A Neurophysiological Approach for Evaluating Noise-Induced Sleep Disturbance: Calculating the Time Constant of the Dynamic Characteristics in the Brainstem
Abstract
:1. Introduction
1.1. Indices for Night-Time Noise
1.2. Neurophysiological View of Wakefulness and Sleep
2. Methods
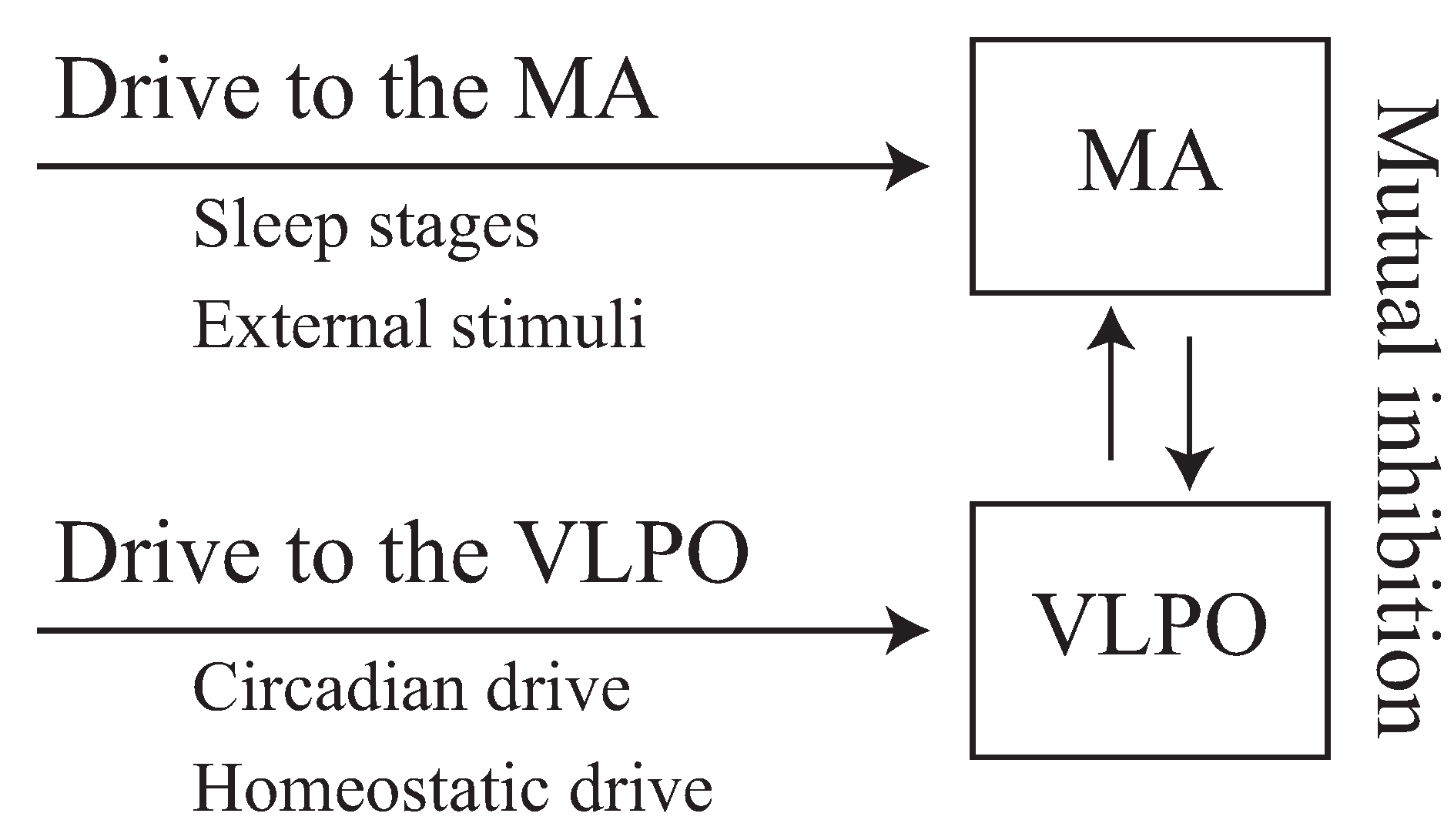
2.1. Phillips–Robinson Model
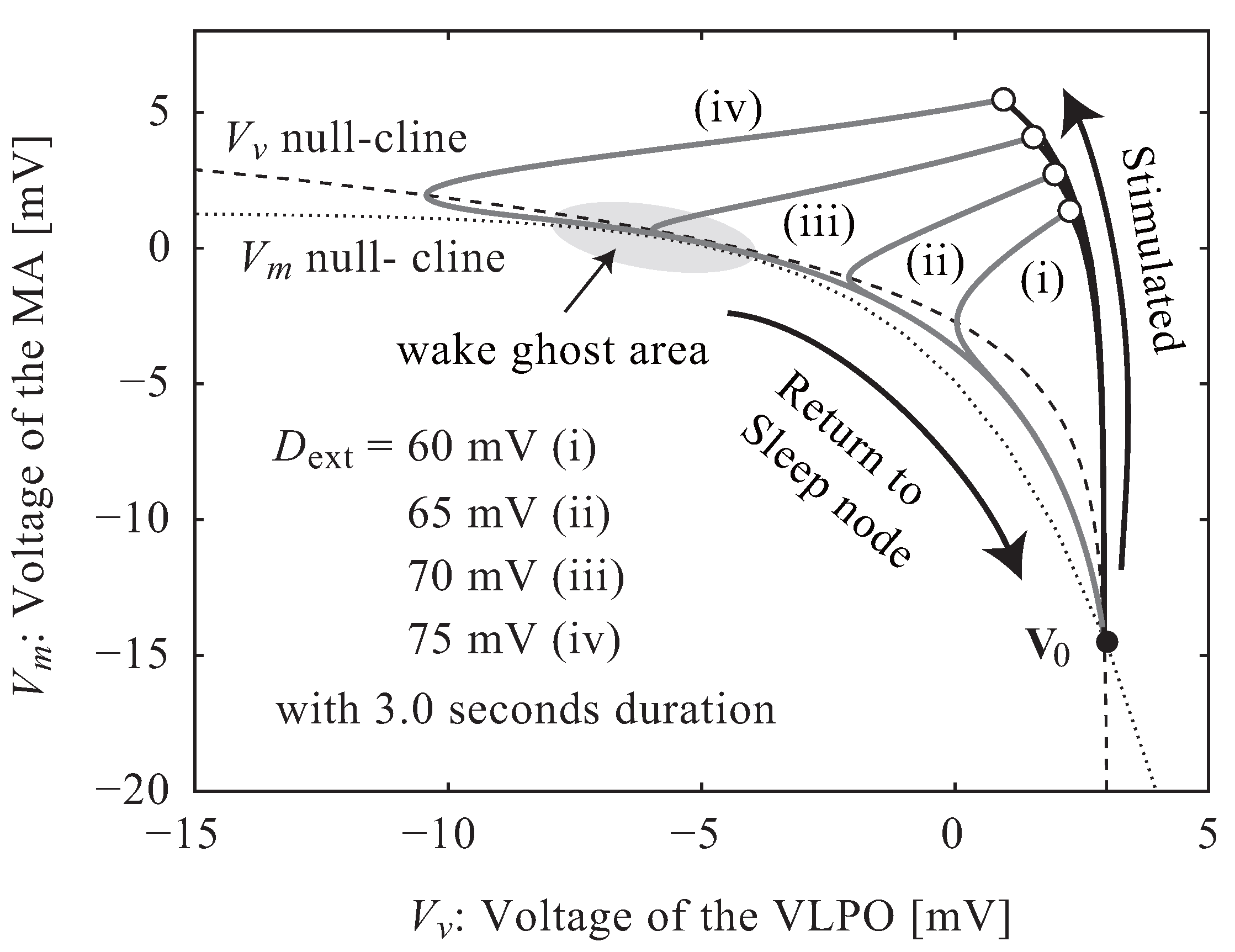
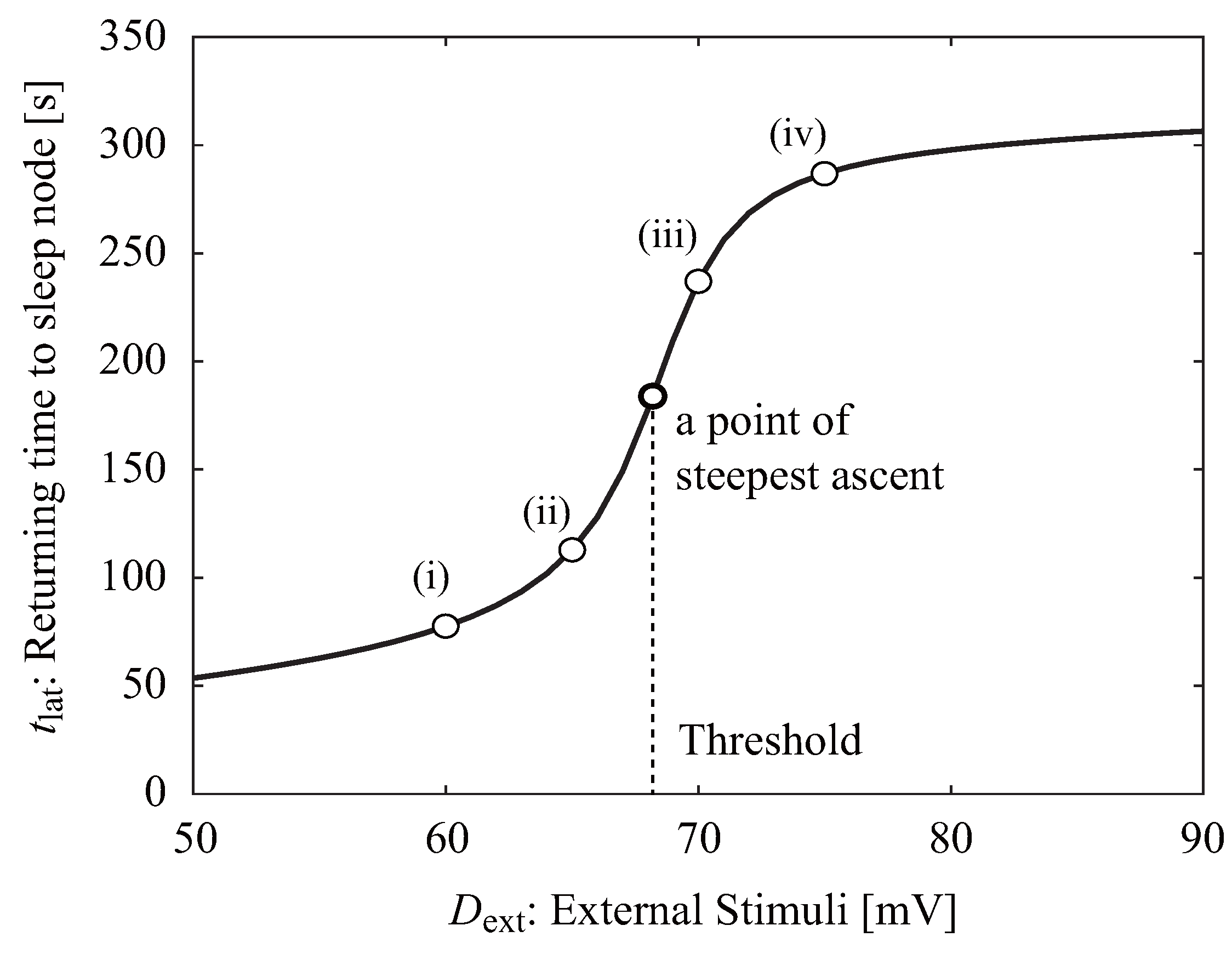
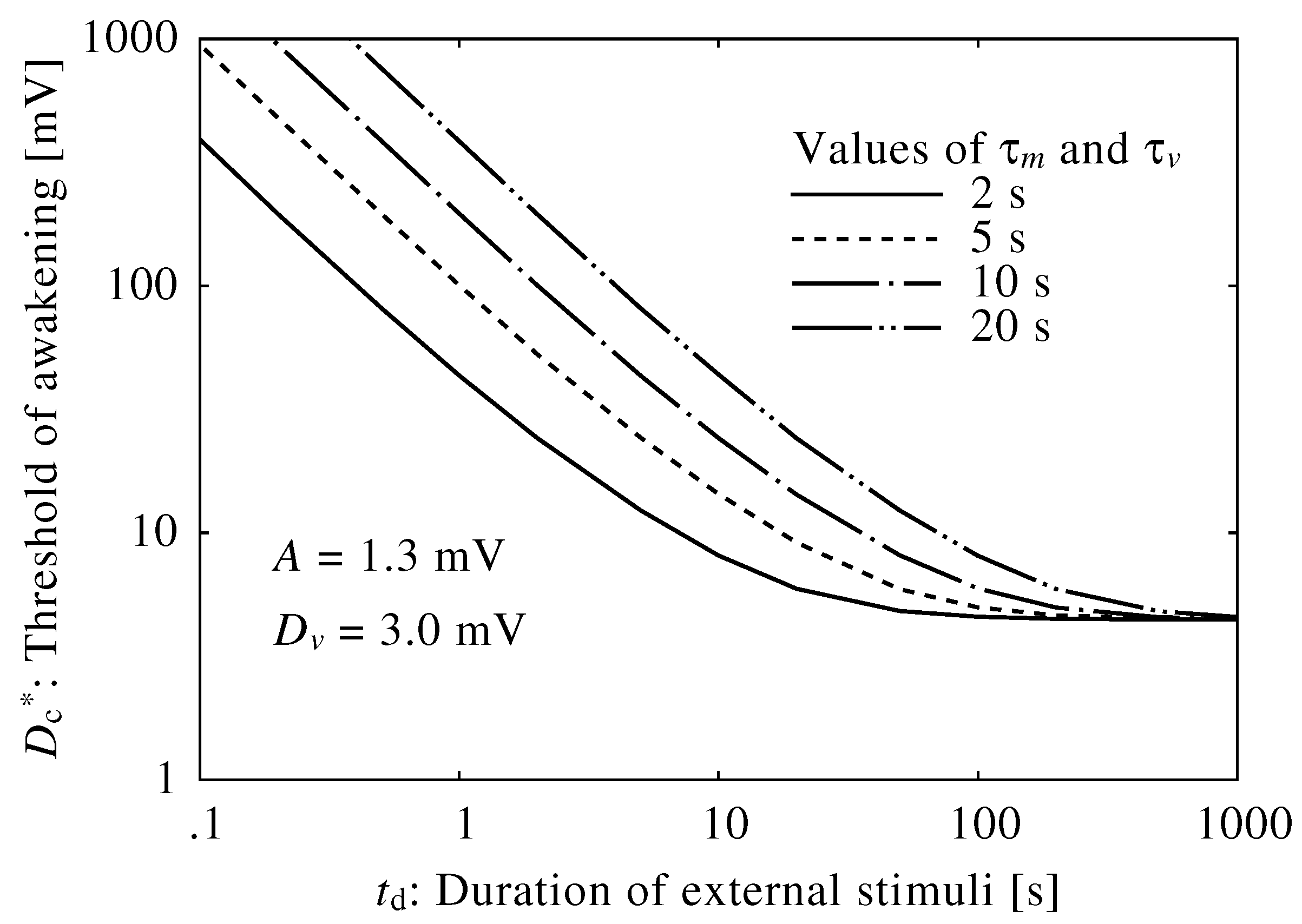
2.2. Neuroelectrical Threshold of Awakening
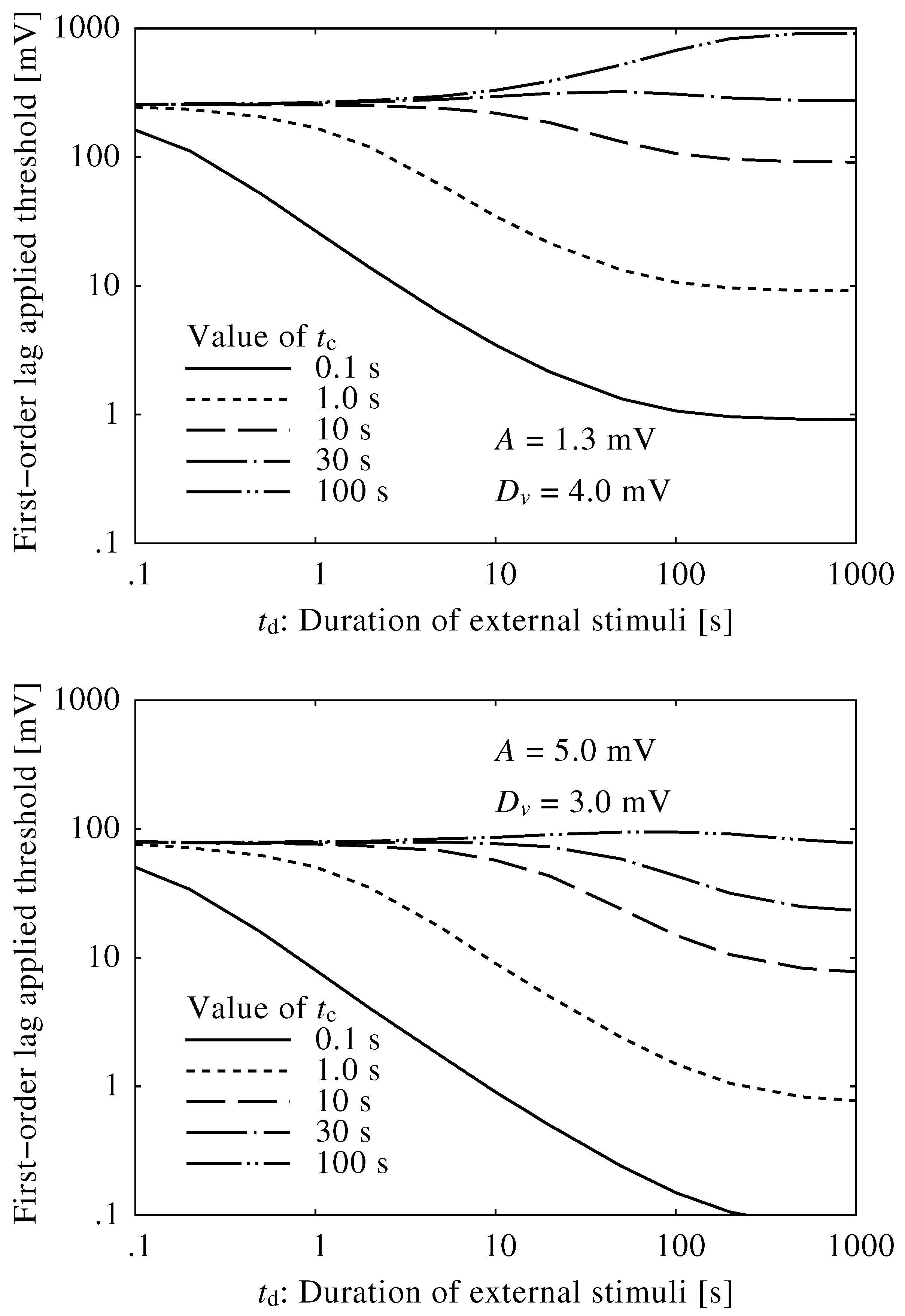
2.3. Conversion of Neuroelectrical Thresholds into Sound Level
3. Results
3.1. Neuroelectrical Threshold of Awakening
3.2. Conversion into Sound Level
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Babisch, W. The noise/stress concept, risk assessment and research needs. Noise Health 2002, 4, 1–11. [Google Scholar] [PubMed]
- Passchier-Vermeer, W. Night-Time Noise Events and Awakening; TNO Inro Report: Delft, The Netherlands, 2003. [Google Scholar]
- Jarup, L.; Babisch, W.; Houthuijs, D.; Pershagen, G.; Katsouyanni, K.; Cadum, E.; Dudley, M.; Savigny, P.; Seiffert, I.; Swart, W.; et al. Hypertension and exposure to noise near airports: The HYENA study. Environ. Health Perspect. 2008, 116, 329. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Europe. Night Noise Guidelines for Europe; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2009. [Google Scholar]
