Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Testicular Testosterone Assay
2.3. Immunohistochemical Staining for Desmin to Calculate the Frequency of Focal Testis Dysgenesis
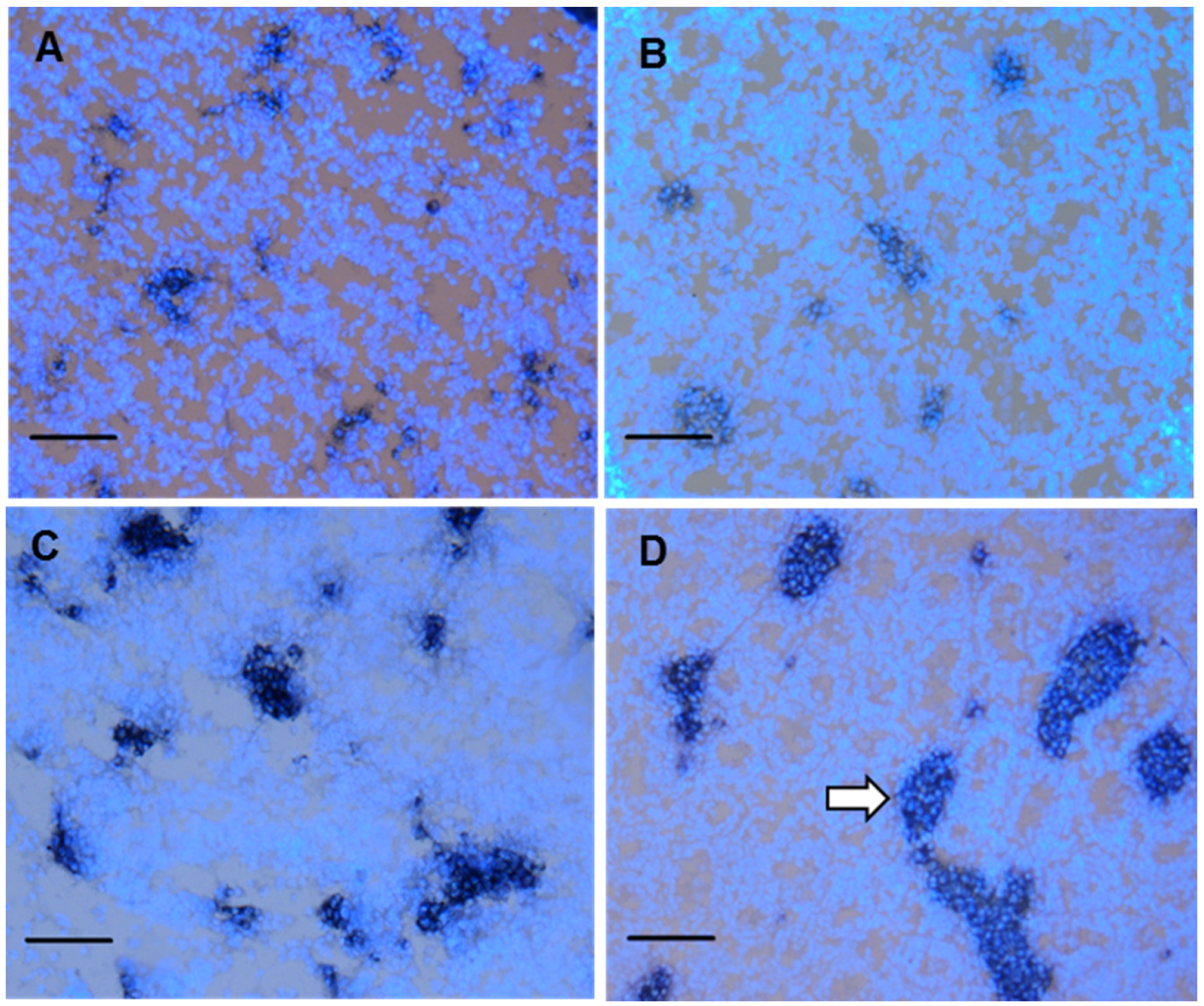
2.4. Hematoxylin and Eosin (HE) Staining for Multinucleated Gonocytes (MNGs)
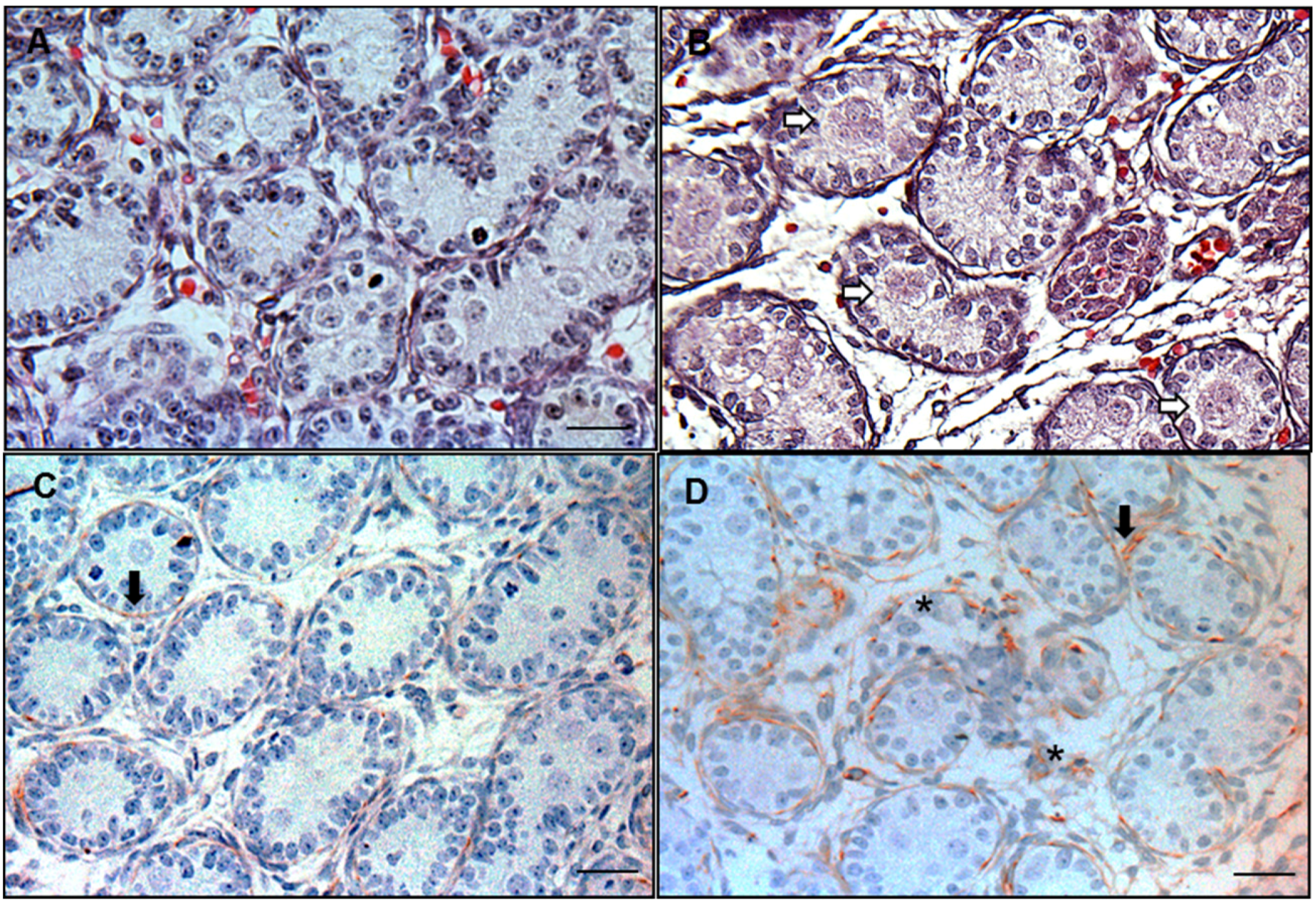
2.5. 3β-HSD Enzymological Staining for Fetal Leydig Cells
2.6. Immunohistochemical Staining of 3β-HSD to Label Fetal Leydig Cells
2.7. Computer-Assisted Image Analysis
2.8. Real-Time PCR (qPCR)
2.9. Quantitative Immunohistochemical Staining of Leydig Cell Specific Proteins
2.10. Statistical Analysis
3. Results
3.1. General Reproductive Toxicology
3.2. Frequency of MNGs and Focal Testis Dysgenesis
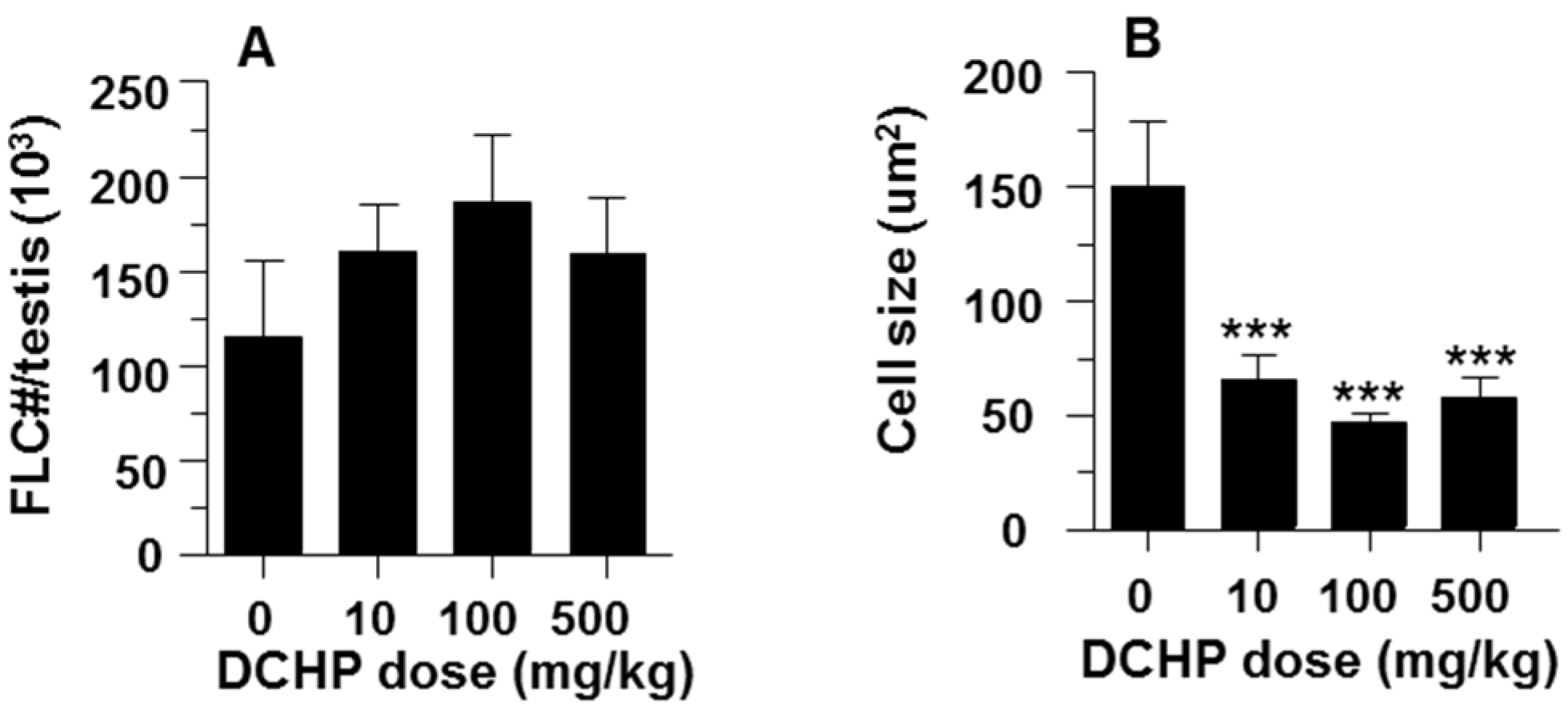
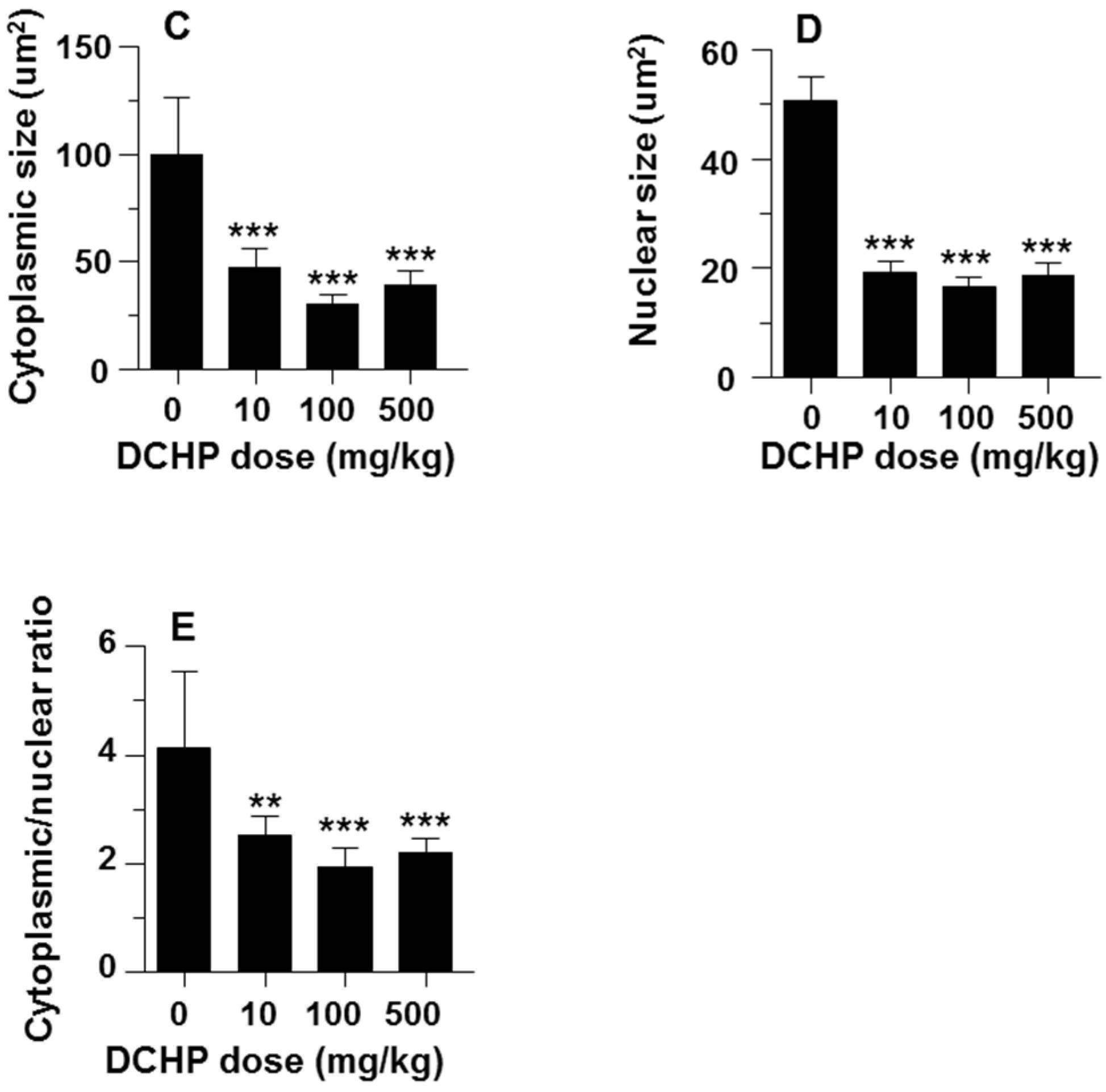
3.3. Fetal Leydig Cell Numbers Per Testis and Leydig Cell Number and Size
3.4. Fetal Leydig cell distribution
3.5. Intratesticular Testosterone Level
3.6. Testicular Cell Gene Expression
3.7. Protein Levels of INSL3 and HSD3B1
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chu, Y.; Huang, Y.; Hardy, D.O.; Lin, S.; Ge, R.S. Structure-dependent inhibition of human and rat 11beta-hydroxysteroid dehydrogenase 2 activities by phthalates. Chem. Biol. Interact. 