c Mice. International Journal of Environmental Research and Public Health, 14(12), 1592. https://doi.org/10.3390/ijerph14121592




