Ethnic Differences in Maternal Adipokines during Normal Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Subjects
2.3. Data and Blood Sample Collection
2.4. Analytic Procedures
2.4.1. Adipokine Assay
2.4.2. Glucose Homeostasis Measures
2.5. Statistical Analysis
3. Results and Discussion
| Characteristics | All Subjects | African American (n = 602) | Hispanic (n = 777) | Caucasian (n = 255) |
|---|---|---|---|---|
| Age (year.) b | 22.03 ± 5.29 | 21.38 ± 5.02 | 22.19 ± 5.27 | 23.11 ± 5.75 |
| Pre-pregnant BMI (kg/m2) c | 25.73 ± 6.30 | 26.15 ± 7.01 | 25.74 ± 5.88 | 24.72 ± 5.65 |
| Obese (BMI ≥ 30) | 329 (20.13) | 135 (22.43) | 148 (19.05) | 46 (18.04) |
| Sum of skinfolds (mm) | 70.62 ± 29.56 | 72.19 ± 32.45 | 70.33 ± 28.05 | 67.95 ± 26.69 |
| Upper arm fat area (mm) | 28.40 ± 16.24 | 29.74 ± 18.75 | 27.59 ± 14.80 | 27.69 ± 13.79 |
| SBP (mmHg) | 112.20 ± 11.35 | 113.81 ± 11.65 | 110.78 ± 10.90 | 112.74 ± 11.49 |
| DBP (mmHg) | 70.21 ± 8.66 | 71.21 ± 9.07 | 69.56 ± 8.18 | 69.84 ± 8.91 |
| Nulliparous | 640 (39.17) | 225 (37.38) | 311 (40.03) | 104 (40.78) |
| Cigarette smoking b | 310 (18.97) | 117 (19.44) | 95 (12.23) | 98 (38.43) |
3.1. Comparison of Adipokines among Ethnic Groups
| Ethnic Group | Adiponectin (µg/mL) | Resistin (ng/mL) | ||
|---|---|---|---|---|
| Entry | 3rd Trimester | Entry | 3rd Trimester | |
| All subjects | 17.66 ± 0.21 | 14.75 ± 0.20 | 47.49 ± 0.63 | 48.22 ± 0.62 |
| African American | 16.49 ± 0.36 b,c | 13.23 ± 0.29 b,e | 50.32 ± 1.10 e | 50.35 ± 1.09 c |
| Hispanic | 17.90 ± 0.32 d | 15.16 ± 0.33 d | 45.28 ±1.11 d | 46.61 ± 0.96 |
| Caucasian | 19.34 ± 0.57 | 16.72 ± 0.52 | 49.43 ± 1.73 | 49.24 ± 1.72 |
3.2. Association of Ethnicity with Low Adiponectin (Lowest Tertile) and High Resistin (Highest Tertile)
| Low Adiponectin a | Unadjusted n (%) | AOR (95% CI) Model 1 b | AOR (95% CI) Model 2 c |
|---|---|---|---|
| Entry | |||
| African American | 248 (41.2) | 2.07 (1.48, 2.89) | 1.86 (1.32, 2.63) |
