Contribution of Drinking Water Softeners to Daily Phosphate Intake in Slovenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical Analysis
2.3. Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verger, E.O.; Armstrong, P.; Nielsen, T.; Chakaroun, R.; Aron-Wisnewsky, J.; Gøbel, R.J.; Schütz, T.; Delaere, F.; Gausseres, N.; Clément, K.; et al. Dietary Assessment in the MetaCardis Study: Development and Relative Validity of an Online Food Frequency Questionnaire. J. Acad. Nutr. Diet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Meyer, A.; Nowak, V.; Hasenegger, V.; Putz, P.; Verstraeten, R.; Remaut-DeWinter, A.M.; Kolsteren, P.; Dostálová, J.; Dlouhý, P.; et al. European Nutrition and Health Report 2009. Forum Nutr. 2009, 62, 1–405. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.T.; Chang, A.R.; Selvin, E.; Rebholz, C.M.; Appel, L.J. Dietary Sources of Phosphorus among Adults in the United States: Results from NHANES 2001–2014. Nutrients 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Increasing Dietary Phosphorus Intake from Food Additives: Potential for Negative Impact on Bone Health. Adv. Nutr. 2014, 5, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Benini, O.; D’Alessandro, C.; Gianfaldoni, D.; Cupisti, A. Extra-Phosphate Load From Food Additives in Commonly Eaten Foods: A Real and Insidious Danger for Renal Patients. J. Renal Nutr. 2011, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Gutekunst, L.; Uribarri, J. Hidden Phosphorus-Enhanced Meats: Part 3. J. Renal Nutr. 2005, 15, e1–e4. [Google Scholar] [CrossRef]
- Van Den Bussche, K.; Herrmann, D.; De Henauw, S.; Kourides, Y.A.; Lauria, F.; Marild, S.; Molnár, D.; Moreno, L.A.; Veidebaum, T.; Ahrens, W.; et al. Urinary mineral concentrations in European pre-adolescent children and their association with calcaneal bone quantitative ultrasound measurements. Int. J. Environ. Res. Public Health 2016, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, S.J.; Lee, S.; Kim, S.; Cho, M.H. Biological effects of inorganic phosphate: Potential signal of toxicity. J. Toxicol. Sci. 2015, 40, 55–69. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Assessment of one published review on health risks associated with phosphate additives in food. EFSA J. 2013, 11, 3444:1–3444:27. [Google Scholar] [CrossRef]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate additives in food—A health risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ellam, T.J.; Chico, T.J. Phosphate: The new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012, 220, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I. Risking public health by approving some health claims?—The case of phosphorus. Food Policy 2011, 36, 725–727. [Google Scholar] [CrossRef]
- Uribarri, J.; Calvo, M.S. Hidden sources of phosphorus in the typical American diet: Does it matter in nephrology? Semin. Dial. 2003, 16, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.C.; Wu, C.C.; Yen, J.F.; Liu, W.C. Vascular Calcification and Renal Bone Disorders. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Winger, R.J.; Uribarri, J.; Lloyd, L. Phosphorus—containing food additives: An insidious danger for people with chronic kidney disease. Trends Food Sci. Technol. 2012, 24, 92–102. [Google Scholar] [CrossRef]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of Serum Phosphate with Vascular and Valvular Calcification in Moderate CKD. JASN 2009, 20, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Dietary phosphorus restriction in dialysis patients: Potential impact of processed meat, poultry and fish products as protein sources. Am. J. Kidney Dis. 2009, 54, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B., Sr.; Gaziano, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. Vascular calcification: In vitro evidence for the role of inorganic phosphate. J. Am. Soc. Nephrol. 2003, 14, S300–S304. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; IShani, A.; Kaira, P.A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. JASN 2009, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.M.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.J.; Lash, J.P.; Chen, J.; He, J.; Navaneethan, S.; et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xie, X.; Wolf, M.; Disthabanchong, S.; Wang, J.; Zha, Y.; Lv, J.; Zhang, L.; Wang, H. Serum phosphorus and progression of CKD and mortality: A meta-analysis of cohort studies. Am. J. Kidney Dis. 2015, 66, 258–265. [Google Scholar] [CrossRef] [PubMed]
- John, G.B.; Cheng, C.-Y.; Kuro-o, M. Role of klotho in aging, phosphate metabolism, and CKD. Am. J. Kidney Dis. 2011, 58, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Li, J.; Liu, J. Serum phosphorus, cardiovascular and all-cause mortality in the general population: A meta-analysis. Clin. Chim. Acta 2016, 461, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. A potential link between phosphate and aging-Lessons from Klotho-deficient mice. Mech. Ageing Dev. 2010, 131, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Tucker, K.L. Is phosphorus intake that exceeds dietary requirements a risk factor in bone health? Ann. N. Y. Acad. Sci. 2013, 1301, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The FGF23 and Klotho system beyond mineral metabolism. Clin. Exp. Nephrol. 2017, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Manzola, A.S.; Mgaidi, A.; Laouali, M.S.; El Maaoui, M. On precipitated calcium and magnesium phosphates during synthetic hard waters softening by monosodium phosphate. Desalination Water Treat. 2014, 52, 4734–4744. [Google Scholar] [CrossRef]
- Lin, Y.P.; Singer, P.C. Inhibition of calcite crystal growth by polyphosphates. Water Res. 2005, 39, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- Dartmann, J.; Alex, T.; Dorsch, T.; Schevalje, E.; Johannsen, K. Influence of decarbonisation and phosphate dosage on copper corrosion in drinking water systems. Acta Hydrochim. Hydrobiol. 2004, 32, 25–32. [Google Scholar] [CrossRef]
- McNeill, L.S.; Edwards, M. Phosphate Inhibitor Use at U.S. Utilities. J. AWWA 2002, 94, 57–63. [Google Scholar]
- McNeill, L.S.; Edwards, M. Iron Pipe Corrosion in Distribution Systems. J. AWWA 2001, 93, 88–100. [Google Scholar]
- Rashchi, F.; Finch, J.A. Polyphosphates: A review. Their chemistry and application with particular reference to mineral processing. Miner. Eng. 2000, 13, 1019–1035. [Google Scholar] [CrossRef]
- Cantor, A.F.; Denig-Chakroff, D.; Vela, R.R.; Oleinik, M.G.; Lynch, D.L. Use of Polyphosphate in Corrosion Control. J. AWWA 2000, 92, 95–102. [Google Scholar]
- Rules on Drinking Water. Official Gazette of RS 2004, 19/04, 35/04, 26/06, 92/06, 25/09 and 74/15. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=PRAV3713 (accessed on 1 March 2017).
- The Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. 1998, L 330, 32–54. [Google Scholar]
- WHO. Guidelines for Drinking-water Quality, 4th ed.; WHO Press: Geneva, Switzerland, 2011; p. 564. [Google Scholar]
- British Standards Institution. Chemicals Used for Treatment of Water Intended for Human Consumption. Sodium Polyphosphate; SIST EN 1212:2005; British Standards Institution (BSI): London, UK, 2005. [Google Scholar]
- ISO. Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method; SIST EN ISO 6878:2004; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Tomažič, I.; Vidic, T. Future science teachers’ understandings of diffusion and osmosis concepts. J. Biol. Educ. 2012, 46, 66–71. [Google Scholar] [CrossRef]
- Nielsen, E.; Ostergaard, G.; Larsen, J.C. Toxicological Risk Assessment of Chemicals: A Practical Guide; CRC Press; Taylor & Francis group: Boca Raton, FL, USA, 2008; pp. 1–334. [Google Scholar]
- U.S. EPA. Risk assessment Guidance for superfund, Volume 1; U.S. Environmental Protection Agency: Washington, DC, USA, 1989; p. 178.
