Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review
Abstract
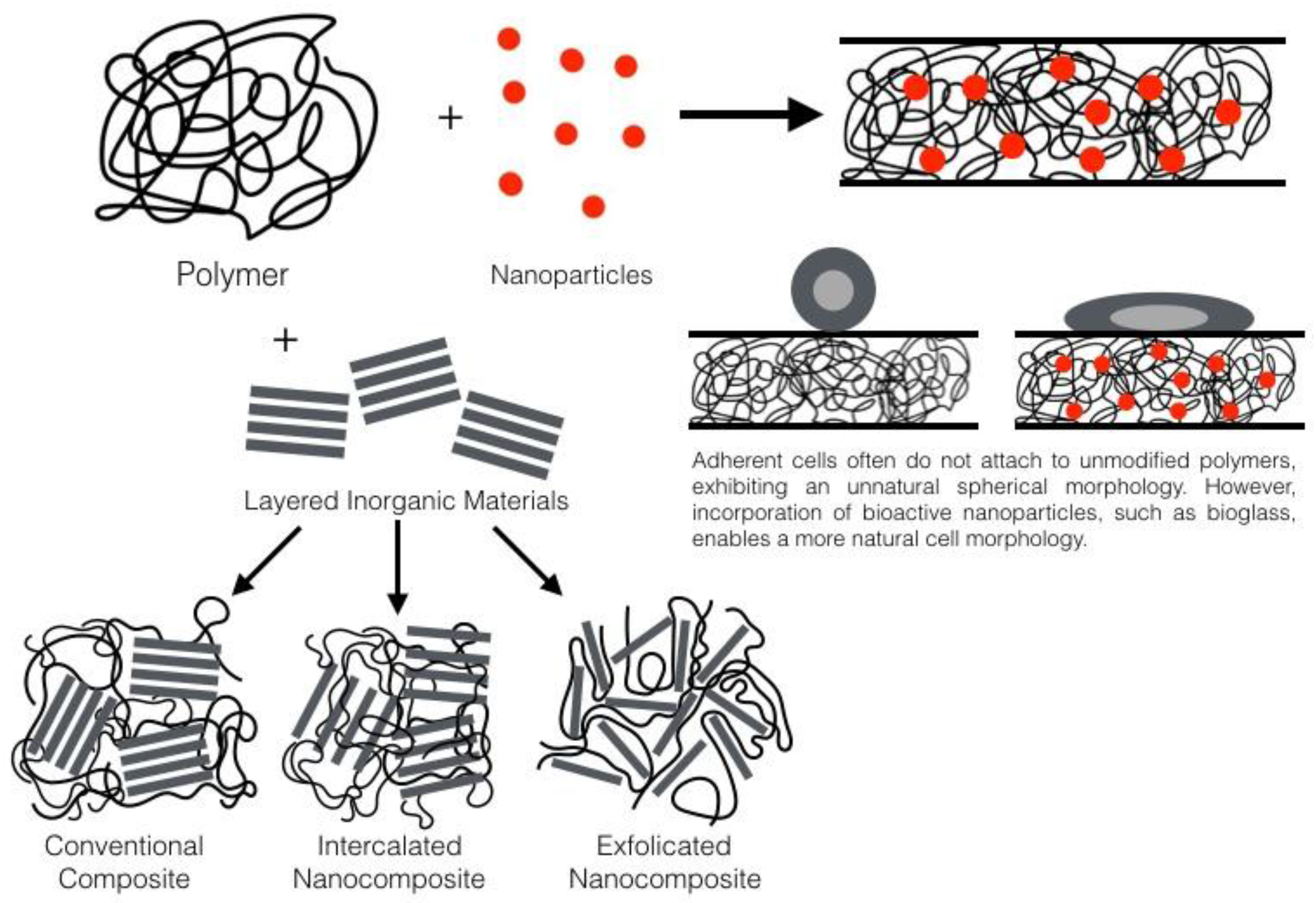
:1. Introduction
2. General Nanocomposite Information
3. Bone Tissue Engineering
- Osteoinduction
- The presence of cells to respond to osteogenesis signals
- A scaffold material to support the growth of cells, osteogenesis and subsequent ECM formation
- Vascularisation of the treatment area
4. Nanocomposite Wound Dressings
5. Tissue Engineering and Regeneration
6. Drug Delivery from Nanocomposites
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fajardo, A.R.; Pereira, A.G.B.; Muniz, E.C. Hydrogels nanocomposites based on crystals, whiskers and fibrils derived from biopolymers. Adv. Struct. Mater. 2015, 74, 43–71. [Google Scholar]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; van de Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K., Jr. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 2, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive ceramics: Theory and clinical applications. Bioceramics 1994, 7, 3–14. [Google Scholar]
- Tilocca, A. Models of structure, dynamics and reactivity of bioglasses: A review. J. Mater. Chem. 2010, 20, 6848–6858. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Loher, S.; Reboul, V.; Brunner, T.J.; Simonet, M.; Dora, C.; Neuenschwander, P.; Stark, W.J. Improved degradation and bioactivity of amorphous aerosol derived tricalcium phosphate nanoparticles in poly(lactide-co-glycolide). Nanotechnology 2006, 17, 2054–2061. [Google Scholar] [CrossRef]
- Couto, D.S.; Alves, N.M.; Mano, J.F. Nanostructured multilayer coatings combining chitosan with bioactive glass nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of novel bioactive glass ceramic nanoparticles. J. Biomed. Mater. Res. A 2009, 88, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.; Mann, A.B.; Pou, J.; Lusquiños, F.; Riveiro, A. Rapid production of ultra long amorphous ceramic nanofibers by laser spinning. Appl. Phys. Lett. 2007, 90, 153109. [Google Scholar] [CrossRef]
- Rohatgi, P.K.; Schultz, B. Lightweight metal matrix nanocomposites—Stretching the boundaries of metals. Mater. Matters 2007, 2, 16. [Google Scholar]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Tampieri, A.; Herrmannsdörfer, T.; Ambrosio, L. A route toward the development of 3D magnetic scaffolds with tailored mechanical and morphological properties for hard tissue regeneration: Preliminary study: A basic approach toward the design of 3D rapid prototyped magnetic scaffolds for hard-tissue regeneration is presented and validated in this paper. Virtual Phys. Prototyp. 2011, 6, 189–195. [Google Scholar]
- Pereira, M.M.; Jones, J.R.; Orefice, R.L.; Hench, L.L. Preparation of bioactive glass-polyvinyl alcohol hybrid foams by the sol-gel method. J. Mater. Sci. Mater. Med. 2005, 16, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, N.; Bhargava, P.; Alam, S.; Pramanik, P. Processing and properties of nano- and macro-hydroxyapatite/poly(ethylene-co-acrylic acid) composites. Polym. Compos. 2006, 27, 633–641. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Woodhead Publishing. Tissue Engineering Using Ceramics and Polymers, 2nd ed.; Boccaccini, A.R., Ma, P.X., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; D’Alessandro, T.; Sandri, M.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Uhlarz, M.; Tampieri, A.; et al. Magnetic poly (ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface 2013, 10, 20120833. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of apatite formation on CaO-SiO2-P2O5 glasses in a simulated body-fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Park, J.B.; Lakes, R.S. Biomaterials, 2nd ed.; Plenum Press: New York, NY, USA, 1992; pp. 185–222. [Google Scholar]