| Hispanic | 235 (30.24) | 1.23 (0.90, 1.74) | 1.15 (0.82, 1.61) |
| Caucasian | 66 (25.88) | Reference | Reference |
| 3rd Trimester | |||
| African American | 258 (42.86) | 2.61 (1.85, 3.66) | 2.41 (1.70, 3.41) |
| Hispanic | 230 (29.60) | 1.43 (1.02, 2.00) | 1.34 (0.95, 1.89) |
| Caucasian | 59 (23.14) | Reference | Reference |
| High Resistin a | Unadjusted n (%) | AOR (95% CI) Model 1 b | AOR (95% CI) Model 2 c |
|---|---|---|---|
| Entry | |||
| African American | 227 (37.71) | 1.49 (1.18, 1.87) | 1.47 (1.17, 1.85) |
| Hispanic | 228 (29.34) | Reference | Reference |
| Caucasian | 91 (35.64) | 1.18 (0.87, 1.61) | 1.21 (0.89, 1.65) |
| 3rd Trimester | |||
| African American | 219 (36.38) | 1.31 (1.04, 1.65) | 1.29 (1.03, 1.62) |
| Hispanic | 233 (29.99) | Reference | Reference |
| Caucasian | 94 (36.86) | 1.24 (0.91, 1.69) | 1.28 (0.94, 1.74) |
| Ethnic Group | Unadjusted | AOR (95% CI) | |
|---|---|---|---|
| Entry | n (%) | Model 1 b | Model 2 c |
| African American | 87 (14.45) | 2.55 (1.48, 4.39) | 2.23 (1.28, 3.87) |
| Hispanic | 55 (7.08) | 1.09 (0.62, 1.92) | 1.02 (0.57, 1.80) |
| Caucasian | 18 (7.06) | Reference | Reference |
| 3rd trimester | |||
| African American | 80 (13.29) | 1.89 (1.12, 3.19) | 1.70 (1.01, 2.89) |
| Hispanic | 58 (7.46) | 1.00 (0.58, 1.72) | 0.93 (0.54, 1.61) |
| Caucasian | 20 (7.84) | Reference | Reference |
| Ethnic Group | Unadjusted | AOR (95% CI) | |
|---|---|---|---|
| Entry | n (%) | Model 1 b | Model 2 c |
| African American | 87 (14.45) | 2.34 (1.63, 3.36) | 2.19 (1.52, 3.17) |
| Hispanic | 55 (7.08) | Reference | Reference |
| Caucasian | 18 (7.06) | 0.92 (0.52, 1.62) | 0.98 (0.56, 1.74) |
| 3rd trimester | |||
| African American | 80 (13.29) | 1.89 (1.31, 2.71) | 1.83 (1.27, 2.63) |
| Hispanic | 58 (7.46) | Reference | Reference |
| Caucasian | 20 (7.84) | 1.00 (0.58, 1.72) | 1.07 (0.62, 1.86) |
3.3. The Correlation between Adipokine Levels with Maternal Factors