- World Health Organization Regional Office for Europe. Burden of Disease from Environmental Noise. Quantification of Healthy Life Years Lost in Europe; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2011. [Google Scholar]
- Babisch, W. Updated exposure-response relationship between road traffic noise and coronary heart diseases: A meta-analysis. Noise Health 2014, 16, 1–9. [Google Scholar] [PubMed]
- Münzel, T.; Gori, T.; Babisch, W.; Basner, M. Cardiovascular effects of environmental noise exposure. Eur. Heart J. 2014, 35, 829–836. [Google Scholar] [PubMed]
- Basner, M.; Brink, M.; Bristow, A.; de Kluizenaar, Y.; Finegold, L.; Hong, J.; Janssen, S.; Klaeboe, R.; Leroux, T.; Liebl, A.; et al. ICBEN review of research on the biological effects of noise 2011-2014. Noise Health 2015, 17, 57–82. [Google Scholar] [PubMed]
- Pearsons, K.S.; Barber, D.S.; Tabachnick, B.G.; Fidell, S. Predicting noise-induced sleep disturbance. J. Acoust. Soc. Am. 1995, 97, 331–338. [Google Scholar] [CrossRef]
- Fidell, S.; Pearsons, K.; Tabachnick, B.; Howe, R.; Silvati, L.; Barber, D.S. Field study of noise-induced sleep disturbance. J. Acoust. Soc. Am. 1995, 98, 1025–1033. [Google Scholar] [CrossRef]