- Hench, L.L.; Andersson, O. An Introduction to Bioceramics. In Bioactive Glasses; Hench, L.L., Wilson, J., Eds.; World Scientific Publishing: Singapore, 1993. [Google Scholar]
- Arcos, D.; Greenspan, D.C.; Vallet-Reg, M. Influence of the Stabilization Temperature on Textural and Structural Features and Ion Release in SiO2-CaO-P2O5 Sol-Gel Glasses. Chem. Mater. 2002, 14, 1515–1522. [Google Scholar] [CrossRef]
- Blencke, B.A.; Bromer, H.; Deutcher, K. Glass ceramic—A new bioactive implant material. Med. Orthop. Technol. 1975, 95, 144–148. [Google Scholar]
- Kokubo, T.; Shigematsu, M.; Nagashima, Y.; Tashiro, M.; Nakamura, T.; Yamamuro, T.; Higashi, S. Apatite- and wollastonite-containing glass–ceramic for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982, 60, 260–267. [Google Scholar]
- Jarcho, M.; Bolen, C.H.; Thomas, M.B.; Bobick, J.; Kay, J.F.; Doremus, R.H. Synthesis and characterization of hydroxyapatite in dense polycrystalline form. J. Mater. Sci. 1976, 11, 2027. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Salinas, A.J. Influence of P2O5 on crystallinity of apatite formed in vitro on surface of bioactive glasses. J. Biomed. Mater. Res. 1999, 46, 560–565. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J.; Gil, M. Effect of magnesium content on the in vitro bioactivity of CaO-MgO-SiO2-P2O5 sol-gel glasses. J. Mater. Chem. 1999, 9, 515–518. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Balas, F.; Vallet-Regí, M. Surface and Chemical Study of SiO2-P2O5-CaO·(MgO) Bioactive Glasses. J. Biomed. Mater. Res. 1999, 12, 750–755. [Google Scholar] [CrossRef]
- Balas, F.; Arcos, D.; Pérez-Pariente, J.; Vallet-Regí, M. Textural properties of SiO2-CaO-P2O5 glasses prepared by the sol-gel method. J. Mater. Res. 2001, 16, 1345–1348. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Laczka, M.; Cholewa, K.; Lazca-Osyczka, A. Gel-derived powders of CaO-P2O5-SiO2 system as a starting material to production of bioactive ceramics. J. Alloy Compd. 1997, 248, 42–51. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Balas, F.; Román, J.; Salinas, A.J. Influence of composition and surface characteristics on the in vitro bioactivity of SiO2-CaO-P2O5-MgO sol-gel glasses. J. Biomed. Mater. Res. 1999, 47, 170–175. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Arcos, D.; Pérez-Pariente, J. Evolution of porosity during in vitro hydroxycarbonate apatite growth in sol-gel glasses. J. Biomed. Mater. Res. 2000, 51, 23–28. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Rámila, A. New bioactive glass and changes in porosity during the growth of a carbonate hydroxyapatite layer on glass surfaces. Chem. Mater. 2000, 12, 961–965. [Google Scholar] [CrossRef]
- Pereira, M.M.; Clark, A.E.; Hench, L.L. Calcium phosphate forma-tion on sol-gel-derived bioactive glasses in vitro. J. Biomed. Mater. Res 1994, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Gil, M.; Nogueroles, E.; Romero, A.; Roma´n, J.; Salinas, A.J.; Ragel, C.V. Bone-like apatite layer formation on sol-gel glasses. In Non-Crystalline and Nanoscale Materials; Rivas, J., Lo’pez Quintela, M.A., Eds.; World Scientific: London, UK, 1998. [Google Scholar]
