Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Workplaces in the Plant Biomass Processing Power Plant
2.2. Plant Biomass
2.3. Tested FFRs
2.4. Microbiological Contamination Analysis
2.5. Identification of Microorganisms
2.5.1. Culture-Dependent Method
2.5.2. Next-Generation Sequencing on the Illumina Platform
DNA Extraction
PCR Amplification and Sequencing
Sequencing Data Analysis
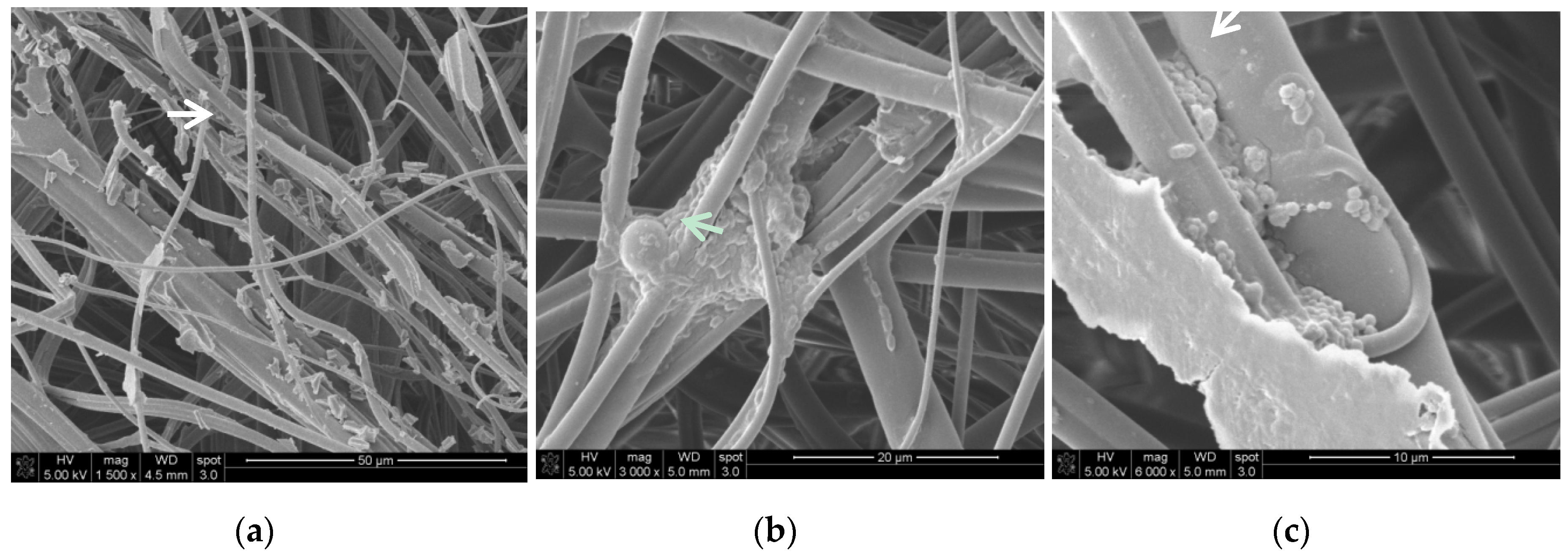
2.6. Scanning Electron Microscopy (SEM) Method
2.7. Analysis of Secondary Metabolites
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kyoto Protocol to the United Nations Framework Convention on Climate Change. Available online: http://unfccc.Int/resource/docs/convkp/kpeng.pdf (accessed on 4 November 2016).
- Institute for Energy Research. Renewable Energy. Available online: http://instituteforenergyresearch.org/topics/encyclopedia/renewable-energy/ (accessed on 4 November 2016).
- The European Parliament and the Council of the European Union. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, 140, 16–62. [Google Scholar]
- Lins, C.; Williamson, L.E.; Leitner, S.; Teske, S. 10 Years of Renevable Energy Progress. Report of Renewable Energy Policy Network for the 21st Century (REN21). Available online: http://www.ren21.net/portals/0/documents/activities/topical%20reports/Ren21_10yr.pdf (accessed on 6 January 2017).
- U.S. Energy Information Administration. Monthly Energy Review 2016. Available online: https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf (accessed on 4 November 2016).