- Fidell, S.; Howe, R.R.; Tabachnick, B.G.; Pearsons, K.S.; Sneddon, M.D. Noise-Induced Sleep Disturbance in Residences Near Two Civil Airports; National Aeronautics and Space Administration, Langley Research Center: Hampton, VA, USA, 1995. [Google Scholar]
- Fidell, S.; Howe, R.; Tabachnick, B.; Pearsons, K.; Silvati, L.; Sneddon, M.; Fletcher, E. Field Studies of Habituation to Change in Nighttime Aircraft Noise and of Sleep Motility Measurement Methods; BBN Technologies: Los Angels, CA, USA, 1998. [Google Scholar]
- Passchier-Vermeer, W.; Vos, H.; Steenbekkers, J.; van der Ploeg, F.; Groothuisoudshoorn, K. Sleep Disturbance and Aircraft Noise Exposure; TNO Inro Report 2002-027; TNO: Delft, The Netherlands, 2002. [Google Scholar]
- Basner, M.; Samel, A.; Isermann, U. Aircraft noise effects on sleep: Application of the results of a large polysomnographic field study. J. Acoust. Soc. Am. 2006, 119, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Miller, N. Alternative analysis of sleep-awakening data. Noise Control Eng. J. 2007, 55, 224–245. [Google Scholar] [CrossRef]