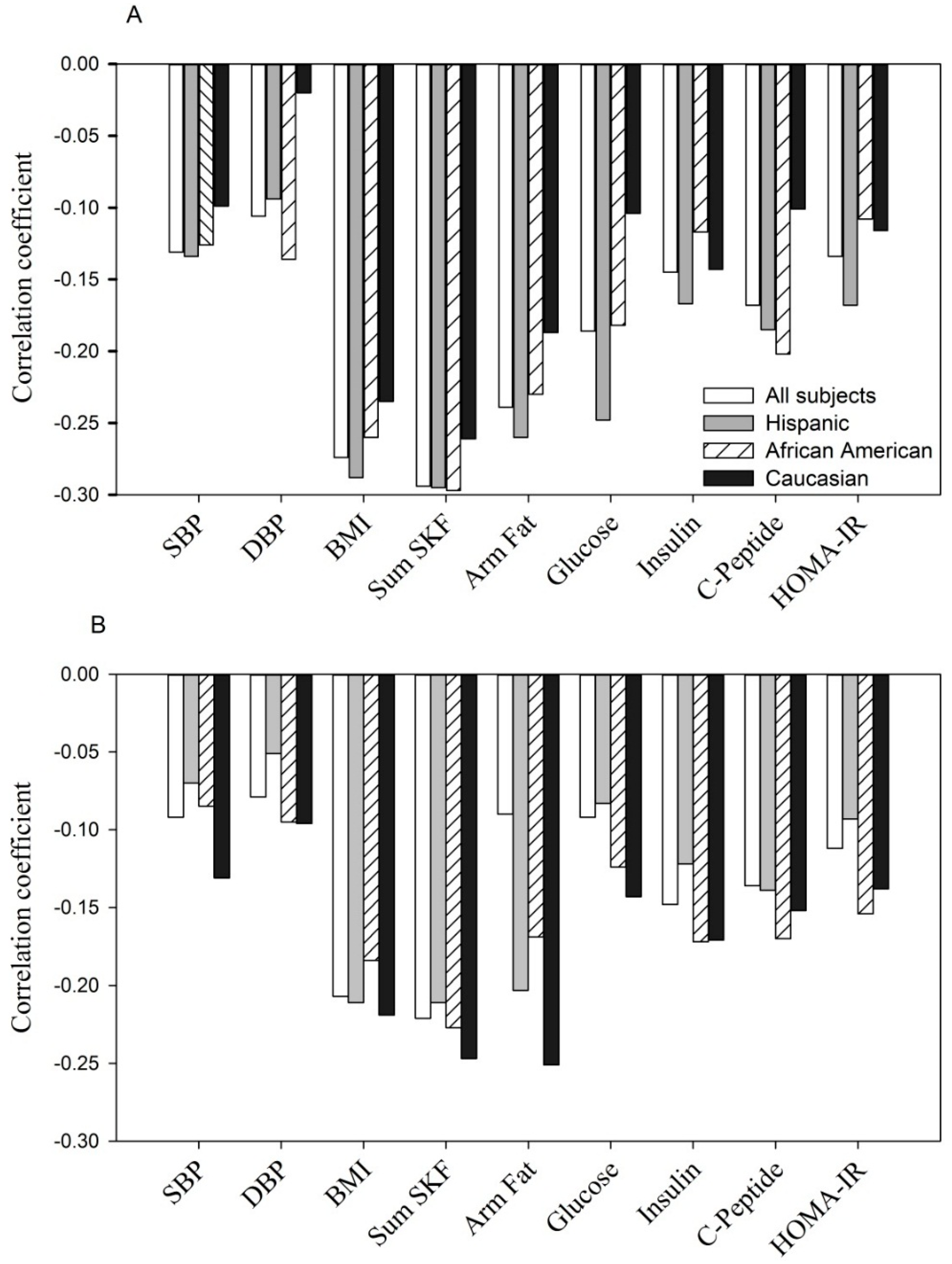
- Negative correlation between adiponectin concentration with selected parameters at entry: p < 0.05 to p < 0.0001 for each of parameters tested in all women, Hispanics and African Americans. In Caucasians, p < 0.05 to p < 0.001 with BMI, sum of skinfolds, arm fat area and insulin; but p > 0.05 for SBP, DBP, C-peptide, glucose and HOMA-IR.
- Negative correlation between adiponectin concentration with selected parameters during the 3rd trimester: p < 0.05 to p < 0.0001 for each of parameters tested in all women, Hispanics, African Americans and Caucasians, except for DBP, fasting glucose in Hispanics (p > 0.05) and DBP, glucose and HOMA-IR in Caucasians (p > 0.05).
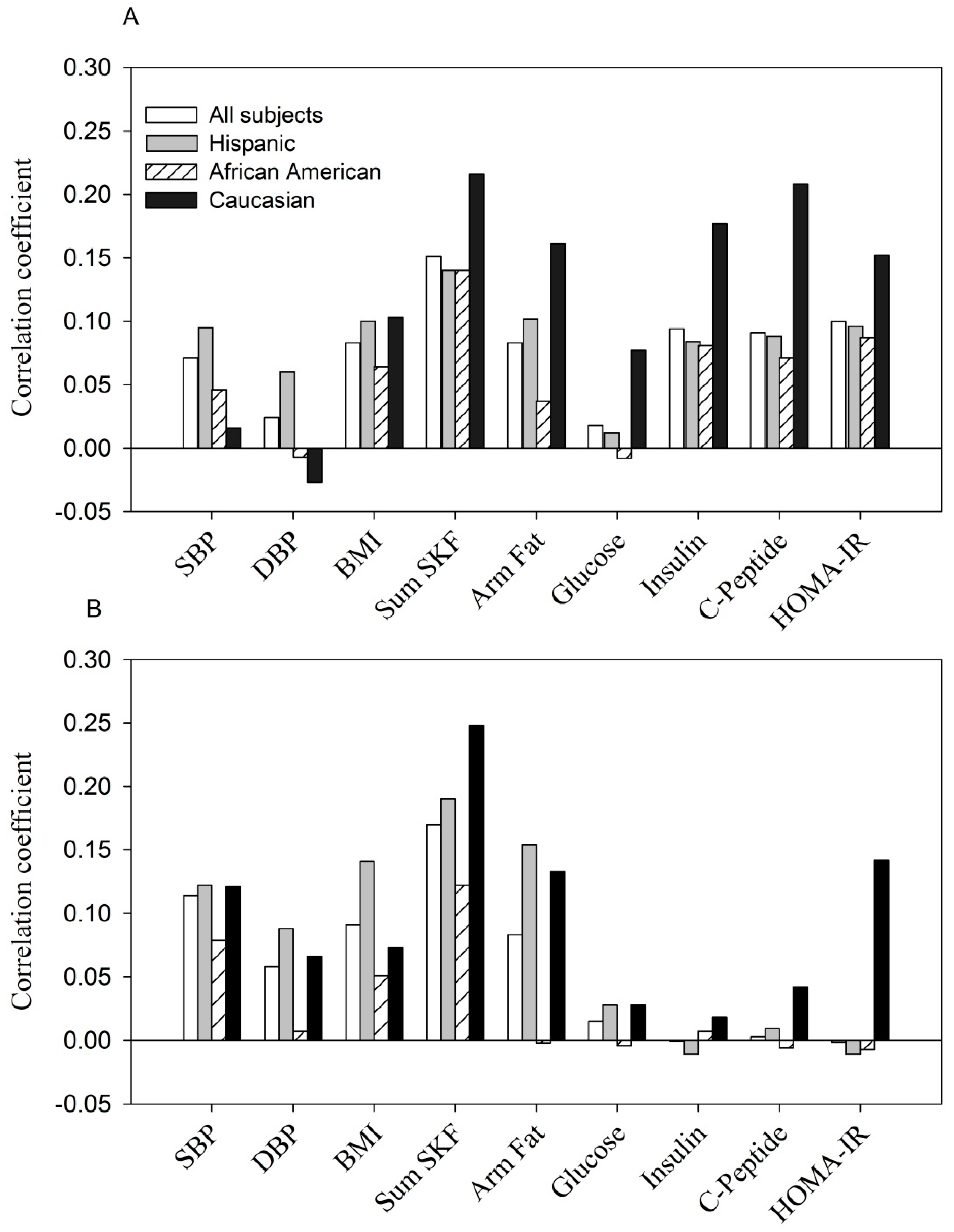
- The positive correlation between resistin concentration with selected parameters at entry: p < 0.05 to p < 0.0001 for each parameter tested in all women, Hispanics and Caucasians, except for DBP, fasting glucose in all women and Hispanics (p > 0.05); for SBP, DBP, BMI and fasting glucose in Caucasians (p > 0.05). The correlations were all non-significant in African Americans except for sum of skinfolds (p < 0.01).
- The positive correlation between resistin concentration with selected parameters at the 3rd trimester: p < 0.05 to p < 0.0001 for SBP, DBP, BMI, sum of skinfolds and arm fat area in all women and Hispanics; p < 0.01 for sum of skinfolds in African Americans; p < 0.001 and p < 0.05 for sum of skinfolds and arm fat area in Caucasians. Other parameters were all non-significant.
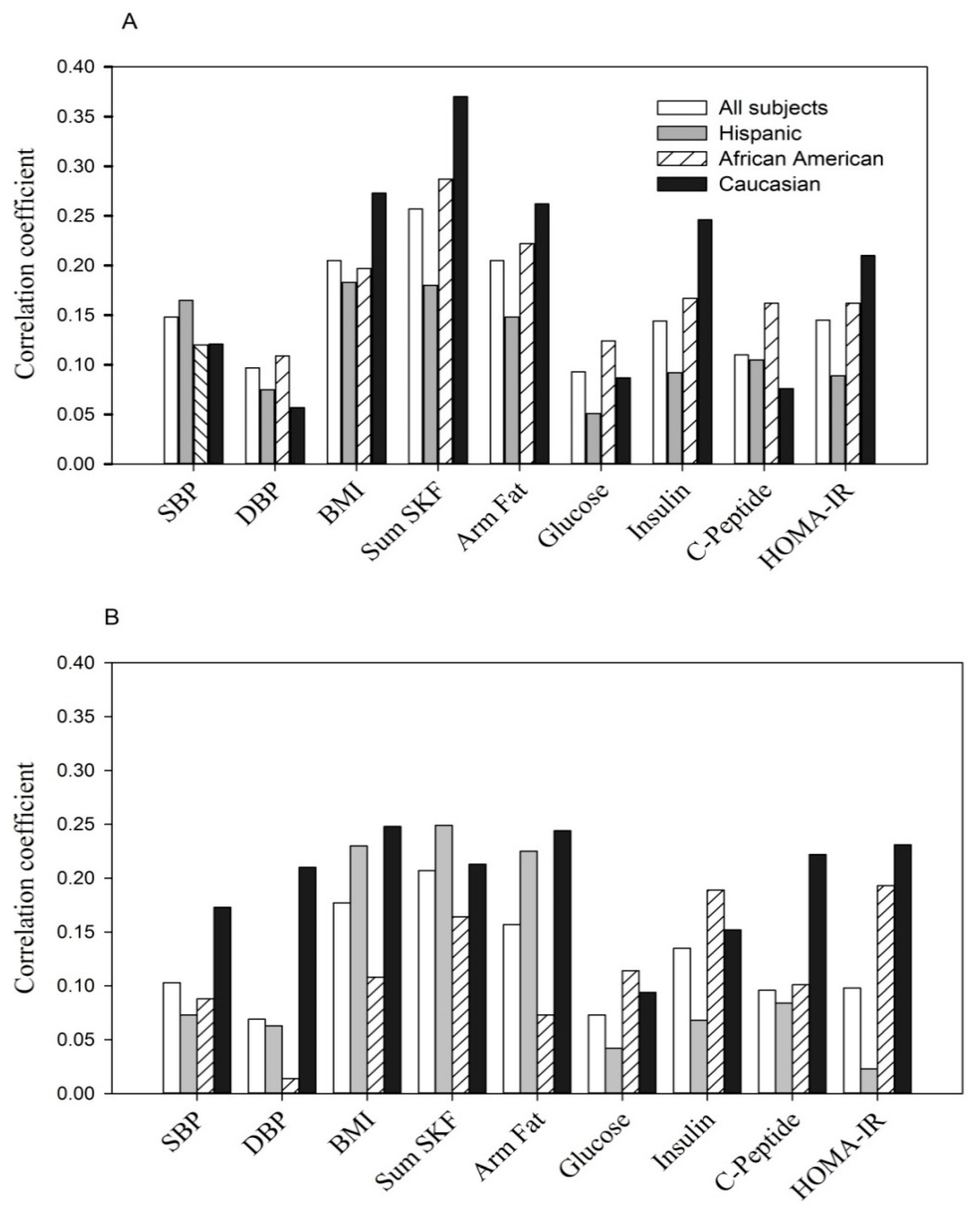
- The positive correlation at entry: p < 0.05 to p < 0.0001 for each of parameters except fasting glucose in Hispanics (p > 0.05) and DBP, fasting glucose and C-peptide in Caucasians (p > 0.05).
- The positive correlation at the 3rd trimester: p < 0.05 to p < 0.0001 for each of parameters with the exception of DBP, fasting glucose and insulin in Hispanics (p > 0.05), DBP and arm fat area in African Americans (p > 0.05) and fasting glucose in Caucasians (p > 0.05).