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Conver. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Ferroukhi, R.; Khalid, A.; Lopez-Peña, A.; Renner, M. Renewable Energy and Jobs: Annual Review 2015; International Renewable Energy Agency (IRENA): Masdar City, United Arab Emirates, 2015. [Google Scholar]
- Wouters, I.M.; Spaan, S.; Douwes, J.; Doekes, G.; Heederik, D. Overview of personal occupational exposure levels to inhalable dust, endotoxin, β(13)-glucan and fungal extracellular polysaccharides in the waste management chain. Ann. Occup. Hyg. 2006, 50, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Lawniczek-Walczyk, A.; Golofit-Szymczak, M.; Cyprowski, M.; Gorny, R.L. Exposure to harmful microbiological agents during the handling of biomass for power production purposes. Med. Pr. 2012, 63, 395–407. [Google Scholar] [PubMed]
- Demers, P.A.; Teschke, K.; Kennedy, S.M. What to do about softwood? A review of respiratory effects and recommendations regarding exposure limits. Am. J. Ind. Med. 1997, 31, 385–398. [Google Scholar] [CrossRef]
- Rusca, S.; Charrière, N.; Droz, P.O.; Oppliger, A. Effects of bioaerosol exposure on work-related symptoms among Swiss sawmill workers. Int. Arch. Occup. Environ. Health 2008, 81, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Schlünssen, V.; Madsen, A.M.; Skov, S.; Sigsgaard, T. Does the use of biofuels affect respiratory health among male Danish energy plant workers? Occup. Environ. Med. 2011, 68, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Madsen, A.M.; Mårtensson, L.; Pomorska, D.; Larsson, L. Assessment of microbial exposure risks from handling of biofuel wood chips and straw—Effect of outdoor storage. Am. J. Med. 2006, 13, 139–145. [Google Scholar]
- Madsen, A.M. Exposure to airborne microbial components in Autumn and Spring during work at Danish biofuel plants. Ann. Occup. Hyg. 2006, 50, 821–831. [Google Scholar] [CrossRef]
- Maus, R.; Goppelsröder, A.; Umhauer, H. Survival of bacterial and mold spores in air filter media. Atmos Environ. 2001, 35, 105–113. [Google Scholar] [CrossRef]
- Jankowska, E.; Reponen, T.; Willeke, K.; Grinshpun, S.A.; Choi, K.J. Collection of fungal spores on air filters and spore reentrainment from filters into air. J. Aerosol Sci. 2000, 31, 969–978. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Skóra, J.; Gutarowska, B. Evaluation of the survivability of microorganisms deposited on filtering respiratory protective devices under varying conditions of humidity. Int. J. Environ. Res. Public Health 2016, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Directive 89/686/EEC—Personal Protective Equipment of 21 December 1989 on the Approximation of the Lawsof the Member States Relating to Personal Protective Equipment. Available online: https://osha.europa.eu/en/legislation/directives/34 (accessed on 4 November 2016).
- EN 149: 2001 + A1: 2009 Respiratory Protective Devices. Filtering Half Masks to Protect against Particles. Requirements, Testing, Marking. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:32928,6062&cs=1FC98AD34A5EE26A0CB5A6155ED4D6E5E (accessed on 18 January 2017).
- EN 13098: 2000 Workplace Atmospheres. Guidelines for Measurement of Airborne Micro-Organisms and Endotoxin. Available online: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT:4740&cs=1DB406DC01FA1F54E6AEC123B75720FF6 (accessed on 18 January 2017).