- Institute of Medicine (U.S.), Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997; p. 448. [Google Scholar]
- Comber, S.; Cassé, F.; Brown, B.; Martin, J.; Hillis, P.; Gardner, M. Phosphate treatment to reduce plumbosolvency of drinking water also reduces discharges of copper into environmental surface waters. Water Environ. J. 2011, 25, 266–270. [Google Scholar] [CrossRef]
- Hayes, C.R.; Incledion, S.; Balch, M. Experience in Wales (UK) of the optimisation of orthophosphate dosing for controlling lead in drinking water. J. Water Health 2008, 6, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dartmann, J.; Sadlowsky, B.; Dorsch, T.; Johannsen, K. Copper corrosion in drinking water systems—Effect of pH and phosphate-dosage. Mater. Corros. 2010, 61, 189–198. [Google Scholar] [CrossRef]
- Batté, M.; Koudjonou, B.; Laurent, P.; Mathieu, L.; Coallier, J.; Prévost, M. Biofilm responses to ageing and to a high phosphate load in a bench-scale drinking water system. Water Res. 2003, 37, 1351–1361. [Google Scholar] [CrossRef]
- Appenzeller, B.M.R.; Duval, Y.B.; Thomas, F.; Block, J.C. Influence of phosphate on bacterial adhesion onto iron oxyhydroxide in drinking water. Environ. Sci. Technol. 2002, 36, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, B.M.R.; Batté, M.; Mathieu, L.; Block, J.C.; Lahoussine, V.; Cavard, J.; Gatel, D. Effect of adding phosphate to drinking water on bacterial growth in slightly and highly corroded pipes. Water Res. 2001, 35, 1100–1105. [Google Scholar] [CrossRef]