3.4. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trujillo, M.E.; Scherer, P.E. Adiponectin—Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Scherer, P.E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2013, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Song, Y.; Ding, E.L.; Roberts, C.K.; Manson, J.E.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Circulating levels of resistin and risk of type 2 diabetes in men and women: Results from two prospective cohorts. Diabetes Care 2009, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Menzaghi, C.; Bacci, S.; Salvemini, L.; Mendonca, C.; Palladino, G.; Fontana, A.; de Bonis, C.; Marucci, A.; Goheen, E.; Prudente, S.; et al. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PLoS ONE 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.F.; Sullivan, L.M.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B., Sr.; Wilson, P.W.; Meigs, J.B. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J. Clin. Endocrinol. Metab. 2008, 93, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Kaplan, R.C.; Wassertheil-Smoller, S.; Cushman, M.; Rohan, T.E.; McGinn, A.P.; Wang, T.; Strickler, H.D.; Scherer, P.E.; Mackey, R.; et al. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: Results from the Women’s Health Initiative. Stroke 2011, 42, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.C.; Zern, T.L.; Taksali, S.E.; Dziura, J.; Cali, A.M.; Wollschlager, M.; Seyal, A.A.; Weiss, R.; Burgert, T.S.; Caprio, S. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 13, 1–7. [Google Scholar]