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002. [Google Scholar]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–173. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilag; Springer: New York, NY, USA, 2009; pp. 299–310. [Google Scholar]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Bálint, M.; Greshake, B.; Bandow, C.; Römbke, J.; Schmitt, I. Illumina metabarcoding of a soil fungal community. Soil Biol. Biochem. 2013, 65, 128–132. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, S.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Meth. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef] [PubMed]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr A 2014, 1362, 145–156. [Google Scholar] [CrossRef]
- The Commission of The European Communities. Commission decision 2002/657/EC of 12 August 2002 implementing Council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences; Houghton Mifflin: Boston, MA, USA, 2005. [Google Scholar]
- Rohr, A.C.; Campleman, S.L.; Long, C.M.; Peterson, M.K.; Weatherstone, S.; Quick, W.; Lewis, A. Potential occupational exposures and health risks associated with biomass-based power generation. Int. J. Environ. Res. Public Health 2015, 12, 8542–8605. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, S.; Laitinen, J.; Fagernäs, L.; Korpijärvi, K.; Korpinen, L.; Ojanen, K.; Aatamila, M.; Jumpponen, M.; Koponen, H.; Jokiniemi, J. Exposure to biological and chemical agents at biomass power plants. Biomass Bioenergy 2016, 93, 78–86. [Google Scholar] [CrossRef]
- Jumpponen, M.; Rönkkömäki, H.; Pasanen, P.; Laitinen, J. Occupational exposure to solid chemical agents in biomass-fired power plants and associated health effects. Chemosphere 2014, 104, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.; Agnew, J.E.; Clarke, S.W. Inhaled aerosols: Deposition and clearance. In Progress Radiopharmacy; Cox, P.H., Mather, S.J., Sampson, C.B., Lazarus, C.R., Eds.; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986; pp. 579–589. [Google Scholar]
- Stobnicka, A.; Górny, R.L. Exposure to flour dust in the occupational environment. Int. J. Occup. Saf. Ergon. 2015, 21, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Baran, S.; Teul, I. Wood dust: An occupational hazard which increases the risk of respiratory disease. J. Physiol. Pharmacol. 2007, 58, 43–50. [Google Scholar] [PubMed]
- Rosenberg, C.; Liukkonen, T.; Kallas-Tarpila, T.; Ruonakangas, A.; Ranta, R.; Nurminen, M.; Welling, I.; Jäppinen, P. Monoterpene and wood dust exposures: Work-related symptoms among Finnish sawmill workers. Am. J. Ind. Med. 2002, 41, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, A.-S.; Chamorro, A.-J.; Hernández-García, I.; Iglesias-de-Sena, H.; Martín-Rodero, H.; Herrera, C.; Marcos, M.; Mirón-Canelo, J.-A. Association between occupational exposure to wood dust and cancer: A systematic review and meta-analysis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Hancock, D.G.; Langley, M.E.; Chia, K.L.; Woodman, R.J.; Shanahan, E.M. Wood dust exposure and lung cancer risk: A meta-analysis. Occup. Environ. Med. 2015, 72, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Bruschweiler, E.D.; Wild, P.; Huynh, C.K.; Savova-Bianchi, D.; Danuser, B.; Hopf, N.B. DNA damage among wood workers assessed with the comet assay. Environ. Health Insights 2016, 10, 105–112. [Google Scholar] [PubMed]
- Pesch, B.; Pierl, C.B.; Gebel, M.; Gross, I.; Becker, D.; Johnen, G.; Rihs, H.-P.; Donhuijsen, K.; Lepentsiotis, V.; Meier, M.; et al. Occupational risks for adenocarcinoma of the nasal cavity and paranasal sinuses in the German wood industry. Occup. Environ. Med. 2008, 65, 191–196. [Google Scholar] [CrossRef] [PubMed]
- International Agency Research Cancer (IARC). Wood dust and formaldehyde. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemical to Humans; IARC: Lyon, France, 1995; Volume 62. [Google Scholar]
- American Association of Governmental Industrial Hygienists. 2014 TLVs and BEIs; American Association of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2014.
- Directive 1999/38/EC Amending for the Second Time Directive 90/394/EEC on the Protection of Workers from the Risks Related to Exposure to Carcinogens at Work and Extending It to Mutagens 1999. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX: 01999L0038-19990601&rid=1 (accessed on 6 January 2017).
- Kauppinen, T.; Vincent, R.; Liukkonen, T.; Grzebyk, M.; Kauppinen, A.; Welling, I.; Arezes, P.; Black, N.; Bochmann, F.; Campelo, F.; et al. Occupational exposure to inhalable wood dust in the member states of the European Union. Ann. Occup. Hyg. 2006, 50, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Occupational Safety and Health Administration. Chemical Sampling Information Wood Dust. Available online: https://www.osha.gov/dts/chemicalsampling/data/CH_276185.html (accessed on 6 January 2017).