2010, 183, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Garcia, J.M.; Lin, H.; Wang, Y.; Yan, P.; Wang, L.; Tan, Y.; Luo, J.; Qiu, Z.; et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS ONE 2014, 9, e87430. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Aydogan Ahbab, M.; Barlas, N. Developmental effects of prenatal di-n-hexyl phthalate and dicyclohexyl phthalate exposure on reproductive tract of male rats: Postnatal outcomes. Food Chem. Toxicol. 2013, 51, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, N.; Iwai, M.; Okazaki, Y. A two-generation reproductive toxicity study of dicyclohexyl phthalate in rats. J. Toxicol. Sci. 2005, 30, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhao, B.; Li, X.W.; Hu, G.X.; Su, Y.; Chu, Y.; Akingbemi, B.T.; Lian, Q.Q.; Ge, R.S. Effects of phthalates on 3 beta-hydroxysteroid dehydrogenase and 17 beta-hydroxysteroid dehydrogenase 3 activities in human and rat testes. Chem. Biol. Interact. 2012, 195, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Gallissot, F.; Sabate, J.P. Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. J. Appl. Toxicol. 2009, 29, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Macpherson, S.; Marchetti, N.; Sharpe, R.M. Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 2003, 18, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.X.; Lian, Q.Q.; Ge, R.S.; Hardy, D.O.; Li, X.K. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol. Metab. 2009, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Skakkebaek, N.E. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil. Steril. 2008, 89 (Suppl. 2), e33–e38. [Google Scholar] [CrossRef] [PubMed]
- Mahood, I.K.; Hallmark, N.; McKinnell, C.; Walker, M.; Fisher, J.S.; Sharpe, R.M. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di(n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology 2005, 146, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ge, R.S.; Chen, G.R.; Hu, G.X.; Dong, L.; Lian, Q.Q.; Hardy, D.O.; Sottas, C.M.; Li, X.K.; Hardy, M.P. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. USA 2008, 105, 7218–7222. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.G.; Servos, G.; Tran, N. Structural and histological analysis of leydig cell steroidogenic function. In The Leydig Cell in Health and Disease; Payne, A.H., Hardy, M.P., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 33–45. [Google Scholar]