- Cossrow, N.; Falkner, B. Race/ethnic issues in obesity and obesity-related comorbidities. J. Clin. Endocrinol. Metab. 2004, 89, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.J.G.; Bowden, D.; Wagenknecht, L.E.; Balasubramanyam, A.; Langfeld, C.; Saad, M.F.; Rotter, J.I.; Guo, X.Q.; Chen, Y.D.I.; Bryer-Ash, M.; et al. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic hispanics and african-americans. J. Clin. Endocrinol. Metab. 2007, 92, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.A. Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr. Rev. 2007, 65, S234–S238. [Google Scholar] [CrossRef] [PubMed]
- Donin, A.S.; Nightingale, C.M.; Owen, C.G.; Rudnicka, A.R.; McNamara, M.C.; Prynne, C.J.; Stephen, A.M.; Cook, D.G.; Whincup, P.H. Ethnic differences in blood lipids and dietary intake between UK children of black African, black Caribbean, South Asian, and white European origin: The Child Heart and Health Study in England (CHASE). Am. J. Clin. Nutr. 2010, 92, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O. Ethnic differences in C-peptide/insulin/glucose dynamics in young pregnant women. J. Clin. Endocrinol. Metab. 2002, 87, 4642–4646. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O.; Stein, T.P. Hypoadiponectinemia: Association with risk of varying degrees of gestational hyperglycemia and with maternal ethnicity. JDM 2012, 2, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Nien, J.K.; Mazaki-Tovi, S.; Romero, R.; Erez, O.; Kusanovic, J.P.; Gotsch, F.; Pineles, B.L.; Gomez, R.; Edwin, S.; Mazor, M.; et al. Adiponectin in severe preeclampsia. J. Perinat. Med. 2007, 35, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Darbinian, J.A.; Ferrara, A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr. Perinat. Epidemiol. 2010, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Savitz, D.A.; Stein, C.R.; Engel, S.M. Maternal ethnicity and pre-eclampsia in New York City, 1995–2003. Paediatr. Perinat. Epidemiol. 2012, 26, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Income, Earnings, and Poverty Data from the 2005 American Community Survey. 2006. Available online: http://www.census.gov/prod/2006pubs/acs-02.pdf (accessed on 6 August 2015).