- Fidell, S.; Tabachnick, B.; Mestre, V.; Fidell, L. Aircraft noise-induced awakenings are more reasonably predicted from relative than from absolute sound exposure levels. J. Acoust. Soc. Am. 2013, 134, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Palmer, A.R. Level and spectrum. In The Oxford Handbook of AUDITORY SCIENCE: The Auditory Brain; Oxford University presss: New York, NY, USA, 2010; Chapter 5; pp. 93–124. [Google Scholar]
- Saper, C.B.; Chou, T.C.; Scammell, T.E. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Merica, H.; Fortune, R.D. State transitions between wake and sleep, and within the ultradian cycle, with focus on the link to neuronal activity. Sleep Med. Rev. 2004, 8, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Gallopin, T.; Fort, P.; Eggermann, E.; Cauli, B.; Luppi, P.H.; Rossier, J.; Audinat, E.; Muhlethaler, M.; Serafin, M. Identification of sleep-promoting neurons in vitro. Nature 2000, 404, 992–995. [Google Scholar] [PubMed]
- Strecker, R.E.; Morairty, S.; Thakkar, M.M.; Porkka-Heiskanen, T.; Basheer, R.; Dauphin, L.J.; Rainnie, D.G.; Portas, C.M.; Greene, R.W.; McCarley, R.W. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav. Brain Res. 2000, 115, 183–204. [Google Scholar] [CrossRef]
- Pace-Schott, E.; Hobson, J. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002, 3, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Borb, A.A.; Achermann, P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythms 1999, 14, 559–570. [Google Scholar] [CrossRef]