- Virtanen, I.; Kallajoki, M.; Narvanen, O.; Paranko, J.; Thornell, L.E.; Miettinen, M.; Lehto, V.P. Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat. Record 1986, 215, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, H.; Su, Z.; Chen, B.; Wang, G.; Wang, C.Q.; Xu, Y.; Ge, R.S. Comparison of cell types in the rat Leydig cell lineage after ethane dimethanesulfonate treatment. Reproduction 2013, 145, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.S.; Dong, Q.; Niu, E.M.; Sottas, C.M.; Hardy, D.O.; Catterall, J.F.; Latif, S.A.; Morris, D.J.; Hardy, M.P. 11b-Hydroxysteroid dehydrogenase 2 in rat Leydig cells: Its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology 2005, 146, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Adham, I.M.; Emmen, J.M.; Engel, W. The role of the testicular factor INSL3 in establishing the gonadal position. Mol. Cell. Endocrinol. 2000, 160, 11–16. [Google Scholar] [CrossRef]
- Tran, N.; Haider, S.G. Ultrastructure of cell contacts of fetal and adult Leydig cells in the rat: A systematic study from birth to senium. Anat. Embryol. (Berl). 2006, 211, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Mahood, I.K.; Scott, H.M.; Brown, R.; Hallmark, N.; Walker, M.; Sharpe, R.M. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: Comparison of fetal and adult end points and their dose sensitivity. Environ. Health Perspect. 2007, 115 (Suppl. 1), 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lian, Q.Q.; Hu, G.X.; Jin, Y.; Zhang, Y.; Hardy, D.O.; Chen, G.R.; Lu, Z.Q.; Sottas, C.M.; Hardy, M.P.; et al. In utero and lactational exposures to diethylhexyl-phthalate affect two populations of leydig cells in male long-evans rats. Biol. Reprod 2009, 80, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Mahood, I.K.; McKinnell, C.; Walker, M.; Hallmark, N.; Scott, H.; Fisher, J.S.; Rivas, A.; Hartung, S.; Ivell, R.; Mason, J.I.; et al. Cellular origins of testicular dysgenesis in rats exposed in utero to di(n-butyl) phthalate. Int. J. Androl. 2006, 29, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.S.; Lambright, C.; Furr, J.; Ostby, J.; Wood, C.; Held, G.; Gray, L.E., Jr. Phthalate ester-induced gubernacular lesions are associated with reduced INSL3 gene expression in the fetal rat testis. Toxicol. Lett. 2004, 146, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.M.; Hutchison, G.R.; Mahood, I.K.; Hallmark, N.; Welsh, M.; De Gendt, K.; Verhoeven, G.; O’Shaughnessy, P.; Sharpe, R.M. Role of