- Hediger, M.L.; Scholl, T.O.; Schall, J.I.; Healey, M.F.; Fischer, R.L. Changes in maternal upper arm fat stores are predictors of variation in infant birth weight. J. Nutr. 1994, 124, 24–30. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Huston-Presley, L.; Kalhan, S.C.; Catalano, P.M. Clinically useful estimates of insulin sensitivity during pregnancy: Validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001, 24, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association: Clinical Practice Recommendations 2000: Position Statement. Diabetes Care 2000, 23, S77–S79.
- Smith, L.M.; Yao-Borengasser, A.; Starks, T.; Tripputi, M.; Kern, P.A.; Rasouli, N. Insulin resistance in African-American and Caucasian women: Differences in lipotoxicity, adipokines, and gene expression in adipose tissue and muscle. J. Clin. Endocrinol. Metab. 2010, 95, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Nyomba, B.L.; Leslie, W.D.; Murphy, L.J. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care 2007, 30, 286–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mente, A.; Razak, F.; Blankenberg, S.; Vuksan, V.; Davis, A.D.; Miller, R.; Teo, K.; Gerstein, H.; Sharma, A.M.; Yusuf, S.; et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 2010, 33, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Deliveliotou, A.; Dimitrakaki, A.; Hassiakos, D.; Panoulis, C.; Deligeoroglou, E.; Creatsas, G.K. Maternal serum resistin concentrations in gestational diabetes mellitus and normal pregnancies. J. Obstet. Gynaecol. Res. 2011, 37, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Vitoratos, N.; Dimitrakaki, A.; Vlahos, N.F.; Gregoriou, O.; Panoulis, K.; Christopoulos, P.; Creatsas, G. Maternal and umbilical resistin levels do not correlate with infant birth weight either in normal pregnancies and or in pregnancies complicated with gestational diabetes. J. Matern. Fetal Neonatal Med. 2010, 23, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Megia, A.; Vendrell, J.; Gutierrez, C.; Sabate, M.; Broch, M.; Fernandez-Real, J.M.; Simon, I. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur. J. Endocrinol. 2008, 158, 173–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lowe, L.P.; Metzger, B.E.; Lowe, W.L., Jr.; Dyer, A.R.; McDade, T.W.; McIntyre, H.D.; HAPO Study Cooperative Research Group. Inflammatory mediators and glucose in pregnancy: Results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J. Clin. Endocrininol. Metab. 2010, 95, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O. Maternal biomarkers of endothelial dysfunction and preterm delivery. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Hendler, I.; Blackwell, S.C.; Mehta, S.H.; Whitty, J.E.; Russell, E.; Sorokin, Y.; Cotton, D.B. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 979–983. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Scholl, T.O. Ethnic Differences in Maternal Adipokines during Normal Pregnancy. Int. J. Environ. Res. Public Health 2016, 13, 8. https://doi.org/10.3390/ijerph13010008
Chen X, Scholl TO. Ethnic Differences in Maternal Adipokines during Normal Pregnancy. International Journal of Environmental Research and Public Health. 2016; 13(1):8. https://doi.org/10.3390/ijerph13010008
Chicago/Turabian StyleChen, Xinhua, and Theresa O. Scholl. 2016. "Ethnic Differences in Maternal Adipokines during Normal Pregnancy" International Journal of Environmental Research and Public Health 13, no. 1: 8. https://doi.org/10.3390/ijerph13010008
APA StyleChen, X., & Scholl, T. O. (2016). Ethnic Differences in Maternal Adipokines during Normal Pregnancy. International Journal of Environmental Research and Public Health, 13(1), 8. https://doi.org/10.3390/ijerph13010008