- Sathasivan, A.; Ohgaki, S. Application of new bacterial regrowth potential method for water distribution system—A clear evidence of phosphorus limitation. Water Res. 1999, 33, 137–144. [Google Scholar] [CrossRef]
- Podtalnica Ljubljanskega Polja, (Geografija Slovenije, 10). Available online: http://giam.zrc-sazu.si/sites/default/files/9616500686.pdf (accessed on 3 July 2017).
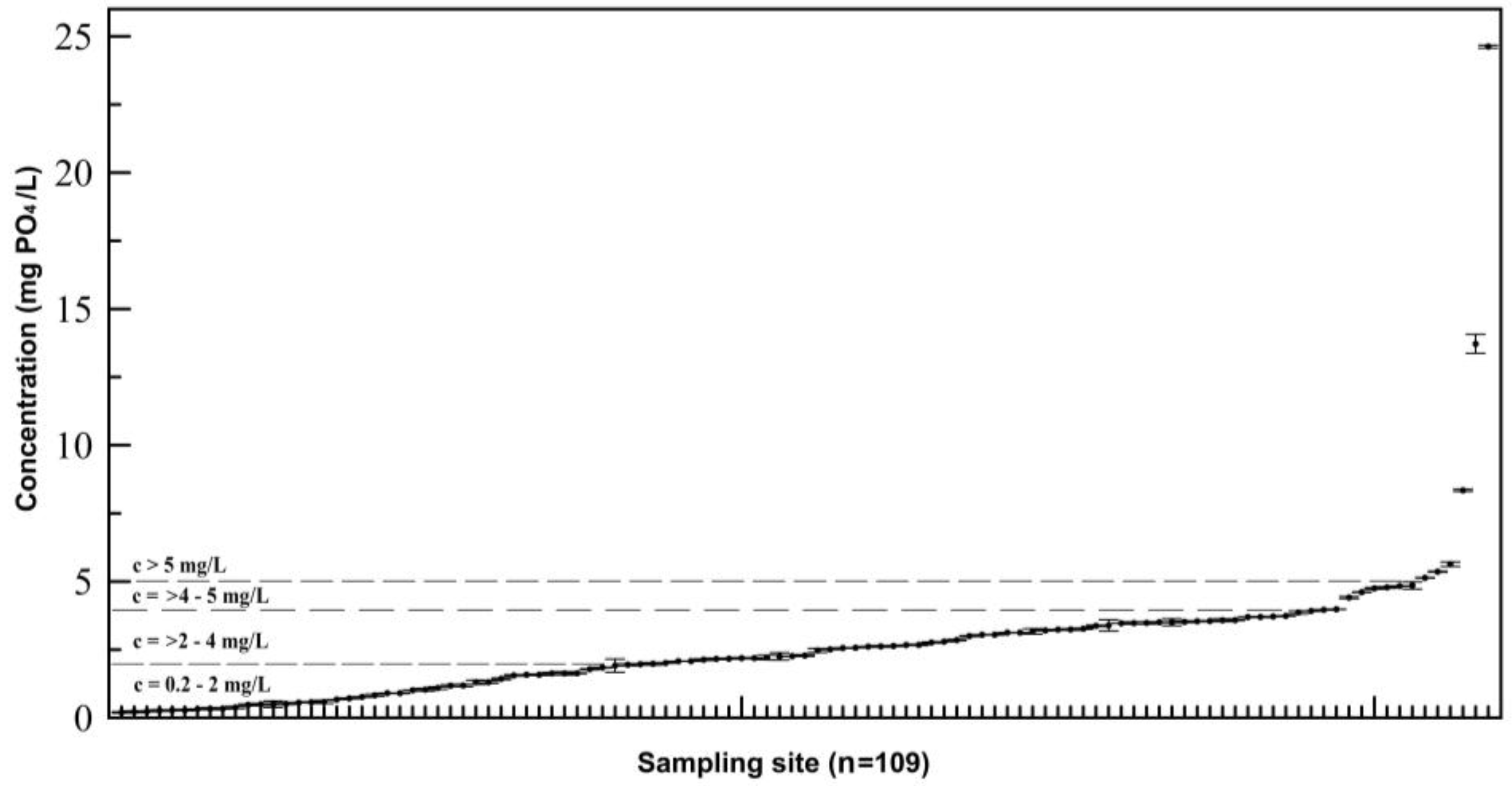
 concentration of PO4 > 0.2 mg PO4/L).
concentration of PO4 > 0.2 mg PO4/L).
 concentration of PO4 > 0.2 mg PO4/L).
concentration of PO4 > 0.2 mg PO4/L).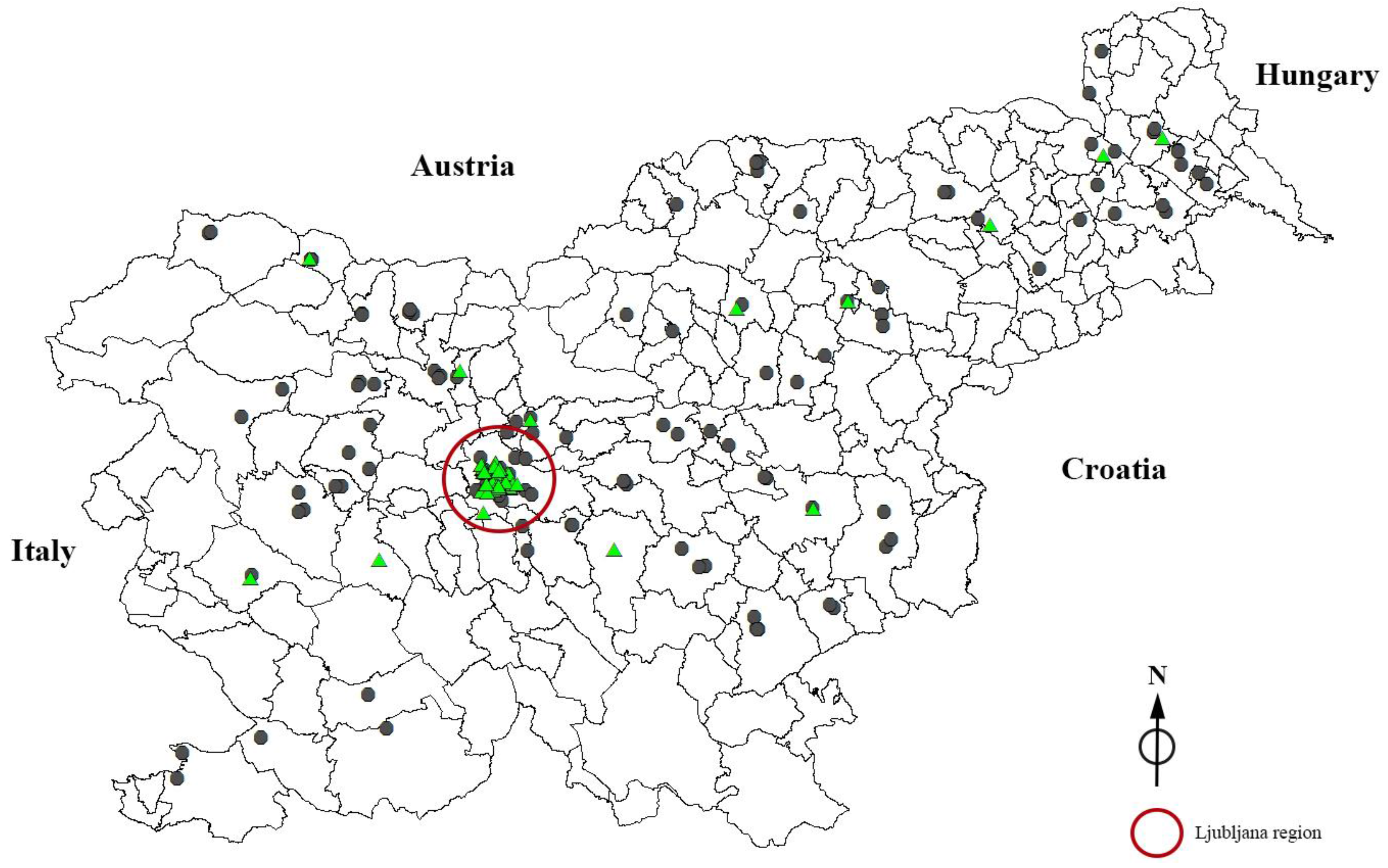
| Measured Concentration (mg PO4/L) * | Respondents’ Answers Regarding Chemical Softening of Their Drinking Water | ||
|---|---|---|---|
| Yes % (n) | No % (n) | Do not Know % (n) | |
| c < 0.2 | 9.5% (23) | 26.0% (63) | 19.4% (47) |
| c > 0.2 | 4.1% (10) | 11.2% (27) | 29.8% (72) |
| Total | 13.6% (33) | 37.2% (90) | 49.2% (119) |
| Age (years) | RDA * (mg/day) | Contribution ** (%) of Phosphates in Drinking Water to RDA | ||
|---|---|---|---|---|
| Worst Case Scenario *** | High Concentration Scenario **** | Realistic Scenario ***** | ||
| 0–6 months | 100 (AI) | 6.0 | 1.3 | 0.6 |