androgens in fetal testis development and dysgenesis. Endocrinology 2007, 148, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I.; Pelliniemi, L.J. Fetal Leydig cells: Cellular origin, morphology, life span, and special functional features. Proc. Soc. Exp. Biol. Med. 1992, 201, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Miyabayashi, K.; Haraguchi, S.; Arakawa, T.; Otake, H.; Baba, T.; Matsuzaki, S.; Shishido, Y.; Akiyama, H.; Tachibana, T.; et al. Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 2013, 27, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Anand-Ivell, R.; Heng, K.; Hafen, B.; Setchell, B.; Ivell, R. Dynamics of INSL3 peptide expression in the rodent testis. Biol. Reprod. 2009, 81, 480–487. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, C.; Sharpe, R.M.; Mahood, K.; Hallmark, N.; Scott, H.; Ivell, R.; Staub, C.; Jegou, B.; Haag, F.; Koch-Nolte, F.; et al. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di(n-Butyl) phthalate. Endocrinology 2005, 146, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Kai, H.; Suita, S.; Nawata, H. Time-specific effects of mono-n-butyl phthalate on the transabdominal descent of the testis in rat fetuses. BJU Int. 2000, 86, 121–125. [Google Scholar] [CrossRef] [PubMed]
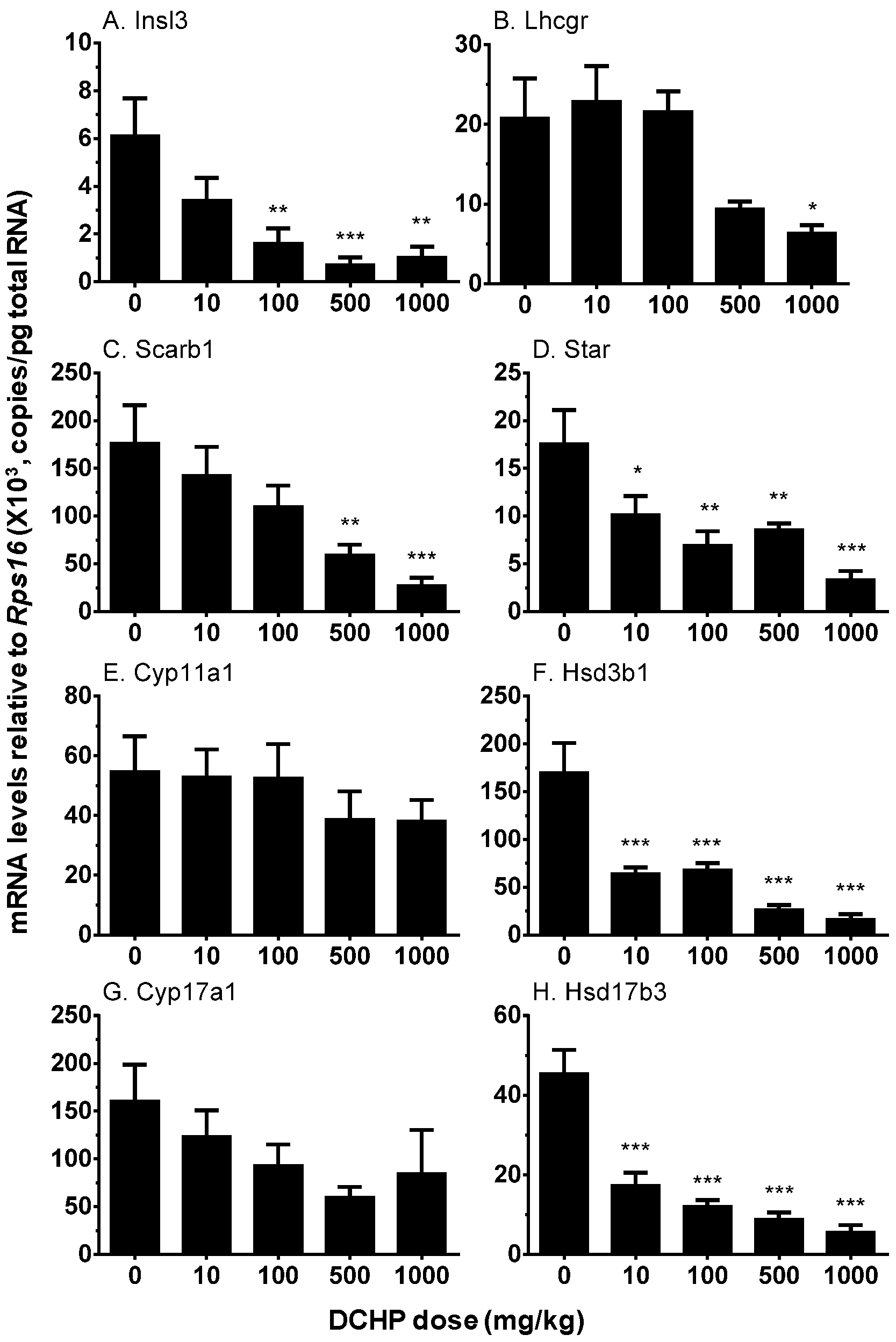
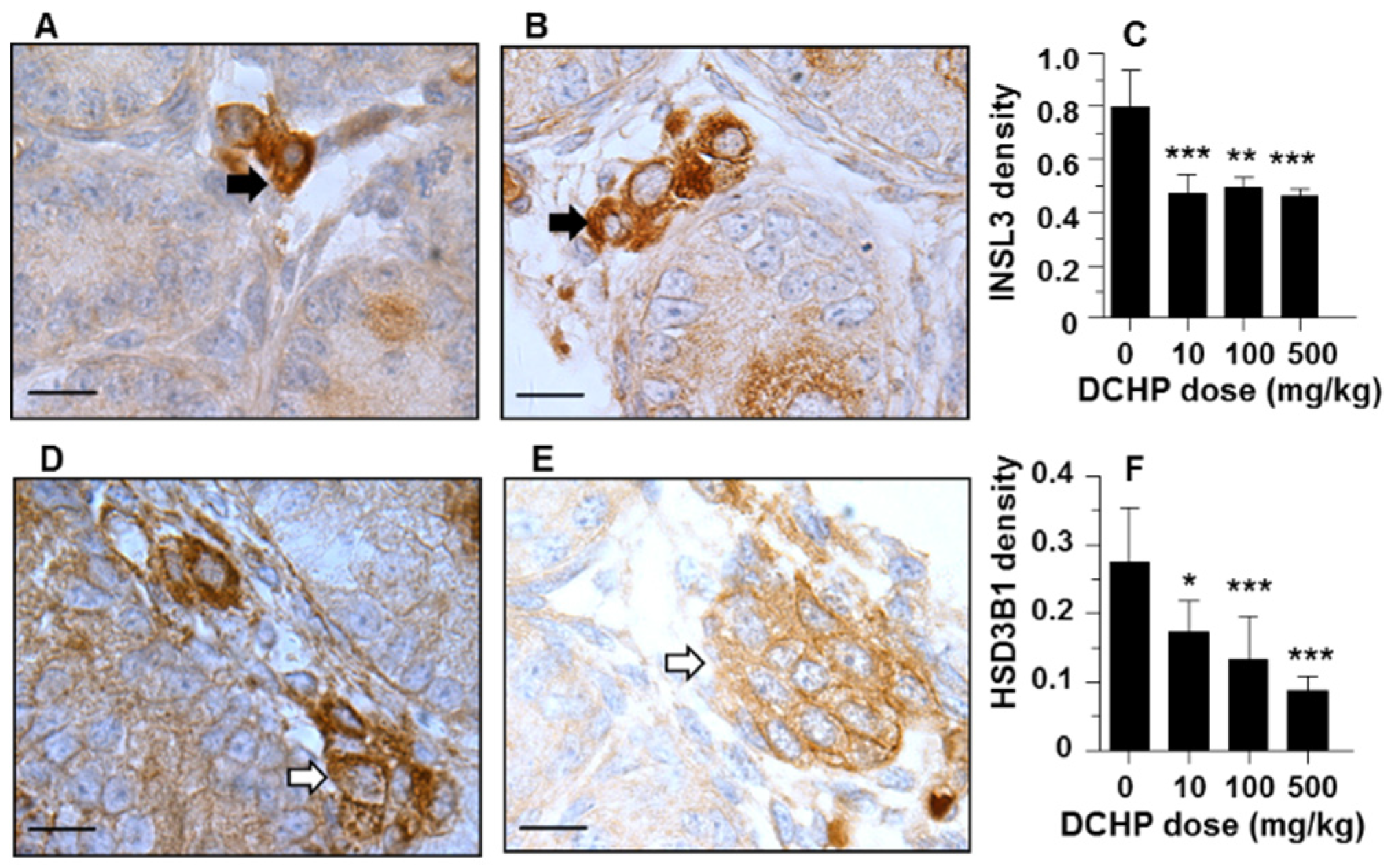
| Parameters | DCHP, mg/kg Per Day | |||
|---|---|---|---|---|
| 0 | 10 | 100 | 500 | |
| Number of dams | 6 | 6 | 6 | 6 |
| Litter size | 13 ± 1 | 13 ± 3 | 16 ± 1 | 12 ± 3 |
| Birth rate | 6/6 | 6/6 | 6/6 | 6/6 |
| Pup male, % | 49 ± 12 | 52 ± 15 | 59 ± 11 | 49 ± 9 |
| Number of pups | 37 | 44 | 51 | 30 |
| Body weight, g | 7.7 ± 0.7 | 6.5 ± 0.4 *** | 6.4 ± 0.9 *** | 6.5 ± 0.7 *** |
| AGD, mm | 3.3 ± 0.3 | 3.0 ± 0.5 | 2.7 ± 0.2 * | 2.6 ± 0.2 * |
| Parameters | DCHP, mg/kg Per Day | |||
|---|---|---|---|---|
| 0 | 10 | 100 | 500 | |
| Number of testes | 6 | 6 | 6 | 6 |
| Testis dysgenesis | 0/6 a | 0/6 | 1/6 | 3/6 |
| MNGs#/Tubule (%) | 0.37 ± 0.24 | 2.08 ± 0.46 | 15.67 ± 2.70 *** | 27.06 ± 2.90 *** |
| Testicular T, ng/mg | 1.90 ± 0.25 | 1.71 ± 0.35 | 1.18 ± 0.23 * | 0.62 ± 0.14 ** |
| Cell No. Per Cluster | Frequency (%); DCHP, mg/kg Per Day | |||
|---|---|---|---|---|
| 0 | 10 | 100 | 500 | |
| 1–4 | 74 ± 6 | 66 ± 4 *** | 47 ± 6 *** | 42 ± 5 *** |
| 5–8 | 18 ± 5 | 17 ± 2 | 17 ± 4 | 14 ± 5 |
| 9–16 | 7 ± 2 | 11 ± 1 ** | 17 ± 3 *** | 16 ± 2 *** |
| >16 | 1 ± 1 | 5 ± 2 *** | 19 ± 7 *** | 28 ± 6 *** |