- Vallet-Regí, M.; Romero, A.M.; Ragel, C.V.; LeGeros, R.Z. XRD, SEM, EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol-gel glasses. J. Biomed. Mater. Res. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Ogawa, K.; Hirano, S.; Miyanishi, T.; Yui, T.; Watanabe, T.A. A new polymorph of chitosan. Macromolecules 1984, 17, 973–975. [Google Scholar] [CrossRef]
- Depan, D.; Venkata Surya, P.K.C.; Girase, B.; Misra, R.D.K. Organic/inorganic hybrid network structure nanocomposite scaffolds based on grafted chitosan for tissue engineering. Acta Biomater. 2011, 7, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yin, Z.; Liu, H.; Chen, X.; Feng, B.; Yuan, H.; Su, B. Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs. Nanotechnology 2012, 23, 485102. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Song, J.H.; Kim, H.E. Nanofiber generation of gelatinhydroxyapatite biomimetics for guided tissue regeneration. Adv. Funct. Mater. 2005, 15, 1988–1994. [Google Scholar] [CrossRef]
- Jayabalan, M.; Shalumon, K.T.; Mitha, M.K.; Ganesan, K.; Epple, M. Effect of hydroxyapatite on the biodegradation and biomechanical stability of polyester nanocomposites for orthopedic applications. Acta Biomater. 2010, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Reis, R.L.; Mano, J.F. Preparation and in vitro characterization of scaffolds of poly(L-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater. 2008, 4, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.K.; Liu, A.X.; Chen, L.; Chen, X.S.; Jing, X.B. Preparation of bioactive glass ceramic nanoparticles by combination of sol-gel and coprecipitation method. J. Non-Cryst. Solids 2009, 355, 368–372. [Google Scholar] [CrossRef]
- Jo, J.H.; Lee, E.J.; Shin, D.S.; Kim, H.E.; Kim, H.W.; Koh, Y.H.; Jang, J.H. In vitro/in vivo biocompatibility and mechanical properties of bioactive glass nanofiber and poly(epsilon-caprolactone) composite materials. J. Biomed. Mater. Res. B 2009, 91B, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Banobre-Lopez, M.; Pineiro-Redondo, Y.; De Santis, R.; Gloria, A.; Ambrosio, L.; Tampieri, A.; Dediu, V.; Rivas, J. Poly(caprolactone) based magnetic scaffolds for bone tissue engineering. J. Appl. Phys. 2011, 109, 07B313. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Cai, H.; Chen, Z.; Wang, T.; Jia, L.; Wang, J.; Wan, Q.; Pei, X. Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: Preparation and in vitro evaluation. Colloids Surf. B Biointerfaces 2016, 147, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Josefina Arellano-Jimenez, M.; Laurencin, C.T.; Sanders, M.M.; Barry Carter, C.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater 2014. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Duran, A.; Carpenter, E.M.; Zur Nieden, N.I.; Malinin, T.I.; Rodríguez-Manzaneque, J.C.; Zanello, L.P. Carboxyl-modified single-wall carbon nanotubes improve bone tissue formation in vitro and repair in an in vivo rat model. Int. J. Nanomed. 2014, 9, 4277–4291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Raj, S.; Kolanthai, E.; Sood, A.K.; Sampath, S.; Chatterjee, K. Chemical functionalization of graphene to augment stem cell osteogenesis and inhibit biofilm formation on polymer composites for orthopedic applications. ACS Appl. Mater. Interfaces 2015, 7, 3237–3252. [Google Scholar] [CrossRef] [PubMed]
- Outten, C.E.; O’Halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 1–7. [Google Scholar]