| 6–12 months | 275 (AI) | 2.2 | 0.5 | 0.2 |
| 1–3 | 460 | 1.7 | 0.4 | 0.2 |
| 4–8 | 500 | 1.6 | 0.3 | 0.2 |
| 9–13 | 1250 | 1.3 | 0.3 | 0.1 |
| 14–18 | 1250 | 1.3 | 0.3 | 0.1 |
| 19–30 | 700 | 2.3 | 0.5 | 0.2 |
| 31–50 | 700 | 2.3 | 0.5 | 0.2 |
| 51–70 | 700 | 2.3 | 0.5 | 0.2 |
| >71 | 700 | 2.3 | 0.5 | 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jereb, G.; Poljšak, B.; Eržen, I. Contribution of Drinking Water Softeners to Daily Phosphate Intake in Slovenia. Int. J. Environ. Res. Public Health 2017, 14, 1186. https://doi.org/10.3390/ijerph14101186
Jereb G, Poljšak B, Eržen I. Contribution of Drinking Water Softeners to Daily Phosphate Intake in Slovenia. International Journal of Environmental Research and Public Health. 2017; 14(10):1186. https://doi.org/10.3390/ijerph14101186
Chicago/Turabian StyleJereb, Gregor, Borut Poljšak, and Ivan Eržen. 2017. "Contribution of Drinking Water Softeners to Daily Phosphate Intake in Slovenia" International Journal of Environmental Research and Public Health 14, no. 10: 1186. https://doi.org/10.3390/ijerph14101186
APA StyleJereb, G., Poljšak, B., & Eržen, I. (2017). Contribution of Drinking Water Softeners to Daily Phosphate Intake in Slovenia. International Journal of Environmental Research and Public Health, 14(10), 1186. https://doi.org/10.3390/ijerph14101186