| Average | 3 ± 0 | 5 ± 1 *** | 11 ± 3 *** | 13 ± 2 *** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, X.; Hu, G.; Li, L.; Su, H.; Wang, Y.; Chen, D.; Zhu, Q.; Li, C.; Li, J.; et al. Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells. Int. J. Environ. Res. Public Health 2016, 13, 246. https://doi.org/10.3390/ijerph13030246
Li X, Chen X, Hu G, Li L, Su H, Wang Y, Chen D, Zhu Q, Li C, Li J, et al. Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells. International Journal of Environmental Research and Public Health. 2016; 13(3):246. https://doi.org/10.3390/ijerph13030246
Chicago/Turabian StyleLi, Xiaoheng, Xiaomin Chen, Guoxin Hu, Linxi Li, Huina Su, Yiyan Wang, Dongxin Chen, Qiqi Zhu, Chao Li, Junwei Li, and et al. 2016. "Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells" International Journal of Environmental Research and Public Health 13, no. 3: 246. https://doi.org/10.3390/ijerph13030246
APA StyleLi, X., Chen, X., Hu, G., Li, L., Su, H., Wang, Y., Chen, D., Zhu, Q., Li, C., Li, J., Wang, M., Lian, Q., & Ge, R.-S. (2016). Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells. International Journal of Environmental Research and Public Health, 13(3), 246. https://doi.org/10.3390/ijerph13030246