- World Health Organization. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment; World Health Organization: Geneva, Switherland, 2006. [Google Scholar]
- Góra, A.; Mackiewicz, B.; Krawczyk, P.; Golec, M.; Skórska, C.; Sitkowska, J.; Sitkowska, J.; Cholewa, G.; Larsson, L.; Jarosz, M.; et al. Occupational exposure to organic dust, microorganisms, endotoxin and peptidoglycan among plants processing workers in Poland. Ann. Agric. Environ. Med. 2016, 16, 143–150. [Google Scholar]
- Wang, Z.; Reponen, T.; Willke, K.; Grinshpun, S.A. Survival of bacteria on respirator filters. Aerosol Sci. Technol. 1999, 30, 300–308. [Google Scholar] [CrossRef]
- Simmons, R.B.; Crow, S.A. Fungal colonization of air filters for use in heating, ventilating, and air conditioning (HVAC) systems. J. Ind. Microbiol. 1995, 14, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/54/EC—Biological Agents at Work. Available online: https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77 (accessed on 4 November 2016).
- Gutarowska, B.; Celikkol-Aydin, S.; Bonifay, V.; Otlewska, A.; Aydin, E.; Oldham, A.L.; Brauer, J.I.; Duncan, K.E.; Adamiak, J.; Sunner, J.A.; et al. Metabolomic and high-throughput sequencing analysis-modern approach for the assessment of biodeterioration of materials from historic buildings. Front Microbiol. 2015, 6, 979. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Papp, A.; Nagymajtényi, L. Effects of 3-nitropropionic acid in rats: General toxicity and functional neurotoxicity. Arch. Hig. Rada Toksikol. 2005, 56, 297–302. [Google Scholar]
- Kozlovsky, A.G.; Zhelifonova, V.P.; Antipova, T.V. Biologically active metabolites of Penicillium fungi. J. Org. Biomol. Chem. 2013, 1, 11–21. [Google Scholar]
- Mahrous, K.F.; Khalil, W.K.B.; Mahmoud, M.A. Assessment of toxicity and clastogenicity of sterigmatocystin in Egyptian Nile tilapia. Afr. J. Biotechnol. 2006, 5, 1180–1189. [Google Scholar]
| Workplace | Description of Performed Activities | Premise Size (Length/Width/Height) (m) | Air Flow Rate M ± SD (m/s) |
|---|---|---|---|
| Tunnel of willow wood chips conveyor | Biomass is transported to a warehouse, unloaded and stored. During storage, workers measure the humidity and temperature of the prism. The biomass is then appropriately processed for energy generation. | 9.0/4.7/3.9 | 0.19 ± 0.01 |
| Tunnel of forest wood chips conveyor | 80.0/3.5/4.1 | 0.13 ± 0.02 | |
| Biomass unloading station | Following the delivery of the biomass to the plant, workers unload it. This disperses organic dust into the air. | 6.3/9.4/9.5 * | 0.13 ± 0.07 |
| Laboratory | Biomass undergoes initial processing (mincing, homogenising, drying) and is then subjected to laboratory analyses. | 5.1/5.5/2.9 | 0.03 ± 0.03 |
| Amplified Sequence | Primer Name | Primer Sequence (5’ > 3’) | Reference |
|---|---|---|---|
| 16S rRNA | 341F 785R | CCTACGGGNGGCWGCAG GACTACHVGGGTATCTAATCC | Klindworth et al. [25] Klindworth et al. [25] |
