Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Reagents
2.3. Synthesis of LMT Nanocomposite
2.4. Adsorption Experiments
2.5. Desorption and Regeneration Experiments
2.6. Characterization
3. Results and Discussion
3.1. Surface Area and Pore Volume
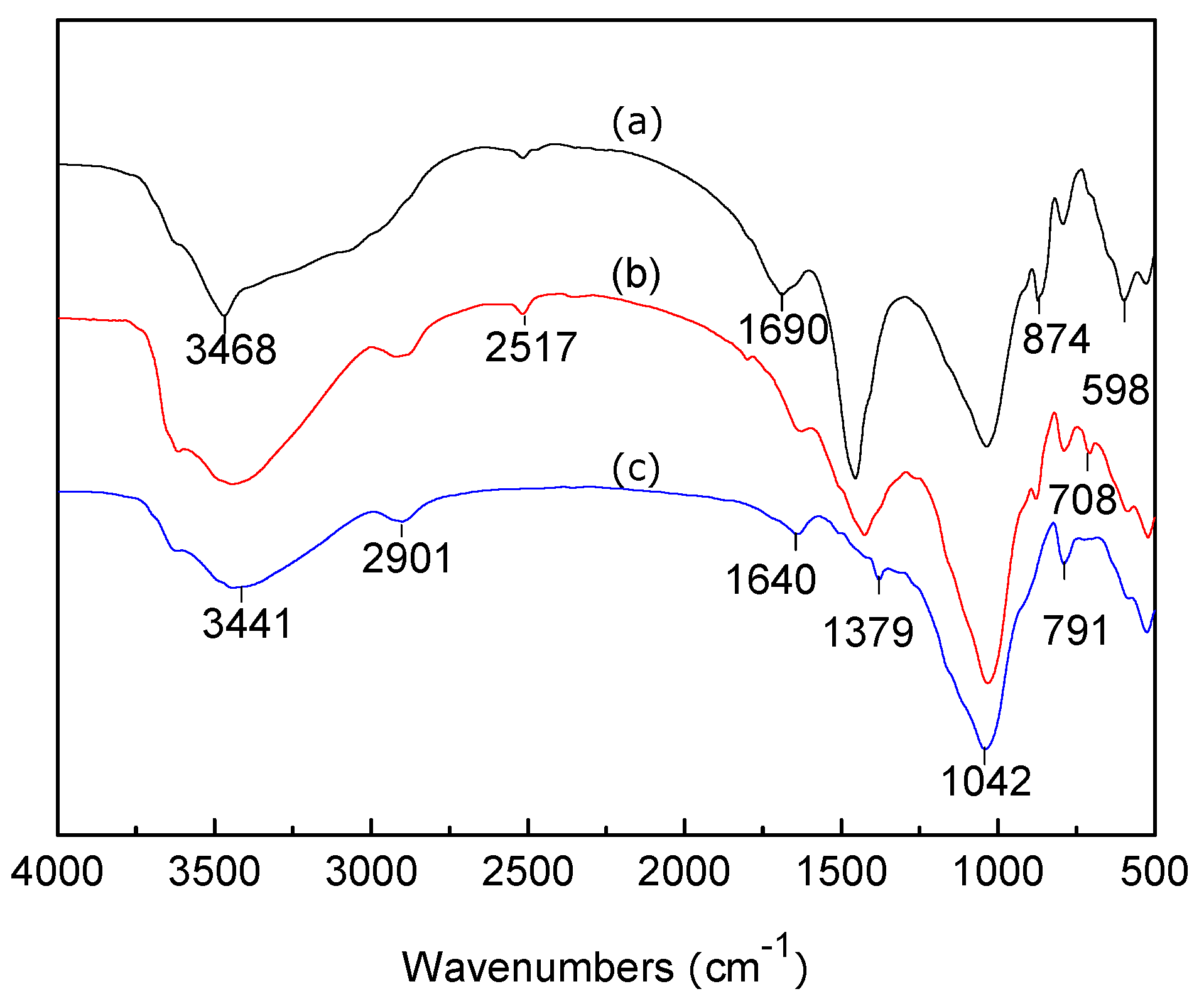
3.2. FTIR Analysis of LC, MT, and LMT
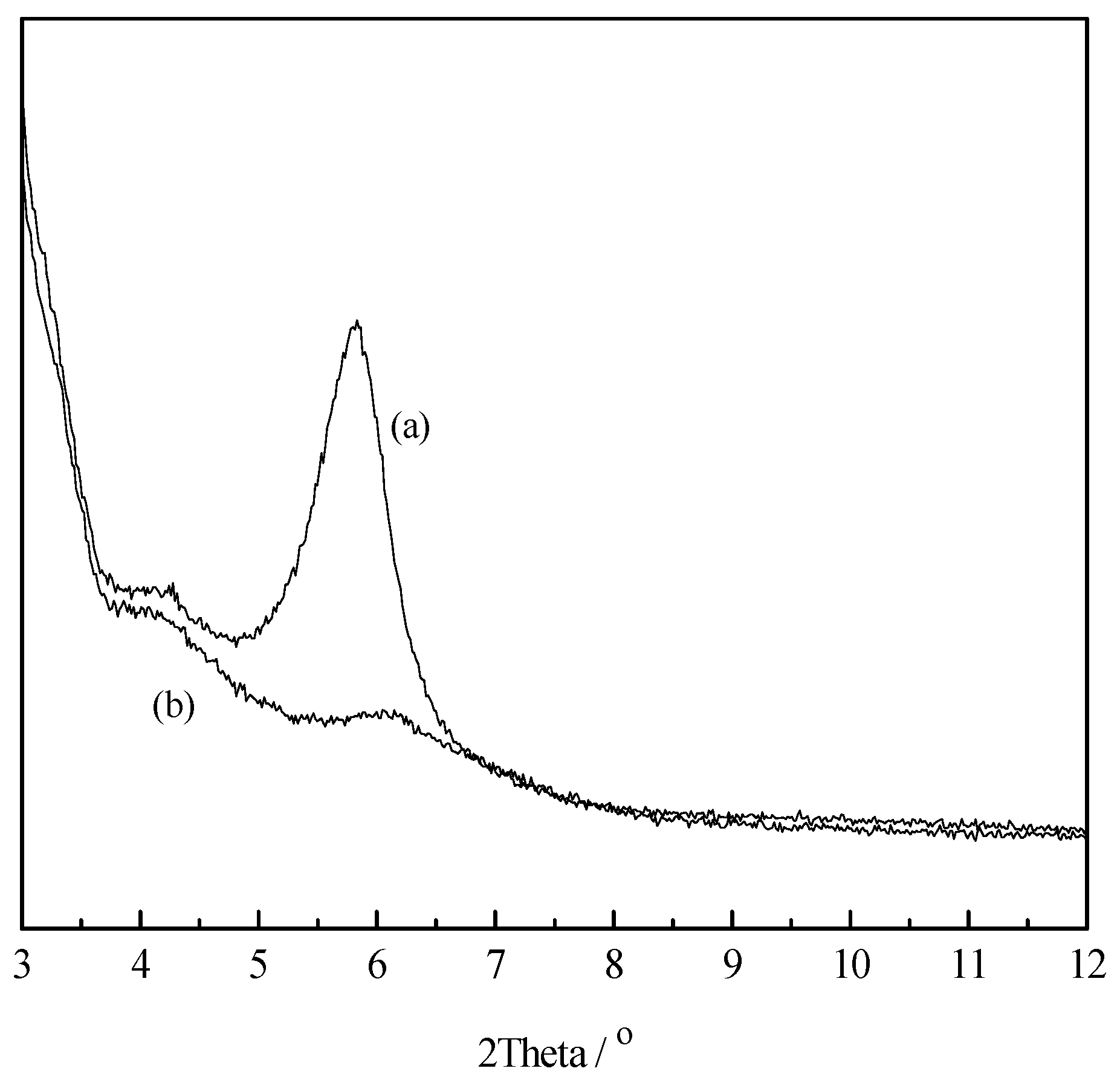
3.3. XRD Analysis of LMT
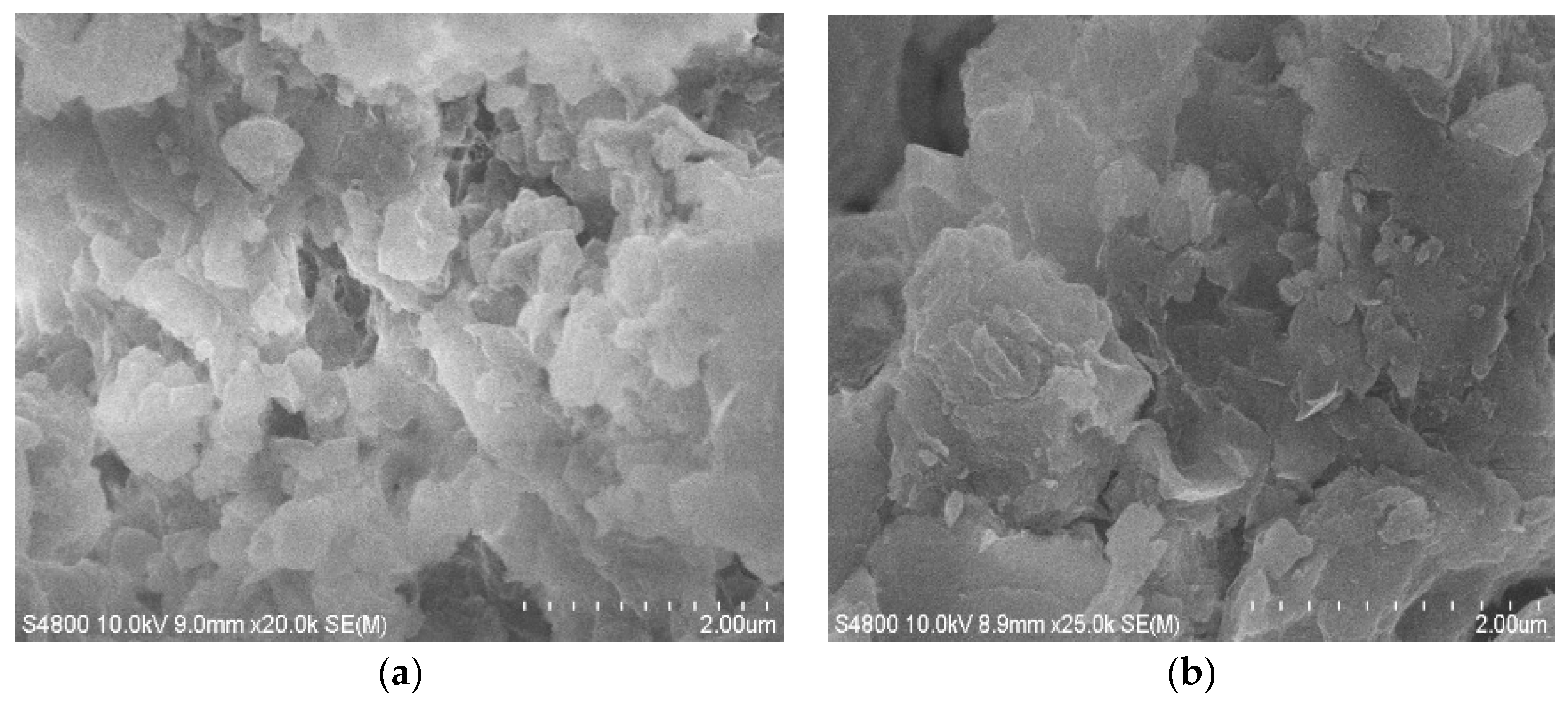
3.4. SEM Image Analysis of LMT
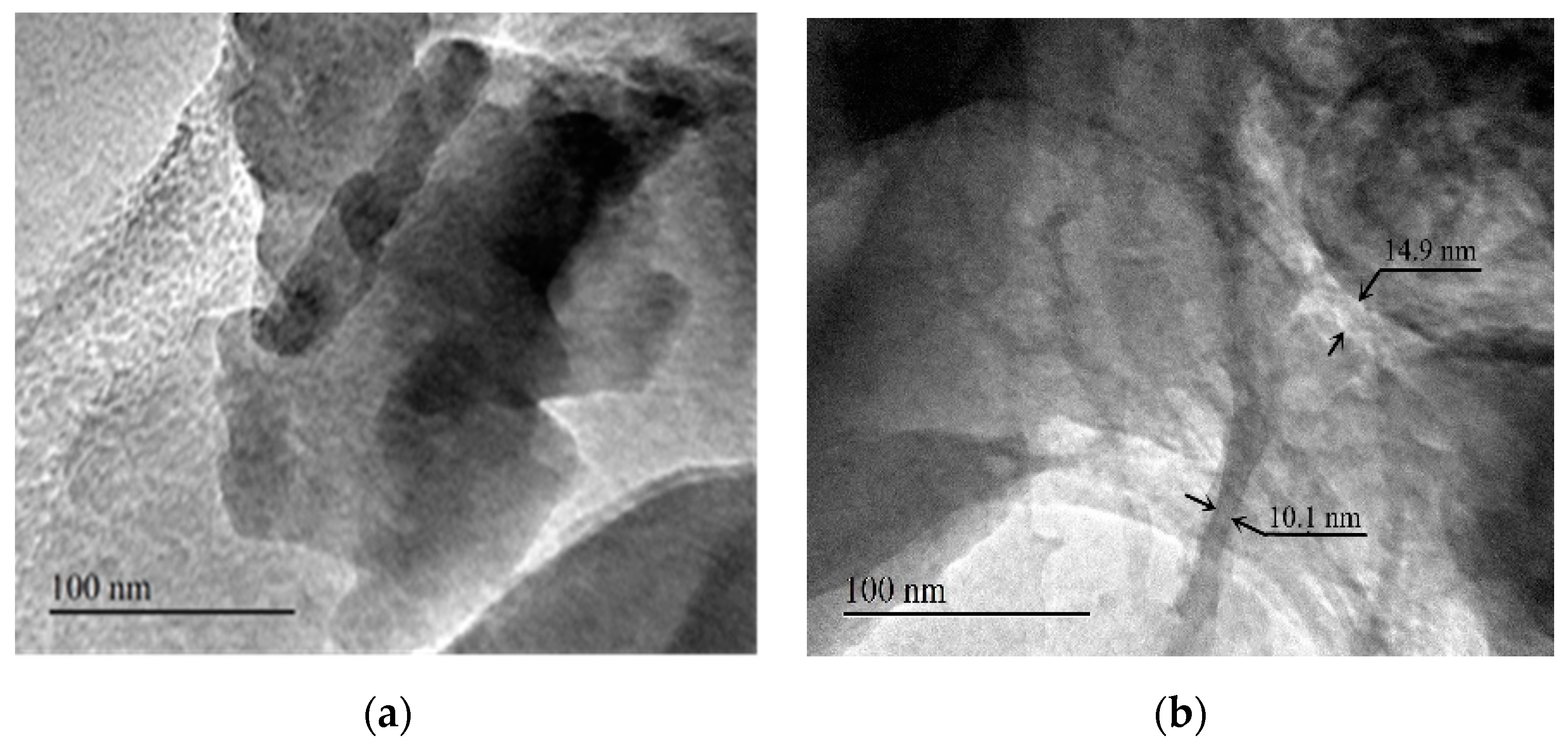
3.5. TEM Image Analysis of LMT
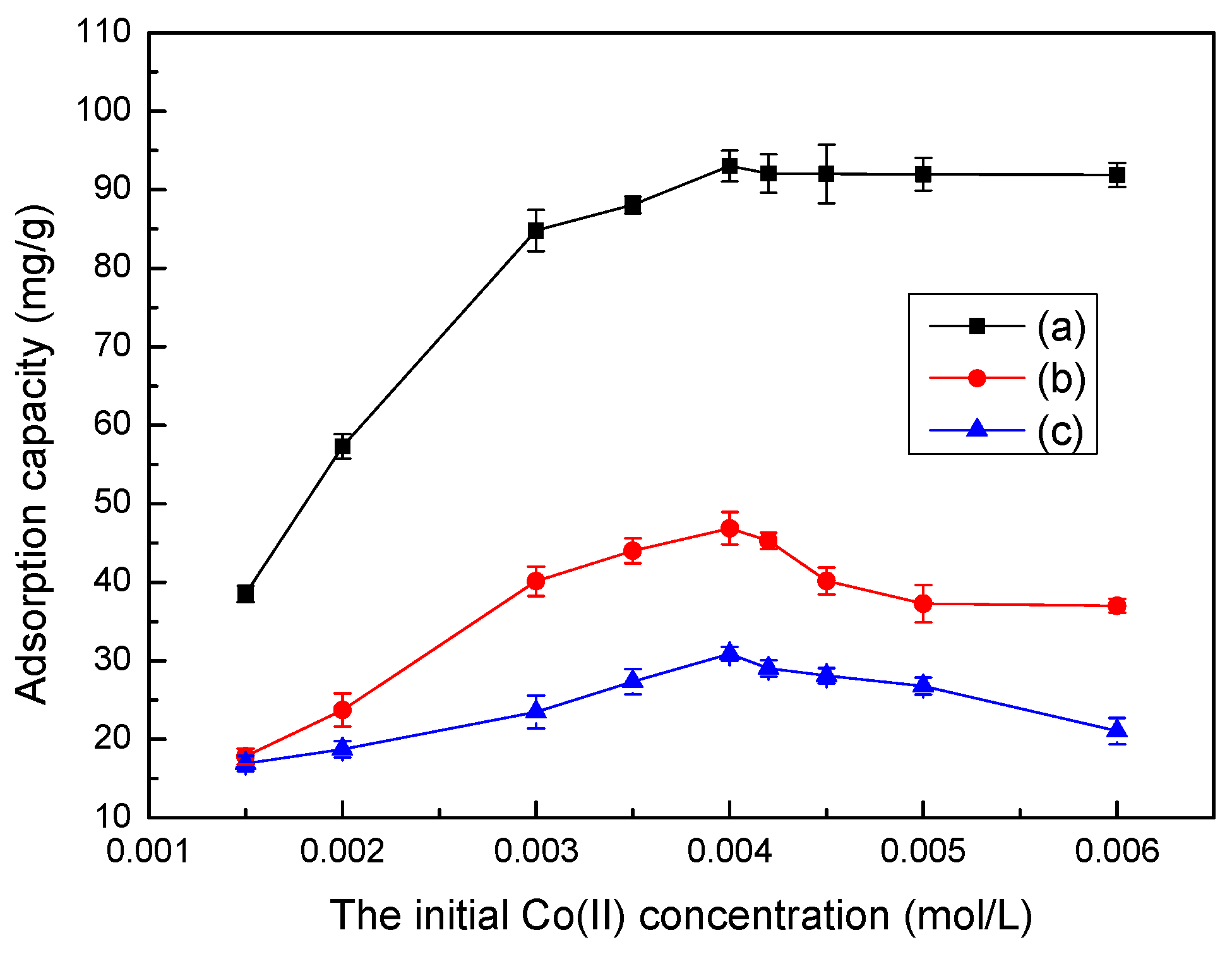
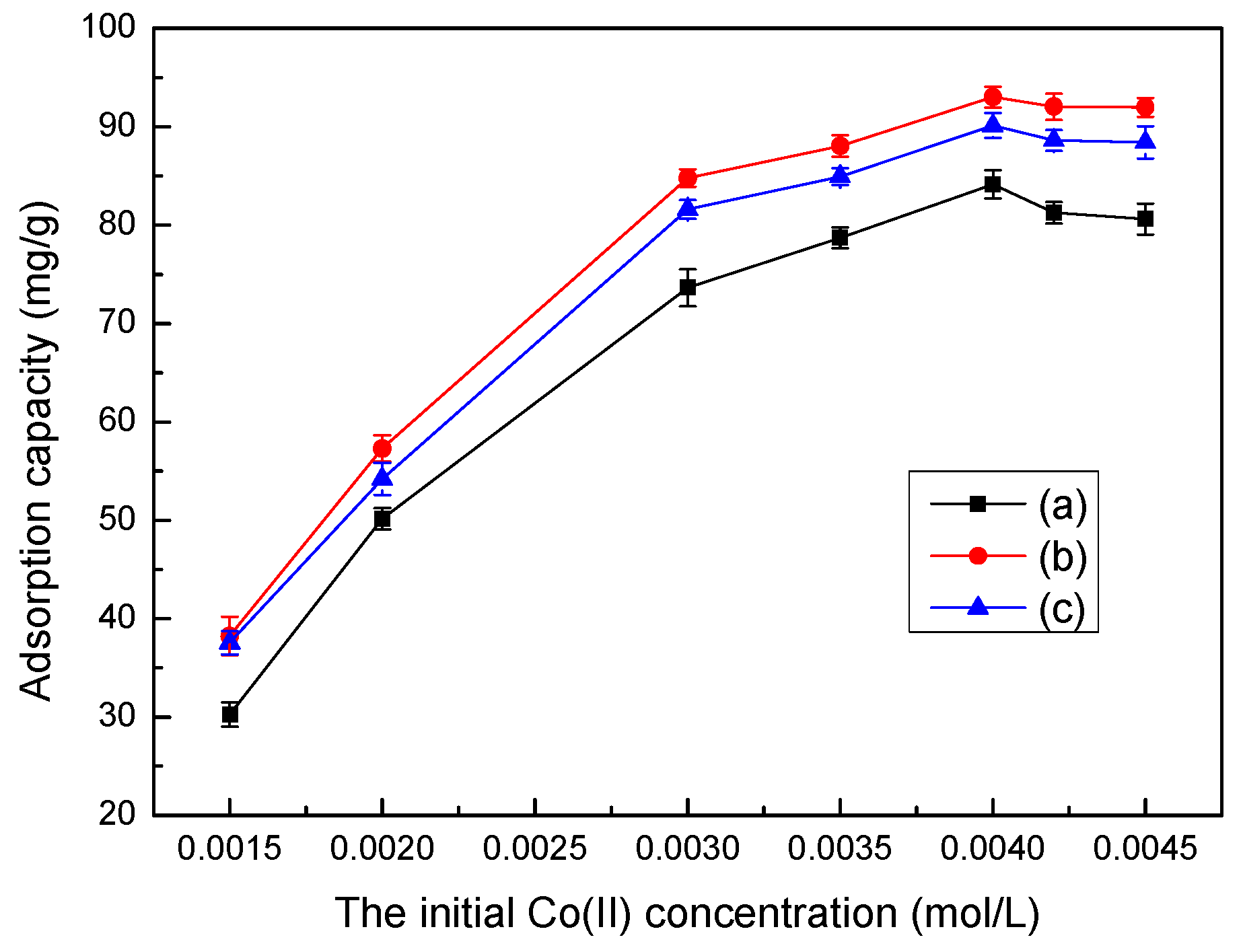
3.6. Effect of Initial Co(II) Concentration on Adsorption
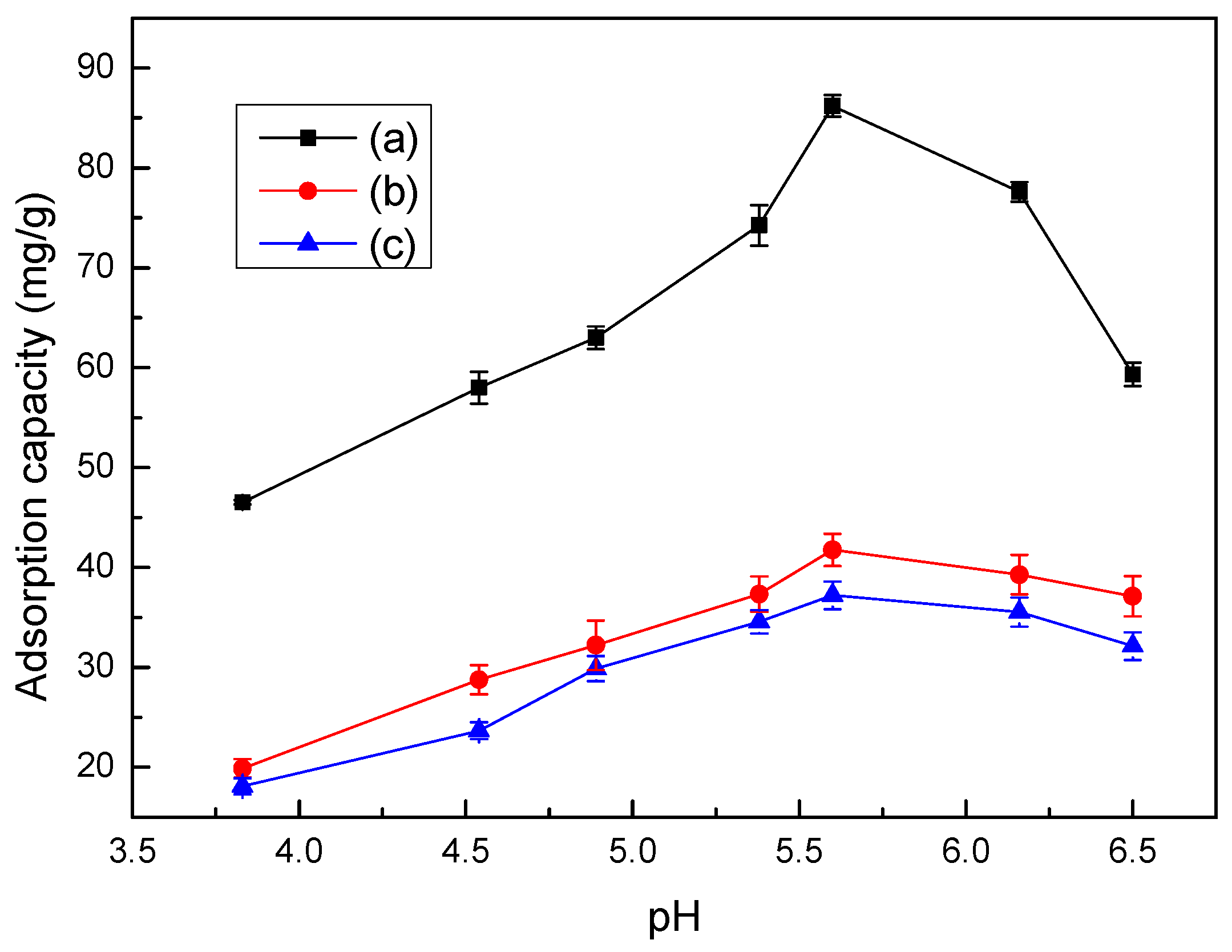
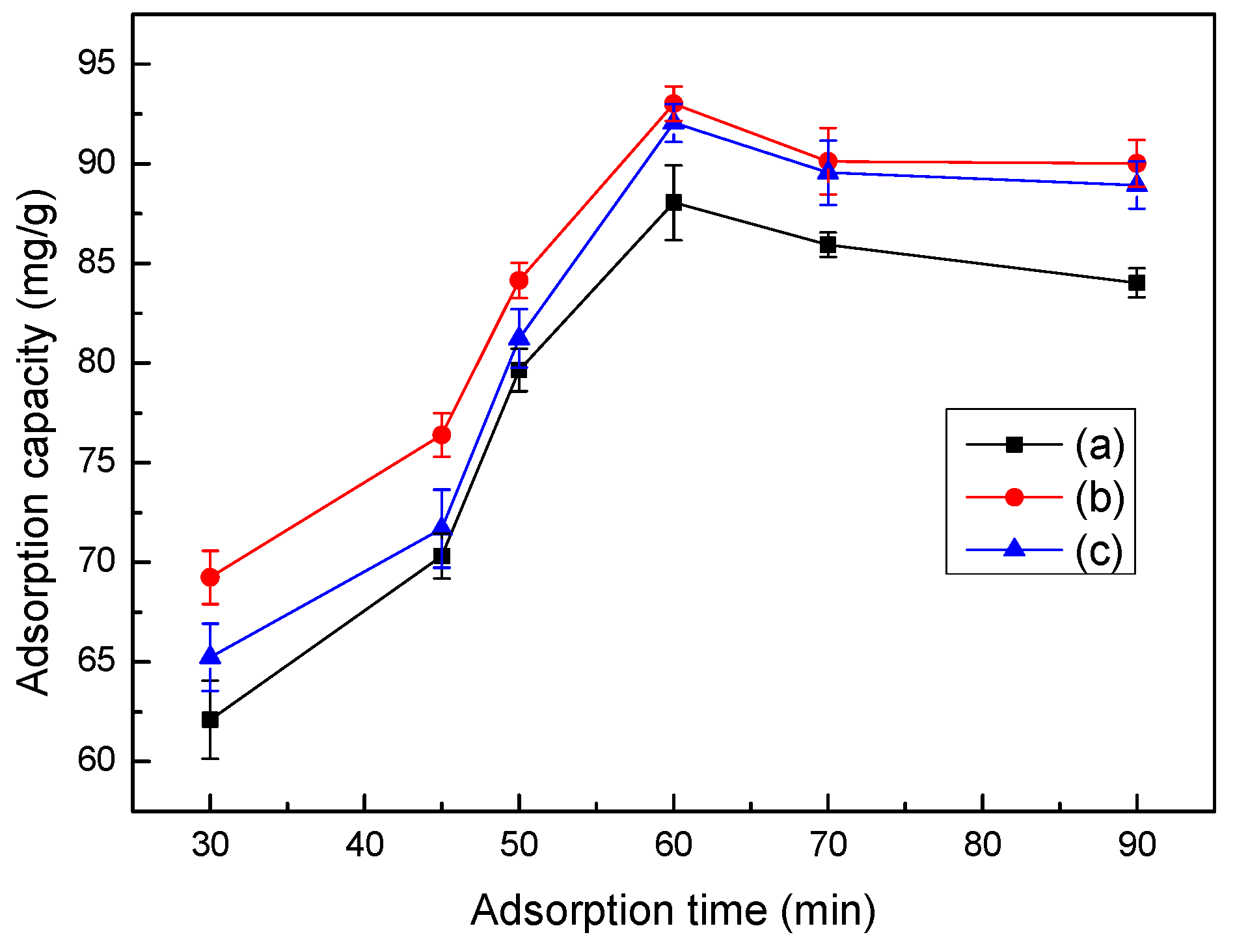
3.7. Effect of pH Values on Adsorption
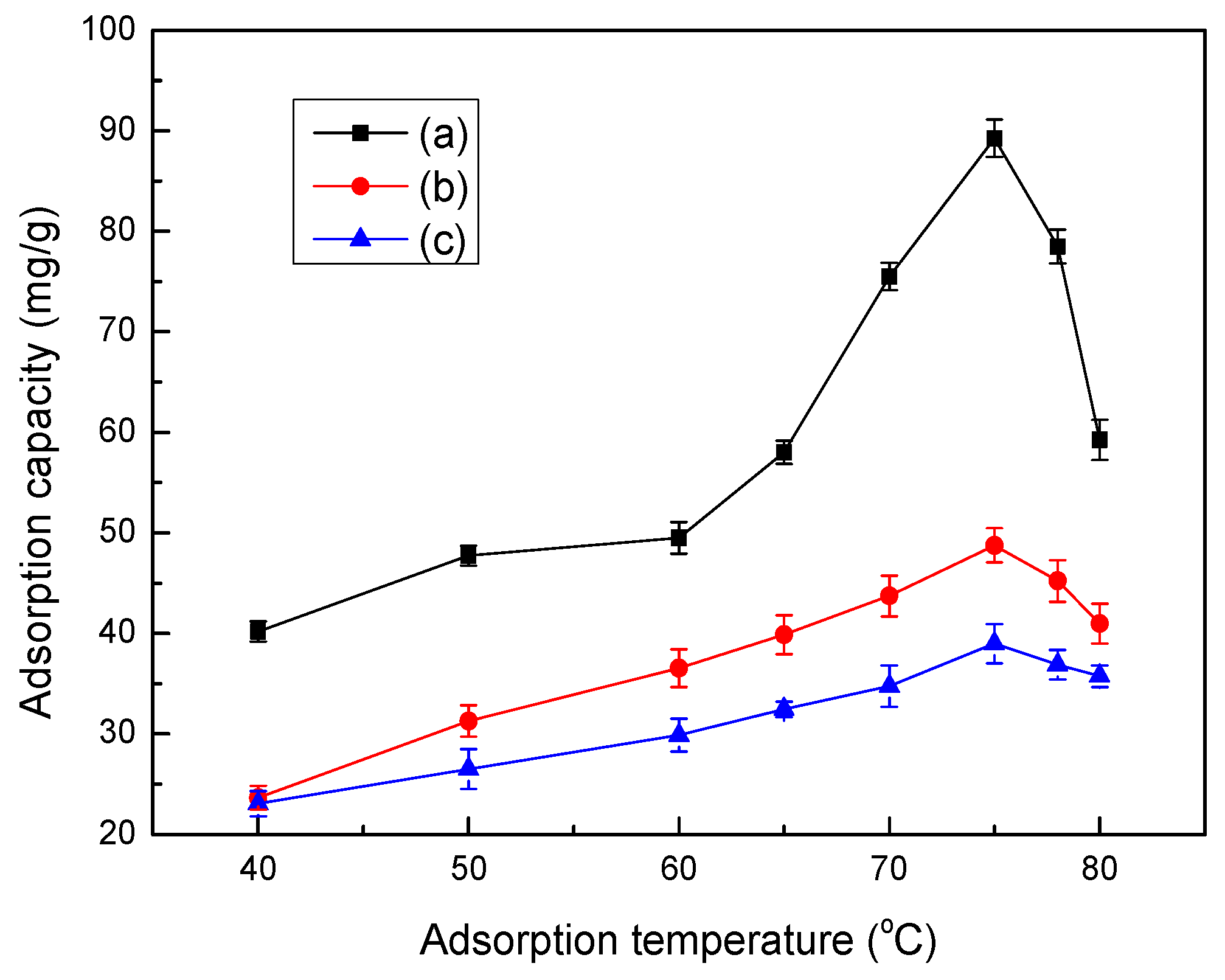
3.8. Effect of Temperature on Adsorption
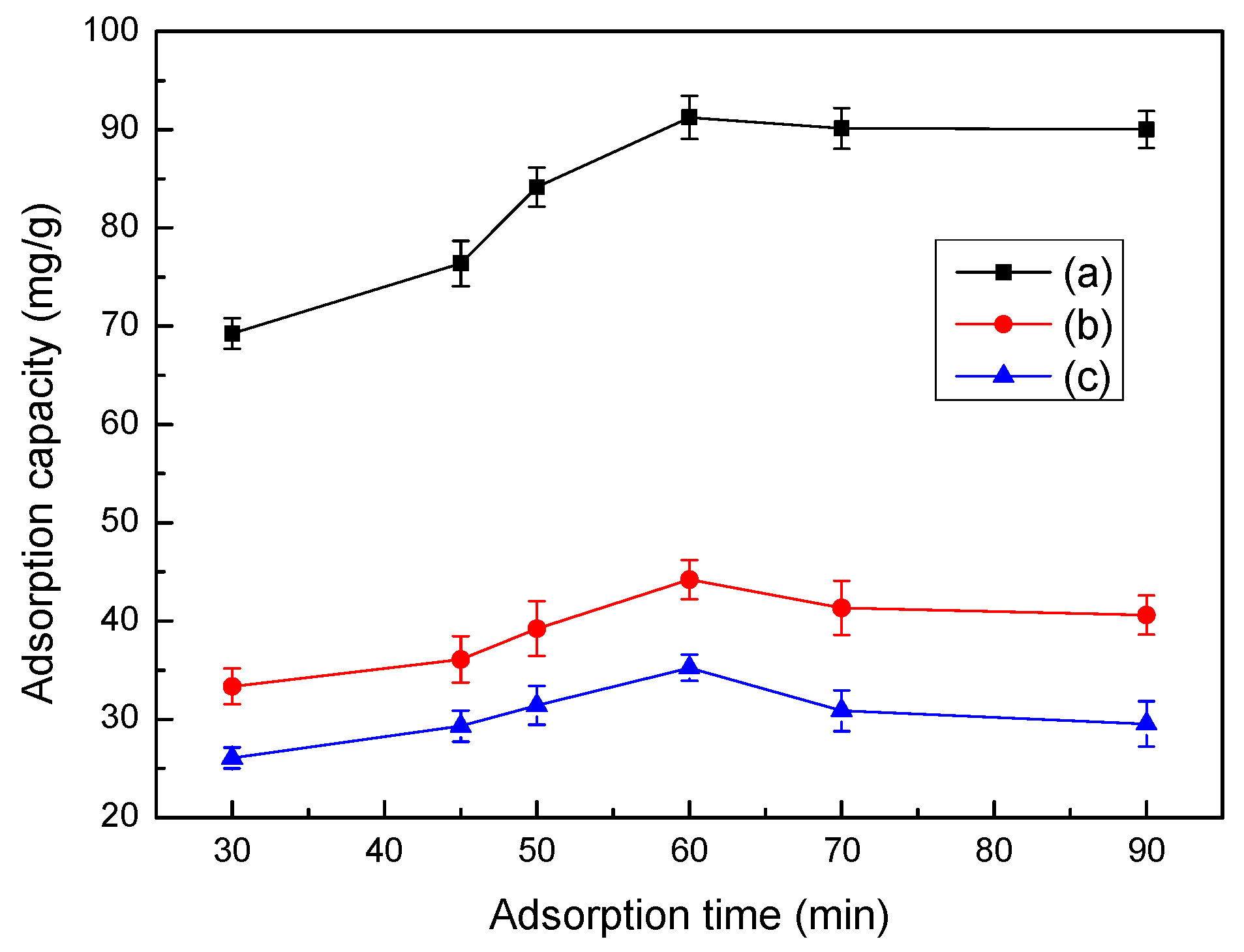
3.9. Effect of Time on Adsorption
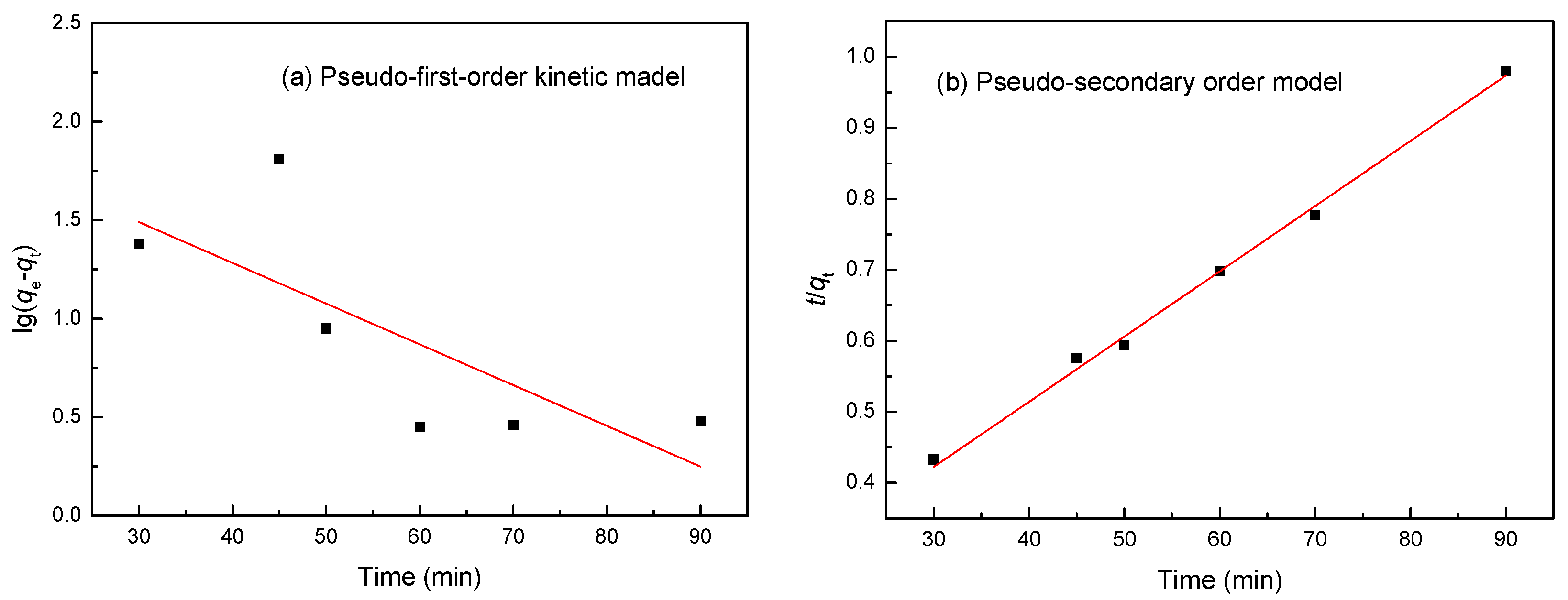
3.10. Kinetic Studies
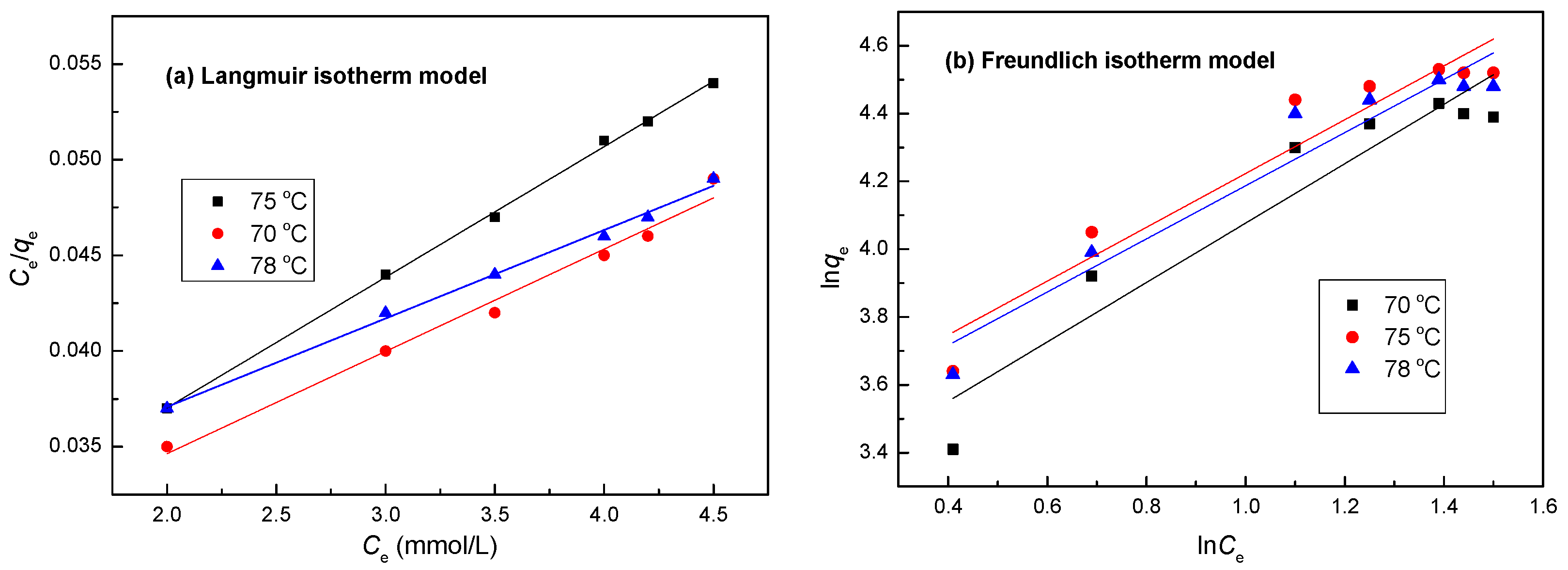
3.11. Isotherm Studies
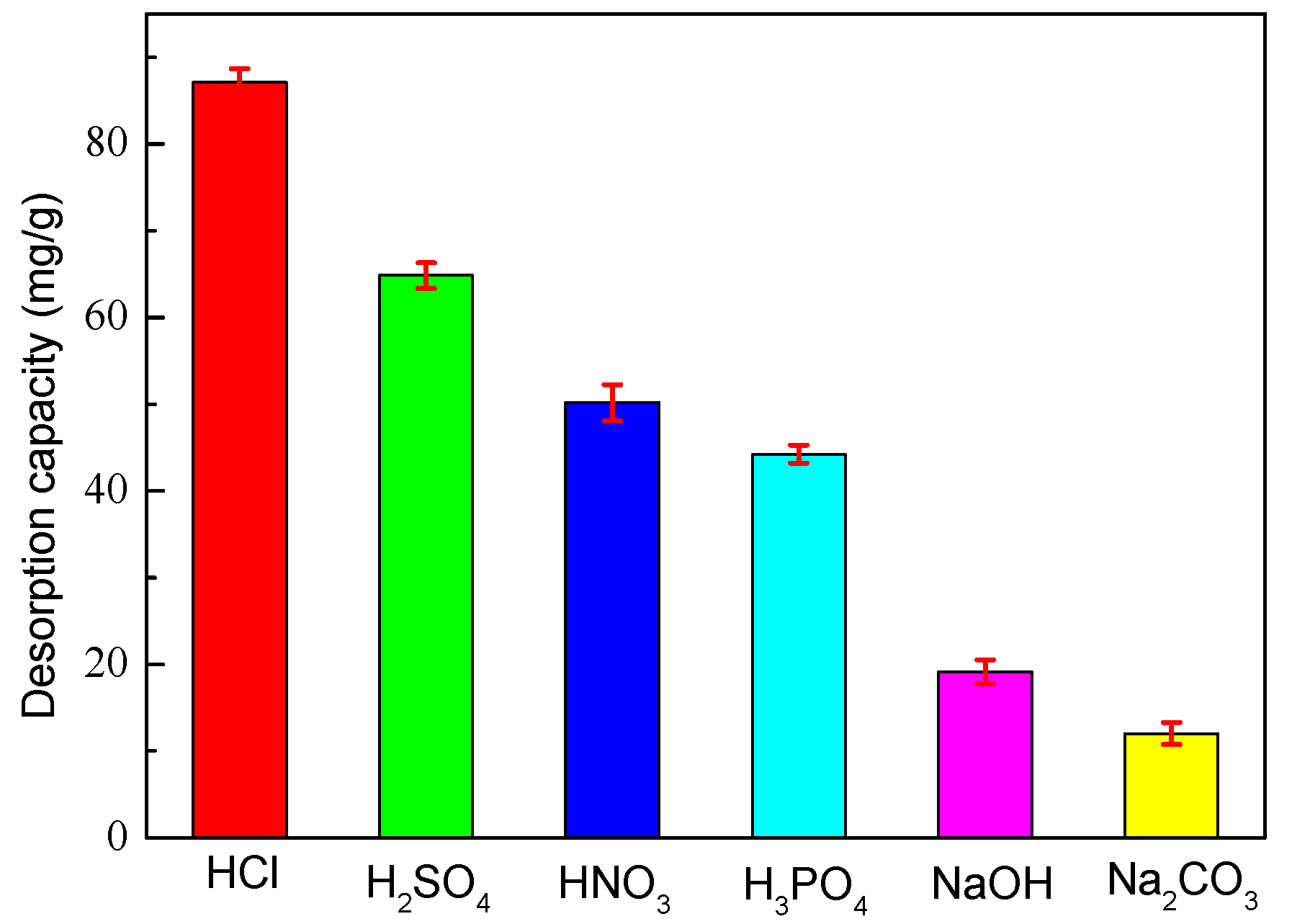
3.12. Effect of Desorption Eluents on Desorption
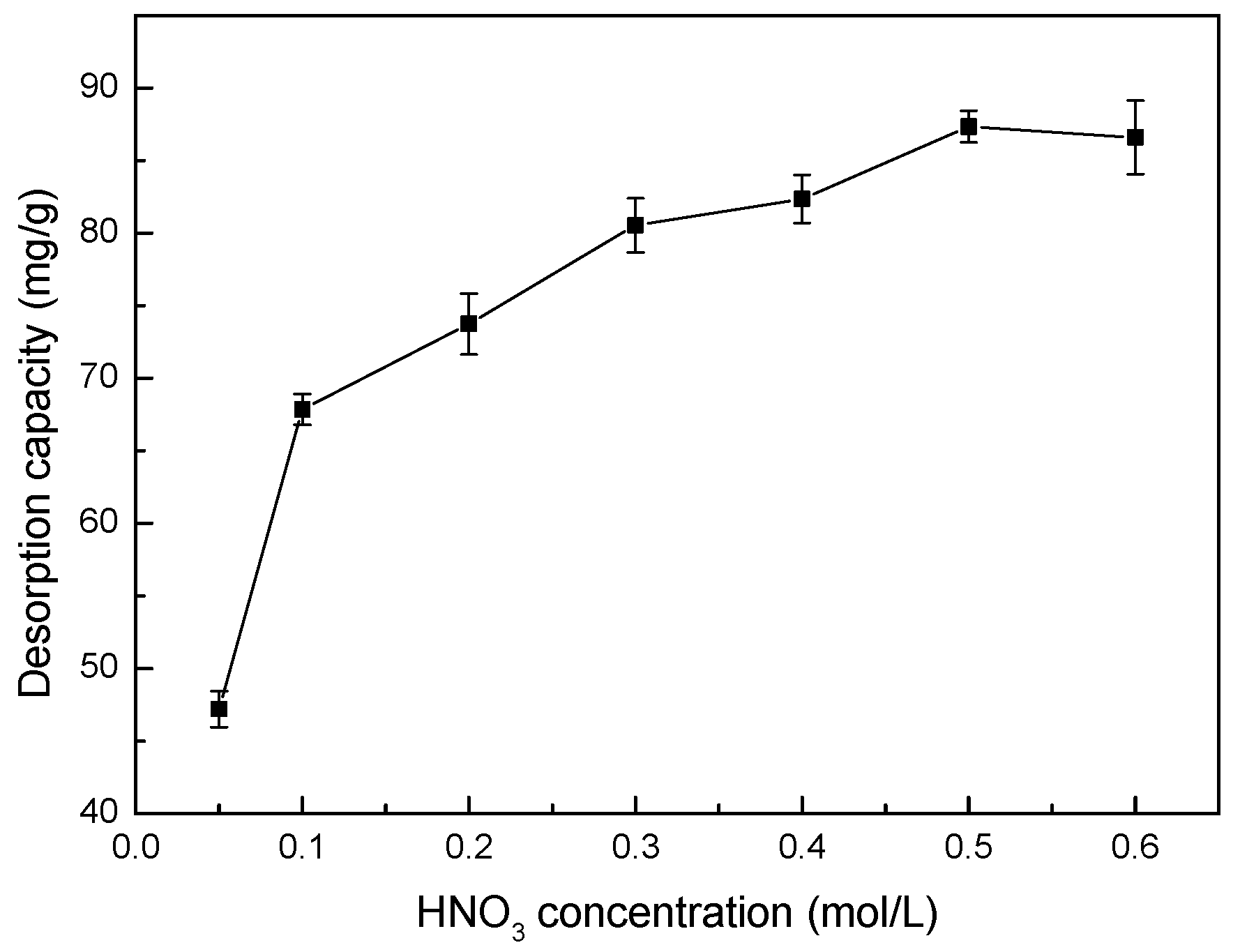
3.13. Effect of HNO3 Concentration on Desorption
3.14. Effect of Temperature on Desorption
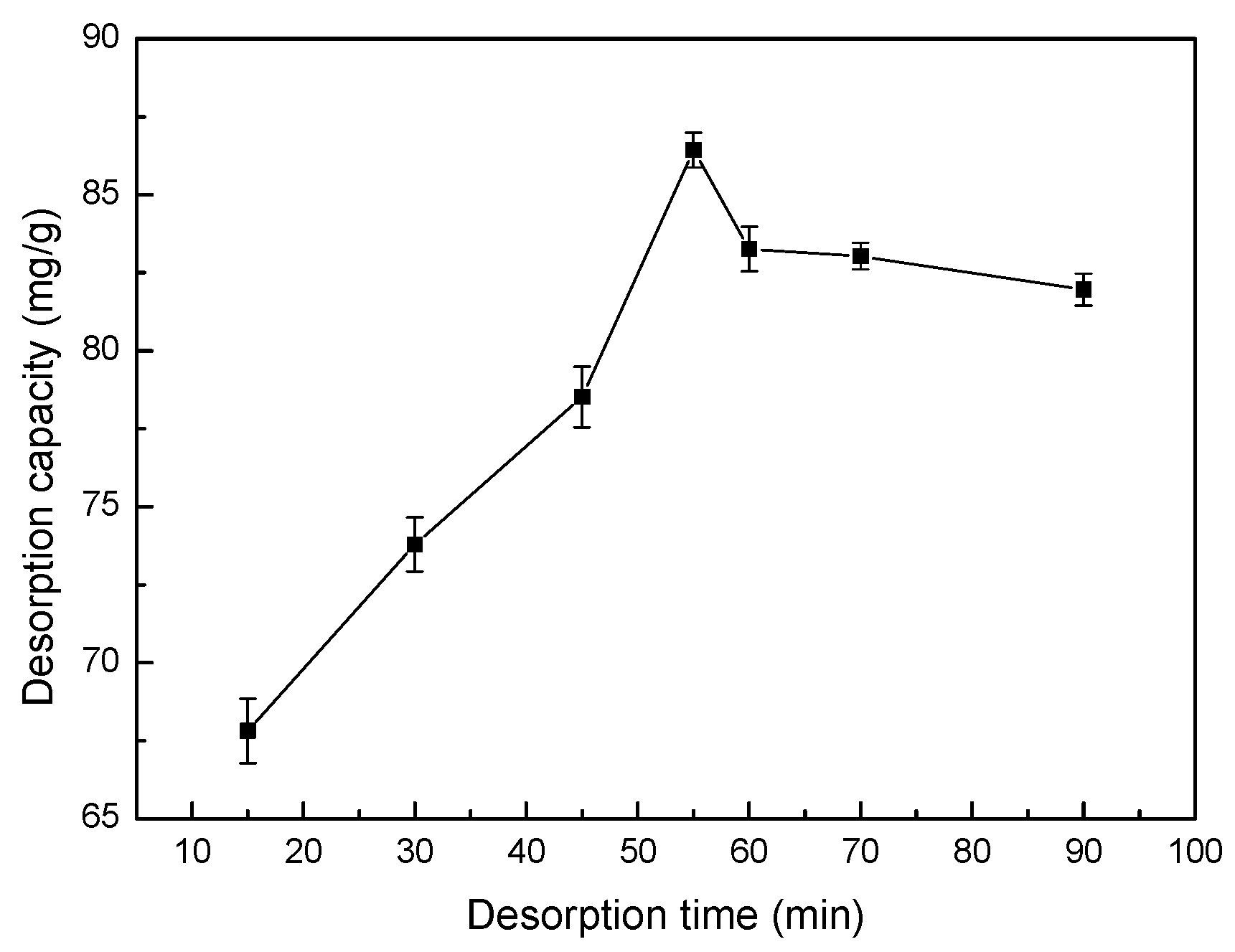
3.15. Effect of Sonication Time on Desorption
3.16. Recycling and Reusability of LMT
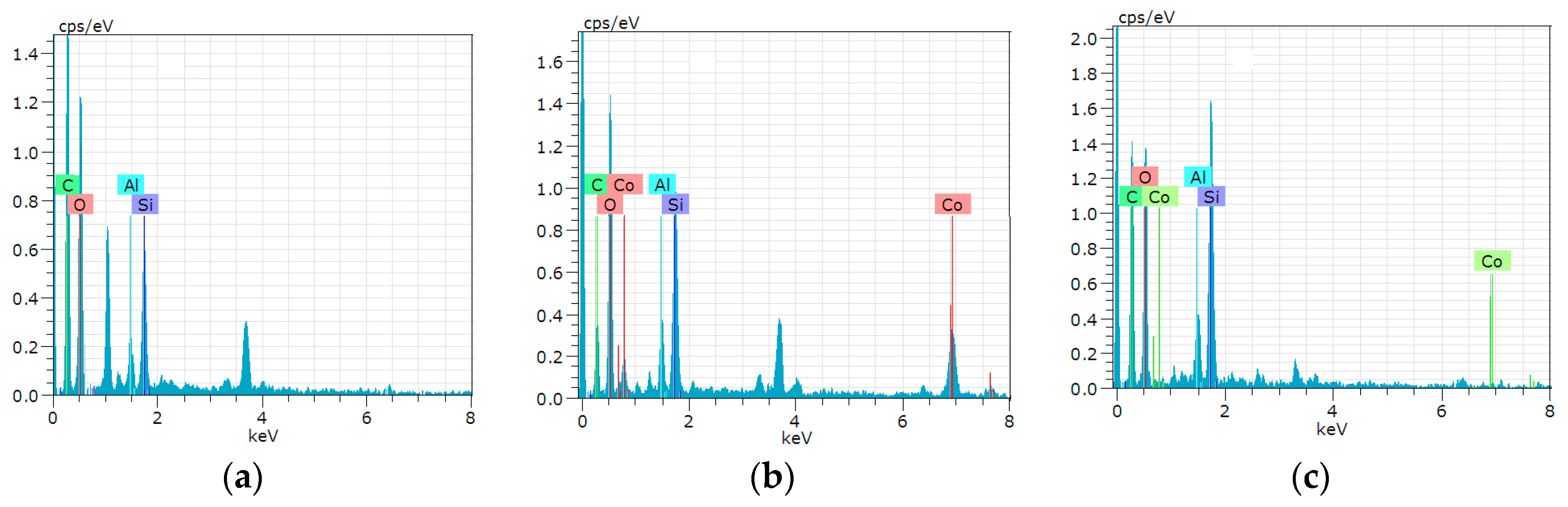
3.17. Adsorption Mechanism of Co(II)
3.18. Comparison with Previously Reported Data for Co(II) Adsorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurniawan, T.A.; Chana, G.Y.S.; Loa, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Malakahmad, A.; Tan, S.; Yavari, S. Valorization of wasted black tea as a low-cost adsorbent for nickel and zinc removal from aqueous solution. J. Chem. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Removal of heavy metals by waste tea leaves from aqueous solution. Eng. Life Sci. 2005, 2, 158–162. [Google Scholar] [CrossRef]