- Luong, L.N.; Ramaswamy, J.; Kohn, D.H. Effects of osteogenic growth factors on bone marrow stromal cell differentiation in a mineral-based delivery system. Biomaterials 2012, 33, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Violant, D.; Galofre, M.; Nart, J.; Teles, R.P. In vitro evaluation of a multispecies oral biofilm on different implant surfaces. Biomed. Mater. 2014, 9, 035007. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Hammer, P.; Vaz, L.G.; Rocha, L.A. Are new TiNbZr alloys potential substitutes of the Ti6Al4V alloy for dental applications? An electrochemical corrosion study. Biomed. Mater. 2013, 8, 065005. [Google Scholar] [CrossRef] [PubMed]
- Ajami, E.; Mahno, E.; Mendes, V.C.; Bell, S.; Moineddin, R.; Davies, J.E. Bone healing and the effect of implant surface topography on osteoconduction in hyperglycemia. Acta Biomater. 2014, 10, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Timucin, E.; Kitajima, T.; Sezerman, O.U.; Ito, Y. Direct in vitro selection of titanium-binding epidermal growth factor. Biomaterials 2014, 35, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Stakleff, K.S.; Lin, F.; Smith Callahan, L.A.; Wade, M.B.; Esterle, A.; Miller, J.; Graham, M.; Becker, M.L. Resorbable, amino acid-based poly(ester urea)s crosslinked with osteogenic growth peptide with enhanced mechanical properties and bioactivity. Acta Biomater. 2013, 9, 5132–5142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Wang, X.F.; Shao, Y.C.; Shi, D.Y.; Chen, T.Y.; Cui, D.F.; Jiang, X.X. Synthetic osteogenic growth peptide promotes differentiation of human bone marrow mesenchymal stem cells to osteoblasts via RhoA/ROCK pathway. Mol. C Biochem. 2011, 358, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.S.; Saska, S.; Martines, M.A.U.; Marchetto, R. Nanostructured materials based on mesoporous silica and mesoporous silica/apatite as osteogenic growth peptide carriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, K.; Zhang, S.-M.; Lee, I.S. Characterization and in vitro biological evaluation of mineral/osteogenic growth peptide nanocomposites synthesized biomimetically on titanium. Appl. Surf. Sci. 2015, 334, 62–68. [Google Scholar] [CrossRef]
- Vacanti, C.A.; Bonassar, L.J. An overview of tissue engineered bone. Clin. Orthop. Relat. Res. 1999, 367, S375–S381. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, T. Wound dressing: Principles and practices. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Sakai, S.; Tsumura, M.; Inoue, M.; Koga, Y.; Fukano, K.; Taya, M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J. Mater. Chem. B 2013, 1, 5067–5075. [Google Scholar] [CrossRef]
- Boateng, S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Woehl, M.A.; Ono, L.; Vidotti, I.C.R.; Wypych, F.; Schreinerc, W.H.; Sierakowski, M.R. Bioactive nanocomposites of bacterial cellulose and natural hydrocolloids. J. Mater. Chem. B 2014, 2, 7034–7044. [Google Scholar] [CrossRef]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. A 2006, 79A, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Desouza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; Desouza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.; Edwards-Jones, V. The role of Acticoat with nanocrystalline silver in the management of burns. Burns 2004, 30, S1–S9. [Google Scholar] [CrossRef]