| ITS1 region | ITS1F12 ITS2 | GAACCWGCGGARGGATCA GCTGCGTTCTTCATCGATGC | Schmidt et al. [26] White et al. [27] |
| Workplace | Air Temperature (°C) | Relative Humidity M ± SD (%) | Concentration of Dust Fraction (mg/m3) M ± SD | ||||
|---|---|---|---|---|---|---|---|
| PM1 | PM2.5 | PM4 | PM10 | PMtotal | |||
| Tunnel of willow wood chips conveyor | 9.2 ± 0.6 | 68.3 ± 3.5 | 0.070 ± 0.015 a | 0.071 ± 0.015 a | 0.073 ± 0.015 a | 0.078 ± 0.018 a | 0.085 ± 0.026 a |
| Tunnel of forest wood chips conveyor | 12.8 ± 0.6 | 66.1 ± 4.8 | 0.148 ± 0.049 b | 0.158 ± 0.052 b | 0.190 ± 0.053 b | 0.260 ± 0.060 b | 0.296 ± 0.071 b |
| Biomass unloading | 8.5 ± 0.3 | 68.4 ± 3.1 | 0.395 ± 0.448 c | 0.460 ± 0.564 c | 0.571 ± 0.711 c | 0.815 ± 1.100 c | 0.967 ± 1.280 c |
| Laboratory | 20.6 ± 2.5 | 46.2 ± 6.3 | 0.030 ± 0.022 d | 0.030 ± 0.022 d | 0.030 ± 0.22 d | 0.032 ± 0.024 d | 0.043 ± 0.039 a |
| Area/Sample Tested | Concentration of Microorganisms | |
|---|---|---|
| Bacteria | Fungi | |
| Air (CFU/m3) | ||
| Tunnel of willow wood chips conveyor | M: 2.67 × 103 c Min: 2.39 × 103 Max: 2.99 × 103 SD: 3.02 × 102 | M: 1.67 × 103 b,c Min: 1.60 × 103 Max: 1.72 × 103 SD: 6.43 × 101 |
| Tunnel of forest wood chips conveyor | M: 2.27 × 103 c Min: 2.18 × 103 Max: 2.37 × 103 SD: 9.61 × 101 | M: 3.25 × 104 b,c Min: 2.63 × 104 Max: 4.20 × 104 SD: 8.33 × 103 |
| Biomass unloading | M: 4.57 × 103 c Min: 4.02 × 103 Max: 5.20 × 103 SD: 5.94 × 102 | M: 3.79 × 104 b,c Min: 2.63 × 104 Max: 5.10 × 104 SD: 1.24 × 104 |
| Laboratory | M: 5.93 × 102 c Min: 8.00 × 101 Max: 8.80 × 102 SD: 4.45 × 102 | M: 6.30 × 102 b,c Min: 4.40 × 102 Max: 7.70 × 102 SD: 1.71 × 102 |
| External background | M: 2.03 × 102 c Min: 1.20 × 102 Max: 3.30 × 102 SD: 1.12 × 102 | M: 2.03 × 102 b,c Min: 1.80 × 102 Max: 2.30 × 102 SD: 2.52 × 101 |
| Biomass (CFU/g) | ||
| Willow wood chips | M: 3.51 × 107 a Min: 2.73 × 107 Max: 4.40 × 107 SD: 7.24 × 106 | M: 2.91 × 105 a,b Min: 2.00 × 105 Max: 4.76 × 105 SD: 1.05 × 105 |
| Forest wood chips | M: 2.28 × 107 b Min: 1.16 × 107 Max: 3.61 × 107 SD: 9.78 × 106 | M: 3.86 × 105 a,b Min: 1.64 × 105 Max: 8.20 × 105 SD: 3.16 × 105 |
| Sunflower pellets | M: 1.68 × 106 c Min: 1.00 × 106 Max: 3.29 × 106 SD: 8.69 × 105 | M: 3.29 × 104 c Min: 1.15 × 104 Max: 7.80 × 104 SD: 2.62 × 104 |
| FFR (CFU/cm2) | ||
| FFR | M: 1.62 × 102 c Min: 1.83 × 101 Max: 3.84 × 102 SD: 1.24 × 102 | M: 2.54 × 101 c Min: 8.50 × 100 Max: 5.75 × 101 SD: 1.71 × 101 |
| Domain | Species | Percentage (%) | ||
|---|---|---|---|---|
| Air | Biomass | FFR | ||
| Bacteria | Aeromonas hydrophila | - | 17.83 | - |
| Bacillus cereus | 1.68 | - | - | |
| Bacillus megaterium | 26.59 | - | 0.18 | |
| Bacillus pumilus | - | 0.01 | - | |
| Bacillus subtilis | 2.24 | 0.11 | 84.48 | |
| Brevundimonas vesicularis | 2.10 | 0.52 | - | |
| Cellulomonas sp. | 22.88 | - | - | |
| Micrococcus sp. | 0.84 | - | - | |