- Achermann, P.; Borbély, A.A. Mathematical models of sleep regulation. Front. Biosci. 2003, 8, S683–S693. [Google Scholar] [CrossRef] [PubMed]
- Achermann, P. The Two-Process Model of Sleep Regulation Revisited. Aviat. Space Environ. Med. 2004, 75, A37–A43(1). [Google Scholar] [PubMed]
- Phillips, A.; Robinson, P. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. J. Biol. Rhythms 2007, 22, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Robinson, P. Sleep deprivation in a quantitative physiologically based model of the ascending arousal system. J. Theor. Biol. 2008, 255, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Rempe, M.J.; Best, J.; Terman, D. A mathematical model of the sleep/wake cycle. J. Math. Biol. 2009, 60, 615–644. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, B.D.; Phillips, A.J.K.; Postnova, S.; Robinson, P.A. A Physiologically Based Model of Orexinergic Stabilization of Sleep and Wake. PLoS ONE 2014, 9, e91982. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, A.C.; Dijk, D.J.; Derks, G. Mathematical Models for Sleep-Wake Dynamics: Comparison of the Two-Process Model and a Mutual Inhibition Neuronal Model. PLoS ONE 2014, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, B.D.; Phillips, A.J.K.; Robinson, P.A. Modeling the impact of impulsive stimuli on sleep-wake dynamics. Phys. Rev. E Stati. Nonlinear Soft Matter Physics 2008, 78, 051920. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, B.; Phillips, A.; Robinson, P. Quantitative physiologically based modeling of subjective fatigue during sleep deprivation. J. Theor. Biol. 2010, 264, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Puckeridge, M.; Fulcher, B.D.; Phillips, A.J.K.; Robinson, P.A. Incorporation of caffeine into a quantitative model of fatigue and sleep. J. Theor. Biol. 2011, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Chen, P.; Robinson, P. Probing the mechanisms of chronotype using quantitative modeling. J. Biol. Rhythms 2010, 25, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, A.C.; Derks, G.; Dijk, D.J. Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Med. Rev. 2016, 28, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Webb, W.; Barnard, G. Effect of flurazepam, pentobarbital, and caffeine on arousal threshold. Sleep 1979, 1, 271–279. [Google Scholar] [PubMed]