- Widgerow, A.D. Nanocrystalline silver, gelatinases and the clinical implications. Burns 2010, 36, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, P.Y.; Ho, C.M.; Lui, V.C.; Chen, Y.; Che, C.M.; Tam, P.K.; Wong, K.K. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. Chem. Med. Chem. 2010, 5, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Koizhaiganova, M.; Lkhagvajav, N.; Yaşa, I.; Çelik, E. New gelatin-hydroxyapatite nanocompositefilms containing nanosilver: Synthesis, and, mechanical and antimicrobial properties. J. Bionanosci. 2011, 5, 8. [Google Scholar] [CrossRef]
- Usman, A.; Hussain, Z.; Riaz, A.; Khan, A.H. Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films. Carbohydr. Polym. 2016, 153, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composite with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA—Clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Kandori, K.; Uoya, Y.; Ishikawa, T. Effects of acetonitrile on adsorption behavior of bovine serum albumin onto synthetic calcium hydroxyapatite particles. J. Colloid Interface Sci. 2002, 252, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Vaníčková, M.; Suttnar, J.; Dyr, J.E. The adhesion of blood platelets on fibrinogen surface: Comparison of two biochemical microplate assays. Platelets 2006, 17, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.P.; Forman, W.B.; Edwards, R.V.; Lindan, O. Fibrin formation: Effect of calcium ions. Blood 1978, 52, 654–658. [Google Scholar] [PubMed]
- Song, L.; Sun, L.; Jiang, N.; Gan, Z. Structural control and hemostatic properties of porous microspheres fabricated by hydroxyapatite-graft-poly(d,l-lactide) nanocomposites. Compos. Sci. Technol. 2016, 134. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014, 6, e99. [Google Scholar] [CrossRef]
- Barabadi, Z.; Azami, M.; Sharifi, E.; Karimi, R.; Lotfibakhshaiesh, N.; Roozafzoon, R.; Joghataei, M.T.; Ai, J. Fabrication of hydrogel based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Mater. Sci. Eng. C 2016, 69, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sharma, M.; Saharia, D.; Sarma, K.K.; Sarma, M.G.; Borthakur, B.B.; Bora, U. In vivo studies of silk based gold nano-composite conduits for functional peripheral nerve regeneration. Biomaterials 2015, 62, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Stahl, K.; Gomis-Perez, C.; Toffanin, S.; Sagnella, A.; Torp, R.; Kaplan, D.L.; Ruani, G.; Omenetto, F.G.; Zamboni, R.; et al. Biofunctional silk/neuron interfaces. Adv. Funct. Mater. 2012, 22, 1871–1884. [Google Scholar] [CrossRef]
- Dinis, T.; Vidal, G.; Marin, F.; Kaplan, D.; Eglès, C. Silk nerve: Bioactive implant for peripheral nerve regeneration. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2016, 1861, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; De Nicola, M.; Mandoli, C.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Ce3+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 2011, 5, 4537–4549. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef] [PubMed]
- Merle, M.; Dellon, A.L.; Campbell, J.N.; Chang, P.S. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989, 10, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Gerhke, S.A.; Shibli, J.A.; Salles, M.B. Potential of the use of an antioxidant compound to promote peripheral nerve regeneration after injury. Neural Regen. Res. 2015, 10, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Sepahvandi, A.; Eskandari, M.; Moztarzadeh, F. Fabrication and characterization of SrAl2O4: Eu2 + Dy3 +/CS-PCL electrospun nanocomposite scaffold for retinal tissue regeneration. Mater. Sci. Eng. C 2016, 66, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, D.; Yuan, Y.; Wu, M. Synthesis of Sr4Al14O25: Eu2+, Dy3+ phosphor nanometer powders by combustion processes and its optical properties. Mater. Sci. Eng. B 2006, 133, 200–204. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, Z.; Gu, W.; Liu, J.; Xu, Z.P. Efficient drug delivery using SiO2-layered double hydroxide nanocomposites. J. Colloid Interface Sci. 2016, 470, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Yun, B.; Lee, K.S.; Chang, Y.-W. Biodegradable shape-memory poly(ε-caprolactone)/polyhedral oligomeric silsequioxane nanocomposites: Sustained drug release and hydrolytic degradation. Mater. Lett. 2016, 166, 125–128. [Google Scholar] [CrossRef]
- Long, J.; Yu, X.; Xu, E.; Wu, Z.; Xu, X.; Jun, Z.; Jiao, A. In situ synthesis of new magnetite chitosan/carrageenan nanocomposites by electrostatic interactions for protein delivery applications. Carbohydr. Polym. 2015, 131, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Mariappan, R.; Piraman, S. Synthesis and characterization of amine modified magnetite nanoparticles as carriers of curcumin-anticancer drug. Powder Technol. 2014, 266, 321–328. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Topham, P.D.; Huang, Y. Fabrication of magnetic drug-loaded polymeric composite nanofibres and their drug release characteristics. RSC Adv. 2012, 2, 2433–2438. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 77, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. Oncol. Rep. 2003, 10, 1663–1683. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Prabha, G. Synthesis, characterization and in vitro drug release of cisplatin loaded Cassava starch acetate–PEG/gelatin nanocomposites. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 21, 10–16. [Google Scholar] [CrossRef]
- Schweta, K.; Manupati, K.; Das, A.; Jha, H. Novel nanocomposites with selective antibacterial action and low cytotoxic effect on eukaryotic cells. Int. J. Biol. Macromol. 2016, 92, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, L.; Szalaty, T.J.; Zdarta, J.; Jesionow, T. Activated lignin and aminosilane-grafted silica as precursors in hybrid material production. Physicochem. Probl. Miner. Process 2016, 52, 459–478. [Google Scholar]
- Ho, L.C.; Hsu, C.H.; Ou, C.M.; Wang, C.W.; Liu, T.P.; Hwang, L.P.; Lin, Y.Y.; Chang, H.T. Unibody coreeshell smart polymer as a theranostic nanoparticle for drug delivery and MR imaging. Biomaterials 2015, 37, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, F. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. Biomed. Res. Int. 2014, 2014, 205–208. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bramhill, J.; Ross, S.; Ross, G. Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review. Int. J. Environ. Res. Public Health 2017, 14, 66. https://doi.org/10.3390/ijerph14010066
Bramhill J, Ross S, Ross G. Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review. International Journal of Environmental Research and Public Health. 2017; 14(1):66. https://doi.org/10.3390/ijerph14010066
Chicago/Turabian StyleBramhill, Jane, Sukunya Ross, and Gareth Ross. 2017. "Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review" International Journal of Environmental Research and Public Health 14, no. 1: 66. https://doi.org/10.3390/ijerph14010066
APA StyleBramhill, J., Ross, S., & Ross, G. (2017). Bioactive Nanocomposites for Tissue Repair and Regeneration: A Review. International Journal of Environmental Research and Public Health, 14(1), 66. https://doi.org/10.3390/ijerph14010066