- Lee, A.Y.W.; Lim, S.F.; Chua, S.N.D.; Baini, K.S.R.; Abdullah, M.O. Adsorption Equilibrium for Heavy Metal Divalent Ions (Cu2+, Zn2+, and Cd2+) into Zirconium-Based Ferromagnetic Sorbent. Adv. Mater. Sci. Eng. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Louzao, M.C.; Abal, P.; Fernández, D.A.; Vieytes, M.R.; Legido, J.L.; Gómez, C.P.; Pais, J.; Botana, L.M. Study of adsorption and flocculation properties of natural clays to remove Prorocentrum lima. Toxins 2015, 7, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, L.Q.; Jiang, J.L.; Chen, J.; Feng, L.D. Preparation and characterization of nanosized TiO2 powder as an inorganic adsorbent for aqueous radionuclide Co(II) ions. J. Radioanal. Nucl. Chem. 2013, 295, 1305–1312. [Google Scholar] [CrossRef]
- Mil, T.D.; Devreese, M.; Baere, S.D.; Ranst, E.V.; Eeckhout, M.; Backer, D.P.; Croubels, S. Characterization of 27 mycotoxin binders and the relation with in vitro zearalenone adsorption at a single concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.; Awual, M.R.; Naushad, M. Functional ligand anchored nanomaterial based facial adsorbent for cobalt(II) detection and removal from water samples. Chem. Eng. J. 2015, 271, 155–163. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhao, D.L.; Zhang, H.; Lu, S.S.; Chen, L.; Yu, X.J. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Huang, Y.; Wang, Q.; Zhai, L.F.; Jiang, S.T. Residue resulted after wheat bran fermentation with Aspergillus niger sorption behavior of cobalt as a function of environmental conditions. Ind. Eng. Chem. Res. 2013, 52, 1352–1358. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Alothman, Z.A.; Alshehri, S.M. Synthesis characterization of Fe3O4@TSC nanocomposite: Highly efficient removal of toxic metal ions from aqueous medium. RSC Adv. 2016, 6, 22679–22689. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.J.; Huang, F.; Li, K.C. Fabrication of high-concentration and stable aqueous suspensions of graphene nanosheets by noncovalent functionalization with lignin and cellulose derivatives. J. Phys. Chem. C 2010, 114, 3811–3816. [Google Scholar] [CrossRef]
- Zhong, L.X.; Peng, X.W.; Yang, D.; Sun, R.C. Adsorption of heavy metals by a porous bioadsorbent from lignocellulosic biomass reconstructed in an ionic liquid. J. Agric. Food Chem. 2012, 60, 5621–5628. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.D.; Neto, V.D.S.; Barros, F.C.D.; Raulino, G.S.C.; Vidal, C.B.; do Nascimento, R.F. Chemical modifications of lignocellulosic materials and their application for removal of cations and anions from aqueous solutions. J. Appl. Poylm. Sci. 2016, 133, 43286. [Google Scholar] [CrossRef]
- Ren, X.M.; Wang, S.W.; Yang, S.T.; Li, J.X. Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J. Radioanal. Nucl. Chem. 2010, 283, 253–259. [Google Scholar] [CrossRef]
- Barati, A.; Asghi, M.; Miri, T.; Eskandari, Z. Removal and recovery of copper and nickel ions from aqueous solution by poly(methacrylamide-co-acrylic acid)/montmorillonite nanocomposites. Environ. Sci. Pollut. R 2013, 20, 6242–6255. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hu, H.Q.; Zhu, J.; Deng, Y.J.; Li, C.S. Adsorption of Cu2+ on Montmorillonite and chitosan-montmorillonite composite toward acetate ligand and the pH dependence. Water Air Soil Poll. 2016, 227, 1–10. [Google Scholar] [CrossRef]
- Ikhsan, J.; Wells, J.D.; Johnson, B.B.; Angove, M.J. Surface complexation modeling of the sorption of Zn(II) by montmorillonite. Colloid. Surf. A Physicochem. Eng. Asp. 2005, 252, 33–41. [Google Scholar] [CrossRef]