- Basner, M.; Griefahn, B.; Berg, M.V.D. Aircraft noise effects on sleep: Mechanisms, mitigation and research needs. Noise Health 2010, 12, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Passchier-Vermeer, W.; Vos, H.; Janssen, S.A.; Miedema, H.M. TNO Summary Report 2007-D-R0012/A Sleep and Traffic Noise Summary Report; Technical Report 2007 L&G N001 64024; TNO: Delft, The Netherlands, 2007. [Google Scholar]
- Lercher, P.; Brink, M.; Rudisser, J.; Van Renterghem, T.; Botteldooren, D.; Baulac, M.; Defrance, J. The effects of railway noise on sleep medication intake: Results from the ALPNAP-study. Noise Health 2010, 12, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Tagusari, J.; Takashima, T.; Furukawa, S.; Matsui, T. Night-Time Noise Index Based on the Integration of Awakening Potential. Int. J. Environ. Res. Public Health 2016, 13, 272. [Google Scholar] [CrossRef] [PubMed]

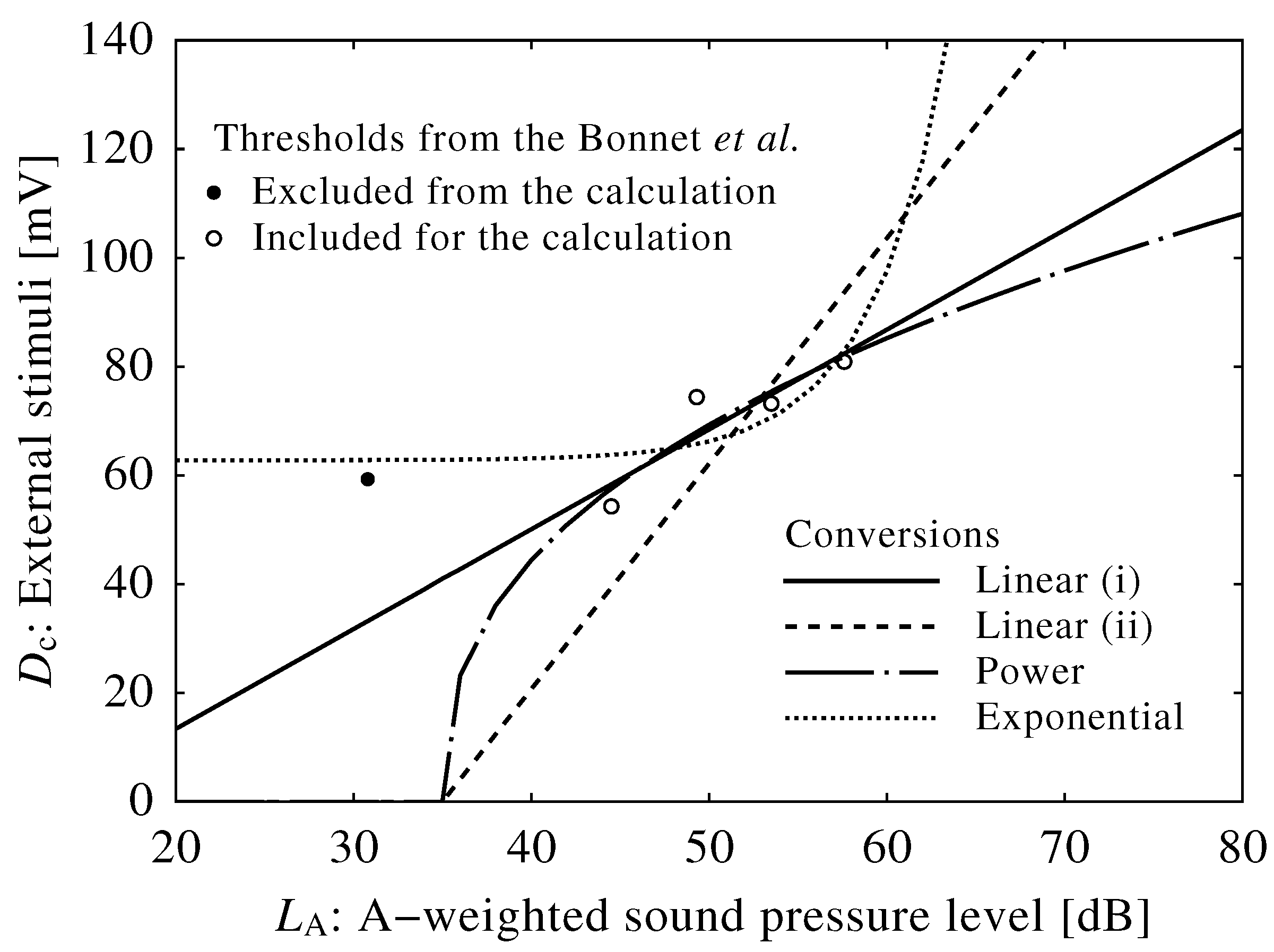

| Results from Bonnet et al. | Results from the Phillips–Robinson model | ||
|---|---|---|---|
| Time after falling asleep (min) | Average awakening levels (dB) | : Drive to the VLPO (mV) | : Neuroelectrical threshold (mV) |
| 5 | 30.8 | 2.62 | 59.3 |
| 110 | 49.3 | 3.24 | 74.4 |
| 220 | 57.6 | 3.50 | 80.9 |
| 330 | 53.5 | 3.19 | 73.2 |
| 420 | 44.5 | 2.46 | 54.3 |
| Conversion Equation | : Range of the Noise Duration (s) | |
|---|---|---|
| SEL | ||
| Linear (i) | ||
| Linear (ii) | ||