| Moraxella sp. | 42.90 | 0.14 | - | |
| Pseudomonas fluorescens | - | 0.42 | - | |
| Pseudomonas luteola | 0.14 | 0.15 | - | |
| Sphingomonas multivunum | - | 0.43 | - | |
| Sphingomonas paucimobilis | - | 0.14 | - | |
| Staphylococcus hominis | 0.42 | - | 18.33 | |
| Staphylococcus sciuri | - | 78.90 | - | |
| Streptomyces sp. | 0.21 | 0.20 | - | |
| Vibrio metschnikovii | - | 1.1 | - | |
| Fungi | Acremonium sp. | - | 0.11 | - |
| Aureobasidium pullulans | - | 0.09 | - | |
| Candida famata | 2.83 | - | - | |
| Candida lusitaniae | 0.38 | - | - | |
| Chrysonilia sitophila | 0.53 | - | 3.72 | |
| Cladosporium cladosporioides | 1.06 | 7.59 | 18.33 | |
| Mucor hiemalis | 2.93 | - | - | |
| Mucor racemosus | - | 0.26 | 54.85 | |
| Paecilomyces variotii | - | 10.56 | - | |
| Penicillium chrysogenum | 86.08 | 6.54 | - | |
| Penicillium commune | 4.22 | - | - | |
| Rhizopus nigricans | 0.86 | 12.25 | 21.91 | |
| Rhodotorula mucilaginosa | - | 33.67 | - | |
| Trichoderma viride | - | 8.46 | - | |
| Verticillium tenerum | 1.10 | 20.45 | 1.20 | |
| Domain | Phylum | Genus | Abundance of Bacterial/Fungal Genera in Samples (%) |
|---|---|---|---|
| Bacteria | Acidobacteria | Acidobacterium | 0.06 |
| Other | 0.05 | ||
| Actinobacteria | Arthrobacter | 1.05 | |
| Corynebacterium | 0.38 | ||
| Micrococcus | 0.41 | ||
| Nonomuraea | 0.10 | ||
| Propionibacterium | 2.83 | ||
| Rubrobacter | 0.20 | ||
| Streptomyces | 0.14 | ||
| Other | 0.38 | ||
| Bacteroidetes | Chitinophaga | 0.18 | |
| Chryseobacterium | 0.25 | ||
| Prevotella | 0.19 | ||
| Other | 0.14 | ||
| Cyanobacteria | Leptolyngbya | 0.53 | |
| Firmicutes | Ammoniphilus | 0.22 | |
| Bacillus * | 43.35 | ||
| Desulfosporomusa | 0.14 | ||
| Geobacillus | 0.60 | ||
| Laceyella | 0.13 | ||
| Lactobacillus | 0.72 | ||
| Paenibacillus | 14.33 | ||
| Staphylococcus * | 0.74 | ||
| Streptococcus | 0.14 | ||
| Thermoactinomyces | 0.25 | ||
| Thermobacillus | 0.15 | ||
| Weissella | 3.00 | ||
| Other | 0.42 | ||
| Nitrospirae | Nitrospira | 0.07 | |
| Planctomycetes | Singulisphaera | 0.12 | |
| Proteobacteria | Acetobacter | 0.16 | |
| Achromobacter | 0.11 | ||
| Acinetobacter | 0.22 | ||
| Azospirillum | 0.26 | ||
| Burkholderia | 1.18 | ||
| Chromohalobacter | 0.13 | ||
| Curvibacter | 0.15 | ||
| Delftia | 0.14 | ||
| Dyella | 0.11 | ||
| Escherichia | 0.18 | ||
| Helicobacter | 0.20 | ||
| Lysobacter | 0.15 | ||
| Oscillospira | 0.16 | ||
| Pseudomonas | 0.16 | ||
| Ralstonia | 16.98 | ||
| Sphingomonas | 0.61 | ||
| Thauera | 0.54 | ||
| Thermomonas | 0.15 | ||
| Xanthomonas | 0.18 | ||
| Other | 0.86 | ||
| Unclassified | 6.10 | ||
| Fungi | Ascomycota | Aspergillus | 51.06 |
| Candida * | 0.70 | ||
| Nakazawaea | 0.45 | ||
| Penicillium * | 2.26 | ||
| Other | 2.21 | ||
| Basidiomycota | Malassezia | 0.19 | |
| Other | 23.77 | ||
| Unclassified | 19.36 | ||
| Secondary Metabolite | Concentration of Secondary Metabolites (μg/kg) | FFR | ||