- Gu, X.Y.; Evans, L.J.; Barabash, S.J. Modeling the adsorption of Cd (II), Cu (II), Ni (II), Pb (II) and Zn (II) onto montmorillonite. Geochim. Cosmochim. Acta 2010, 74, 5718–5728. [Google Scholar] [CrossRef]
- Hua, S.B.; Yang, H.X.; Wang, W.B.; Wang, A.Q. Controlled release of ofloxacin from chitosan-montmorillonite hydrogel. Appl. Clay Sci. 2010, 50, 112–117. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Naushad, M.; Shiwaku, H.; Yaita, T. Preparation of new class composite adsorbent for enhanced palladium(II) detection and recovery. Sens. Actuators B Chem. 2015, 209, 790–797. [Google Scholar] [CrossRef]
- Bunhu, T.; Tichagwa, L. Adsorption of methyl orange, Pb2+ and Cd2+ from aqueous solution by composites of lignocellulose-montmorillonite modified with methacryloxypropyl trimethoxysilane. Macromol. Symp. 2012, 313–314, 146–156. [Google Scholar] [CrossRef]
- Du, M.; Li, Q.; Wang, L. Adsorption removal of Pb2+ and Cd2+ on lignocellulose-g-acrylic acid/montmorillonite nanocomposite from aqueous solutions. Desalin. Water Treat. 2016, 57, 3216–3269. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, K.; Huo, L.X.; Li, X.X.; Qi, X.B. Modification of 13X molecular sieve by chitosan for adsorptive removal of cadmium from simulated wastewater. Materials 2017, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.H.; Li, L.L. Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process. Chem. Eng. J. 2013, 223, 688–695. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Al-Maswari, B.M.; Alqadami, A.A.; Alshehri, S.M. Nickel ferrite bearing nitrogen-doped mesoporous carbon as efficient adsorbent for the removal of highly toxic metal ion from aqueous medium. Chem. Eng. J. 2017, 330, 1351–1360. [Google Scholar] [CrossRef]
- Zkahraman, B.O.; Acar, I.; Emik, S. Removal of Cu2+ and Pb2+ ions using CMC based thermoresponsive nanocomposite hydrogel. CLEAN-Soil Air Water 2011, 39, 658–664. [Google Scholar] [CrossRef]