| Power | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagusari, J.; Matsui, T. A Neurophysiological Approach for Evaluating Noise-Induced Sleep Disturbance: Calculating the Time Constant of the Dynamic Characteristics in the Brainstem. Int. J. Environ. Res. Public Health 2016, 13, 369. https://doi.org/10.3390/ijerph13040369
Tagusari J, Matsui T. A Neurophysiological Approach for Evaluating Noise-Induced Sleep Disturbance: Calculating the Time Constant of the Dynamic Characteristics in the Brainstem. International Journal of Environmental Research and Public Health. 2016; 13(4):369. https://doi.org/10.3390/ijerph13040369
Chicago/Turabian StyleTagusari, Junta, and Toshihito Matsui. 2016. "A Neurophysiological Approach for Evaluating Noise-Induced Sleep Disturbance: Calculating the Time Constant of the Dynamic Characteristics in the Brainstem" International Journal of Environmental Research and Public Health 13, no. 4: 369. https://doi.org/10.3390/ijerph13040369
APA StyleTagusari, J., & Matsui, T. (2016). A Neurophysiological Approach for Evaluating Noise-Induced Sleep Disturbance: Calculating the Time Constant of the Dynamic Characteristics in the Brainstem. International Journal of Environmental Research and Public Health, 13(4), 369. https://doi.org/10.3390/ijerph13040369