|---|---|---|---|---|
| Willow Wood Chips | Forest Wood Chips | Sunflower Pellets | ||
| 3-Nitropropionic acid | 11.6 | 1.96 | 2.73 | - |
| Alternariol | 22.3 | - | 66.43 | - |
| Alternariolmethylether | 6.09 | 2.65 | 50.67 | - |
| Ascochlorin | 0.77 | 4.16 | 4.00 | - |
| Altersetin | - | - | 90.63 | 3.39 |
| Asperglaucide | 22.3 | 0.229 | - | 399.9 |
| Andrastin A | - | - | 1878.2 | - |
| Averantin | - | 1.67 | 1.24 | - |
| Averufin | 0.11 | 1.28 | - | - |
| Citreorosein | 5.70 | 37.12 | 83.57 | 14.44 |
| Cladosporin | 3.44 | - | - | - |
| Cyclopenol | 8.10 | - | - | - |
| Emodin | 4.37 | 45.59 | 180.9 | 15.66 |
| Fallacinol | - | - | - | 1.67 |
| Fumigaclavine C | - | 60,698 | 33,479 | - |
| Fumigaclavine A | - | 12.55 | 7.52 | - |
| Fumiquinazolin A | - | 1959 | - | - |
| Fumiquinazolin D | - | 21,073 | 11,992 | - |
| Fumiquinazolin F | - | 6586 | 3599 | - |
| Fumitremorgin C | - | 3.15 | - | - |
| Infectopyron | 35.27 | - | - | - |
| Integracin A | - | 1.99 | 5.97 | - |
| Integracin B | 0.08 | 3.50 | 42.35 | - |
| Isofusidienol | - | - | 30.46 | - |
| Macrosporin | 6.85 | 10.42 | - | - |
| Methylsulochrin | - | 59.72 | 40.75 | - |
| Monocerin | - | 1.11 | 145.59 | - |
| Neoechinulin A | 58.92 | 0.39 | - | - |
| Norsolorinic acid | - | 10.82 | 0.723 | - |
| Orsellinic acid | 3795 | - | - | - |
| Pseurotin A | - | 16.33 | 30.02 | - |
| Quinocitrinine A | 2.90 | 0.130 | 1.80 | - |
| Pyripyropene A | - | 3.10 | - | - |
| Skyrin | 0.489 | 30.92 | 32.81 | - |
| Sydonic acid | - | - | 5.94 | - |
| Sterigmatocystin | 0.694 | 1.03 | - | - |
| Tentoxin | 47.16 | - | - | - |
| Tenuazonic acid | 939.5 | - | - | - |
| Viridicatum toxin | 2.09 | - | - | - |
| Versicolorin C | - | 60.87 | - | - |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc, J.; Otlewska, A.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Gutarowska, B. Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass. Int. J. Environ. Res. Public Health 2017, 14, 99. https://doi.org/10.3390/ijerph14010099
Szulc J, Otlewska A, Okrasa M, Majchrzycka K, Sulyok M, Gutarowska B. Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass. International Journal of Environmental Research and Public Health. 2017; 14(1):99. https://doi.org/10.3390/ijerph14010099
Chicago/Turabian StyleSzulc, Justyna, Anna Otlewska, Małgorzata Okrasa, Katarzyna Majchrzycka, Michael Sulyok, and Beata Gutarowska. 2017. "Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass" International Journal of Environmental Research and Public Health 14, no. 1: 99. https://doi.org/10.3390/ijerph14010099
APA StyleSzulc, J., Otlewska, A., Okrasa, M., Majchrzycka, K., Sulyok, M., & Gutarowska, B. (2017). Microbiological Contamination at Workplaces in a Combined Heat and Power (CHP) Station Processing Plant Biomass. International Journal of Environmental Research and Public Health, 14(1), 99. https://doi.org/10.3390/ijerph14010099