- Kamblea, G.S.; Gharea, A.A.; Kolekara, S.S.; Hanb, S.H.; Anuse, M.A. Development of an reliable analytical method for synergistic extractive spectrophotometric determination of cobalt(II) from alloys and nano composite samples by using chromogenic chelating ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 84, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, X.; Qinggeletu; Wu, Y. Health risks and contamination levels of heavy metals in dusts from parks and squares of an industrial city in semi-arid area of China. Int. J. Environ. Res. Public Health 2017, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wen, G.H. Absorbed Pb2+ and Cd2+ ions in water by cross-linked starch xanthate. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Ghfar, A.A.; Naushad, M.; Alothman, Z.A.; Habila, M.A.; Albadarin, A.B. Efficient removal of Co(II) metal ion from aqueous solution using cost-effective oxidized activated carbon: Kinetic and isotherm studies. Desalin. Water Treat. 2017, 70, 220–226. [Google Scholar] [CrossRef]
- Pan, X.; Wang, J.; Zhang, D. Sorption of cobalt to bone char: Kinetics, competitive sorption and mechanism. Desalination 2009, 249, 609–614. [Google Scholar] [CrossRef]
- Mou, J.; Wang, G.J.; Shi, W.X.; Zhang, S.W. Sorption of radiocobalt on a novel γ-MnO2 hollow structure: Effects of pH, ionic strength, humic substances and temperature. J. Radioanal. Nucl. Chem. 2012, 292, 293–303. [Google Scholar] [CrossRef]
- Simsek, E.B.; Duranoglu, D.; Beker, U. Heavy metal adsorption by magnetic hybrid-sorbent: An experimental and theoretical approach. Sep. Sci. Technol. 2012, 47, 1334–1340. [Google Scholar] [CrossRef]
- El-Kholy, N.G.; Badawy, N.A.; El-Said, A.G.; Pasir, A.A.E. Competitive adsorption of Co(II) in a binary and tertiary system with metal ions Cr(III) and Ni(II) on Lewatite S-100 cation exchange resin. Nat. Sci. 2013, 11, 41–48. [Google Scholar]
- Corneliu, C.; Laura, B.; Matei, M. Adsorption characteristics of Co(II) ions from aqueous solutions on romanian peat moss. Environ. Eng. Manag. J. 2009, 8, 1089–1095. [Google Scholar]
- Caramalau, C.; Bulgariu, L.; Macoveanu, M. Kinetic study of cobalt(II) adsorption on peat activated by simple chemical treatments. Environ. Eng. Manag. J. 2009, 8, 1351–1358. [Google Scholar]
- Chen, L.; Lu, S. Sorption and desorption of radiocobalt on montmorillonite-effects of pH, ionic strength and fulvic acid. Appl. Radiat. Isot. 2008, 66, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, M.; Hu, R.; Tan, X.; Li, J. Water-soluble polyacrylamide coated-Fe3O4 magnetic composites for high-efficient enrichment of U(VI) from radioactive wastewater. Chem. Eng. J. 2014, 246, 268–276. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.N.; Kumar, K.K. Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)-a comparative study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Bas, N.; Yakar, A.; Bayramgil, P.N. Removal of cobalt ions from aqueous solutions by using poly(N,N-dimethylaminopropyl methacrylamide/itaconic acid) hydrogels. J. Appl. Polym. Sci. 2014, 131, 39569. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Bajwa, I.I.; Hanif, M.A.; Bukhari, I.H. Removal of lead and cobalt using lignocellulosic fiber derived from Citrus reticulata waste biomass. Korean J. Chem. Eng. 2010, 27, 218–227. [Google Scholar] [CrossRef]
- Salman, M.; Athar, M.; Farooq, U. Biosorption of heavy metals from aqueous solutions using indigenous and modified lignocellulosic materials. Rev. Environ. Sci. Bio Technol. 2015, 14, 211–228. [Google Scholar] [CrossRef]
- Toncelli, C.; Haijer, A.; Alberts, F.; Broekhuis, A.A.; Picchioni, F. The green route from carbon monoxide fixation to functional polyamines: A class of high-performing metal ion scavengers. Ind. Eng. Chem. Res. 2015, 54, 9450–9457. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.K.; Sillanpaa, M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 2010, 48, 181–186. [Google Scholar] [CrossRef]
- Sandesh, K.; Kumar, R.S.; Jagadeeshbabu, P.E. Rapid removal of cobalt(II) from aqueous solution using cuttlefish bones; equilibrium, kinetics, and thermodynamic study. Asia-Pac. J. Chem. Eng. 2013, 8, 144–153. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Tahmasbi, M.; Bastami, T.R.; Besharati, J.A. Rapid removal of cobalt ion from aqueous solutions by almond green hull. J. Hazard. Mater. 2009, 166, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Vilvanathan, S.; Shanthakumar, S. Biosorption of Co(II) ions from aqueous solution using Chrysanthemum indicum: Kinetics, equilibrium and thermodynamics. Process Saf. Environ. Prot. 2015, 96, 98–110. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Hayrunnisa, N.; Ekrem, K. Removal of cobalt(II) ions from aqueous solution by using alternative adsorbent industrial red mud waste material. Int. J. Phys. Sci. 2012, 7, 1386–1394. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]



| Sample | SBET (m2/g) | Sext (m2/g) | Sext/SBET (%) | Smic (m2/g) | Vtot (cm3/g) | Vmeso (cm3/g) | Vmeso/Vtot (%) | Dp (nm) |
|---|---|---|---|---|---|---|---|---|
| MT | 87.6 | 50.2 | 57.3 | 19.1 | 1.013 | 0.651 | 64.3 | 103.8 |
| LMT | 245.1 | 177.4 | 73.4 | 52.0 | 1.692 | 1.175 | 69.4 | 57.7 |
| Co(II)-loaded LMT | 112.3 | 46.2 | 41.1 | 29.4 | 1.117 | 0.395 | 35.4 | 30.6 |
| Metal | Parameter | Pseudo-First-Order | Pseudo-Second-Order | ||
|---|---|---|---|---|---|
| Co(II) | R2 | 0.4667 | 0.9995 | ||
| Constants | k1 | 0.0013 min−1 | k2 | 0.0001 min−1 | |
| qec | 78.15 mg/g | qec | 92.98 mg/g | ||
| qe | 93.02 mg/g | qe | 93.02 mg/g | ||
| Isotherm | Parameter | Temperature (°C) | ||
|---|---|---|---|---|
| 70 | 75 | 78 | ||
| Langmuir Model | qmax (mg/g) | 87.14 | 93.43 | 91.60 |
| KL (L/mg) | 0.0017 | 0.0065 | 0.0043 | |
| RL | 0.84 | 1.06 | 0.62 | |
| R2 | 0.9543 | 0.9987 | 0.9702 | |
| Freundlich Model | Kf (mg g−1 (L mg−1)1/n) | 2.8 | 9.7 | 3.5 |
| n | 1.05 | 1.87 | 1.33 | |
| R2 | 0.8903 | 0.9080 | 0.9201 | |
| Cycle Number | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Adsorption qe (mg/g) | 93.02 | 90.51 | 85.44 | 80.07 | 51.17 |
| Desorption qe (mg/g) | 87.34 | 81.64 | 78.46 | 68.43 | 37.26 |
| Adsorbent Material | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| LMT | 93.02 | This study |
| Cuttlefish bones | 76.76 | [47] |
| Almond green hull | 45.5 | [48] |
| CIF-BC | 45.44 | [49] |
| ZrO-Montmorillite | 22.8 | [50] |
| Red mud waste material | 18.05 | [51] |
| Natural zeolites | 14.38 | [52] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Chen, Z. Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study. Int. J. Environ. Res. Public Health 2017, 14, 1453. https://doi.org/10.3390/ijerph14121453
Zhang X, Wang X, Chen Z. Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study. International Journal of Environmental Research and Public Health. 2017; 14(12):1453. https://doi.org/10.3390/ijerph14121453
Chicago/Turabian StyleZhang, Xiaotao, Ximing Wang, and Zhangjing Chen. 2017. "Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study" International Journal of Environmental Research and Public Health 14, no. 12: 1453. https://doi.org/10.3390/ijerph14121453
APA StyleZhang, X., Wang, X., & Chen, Z. (2017). Radioactive Cobalt(II) Removal from Aqueous Solutions Using a Reusable Nanocomposite: Kinetic, Isotherms, and Mechanistic Study. International Journal of Environmental Research and Public Health, 14(12), 1453. https://doi.org/10.3390/ijerph14121453





