Chronic Exposure to Static Magnetic Fields from Magnetic Resonance Imaging Devices Deserves Screening for Osteoporosis and Vitamin D Levels: A Rat Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Exposure Procedure
2.2. Biochemistry Analysis
2.3. Electron Microscopy and FESEM Characterization

2.4. Transmission Electron Microscopy
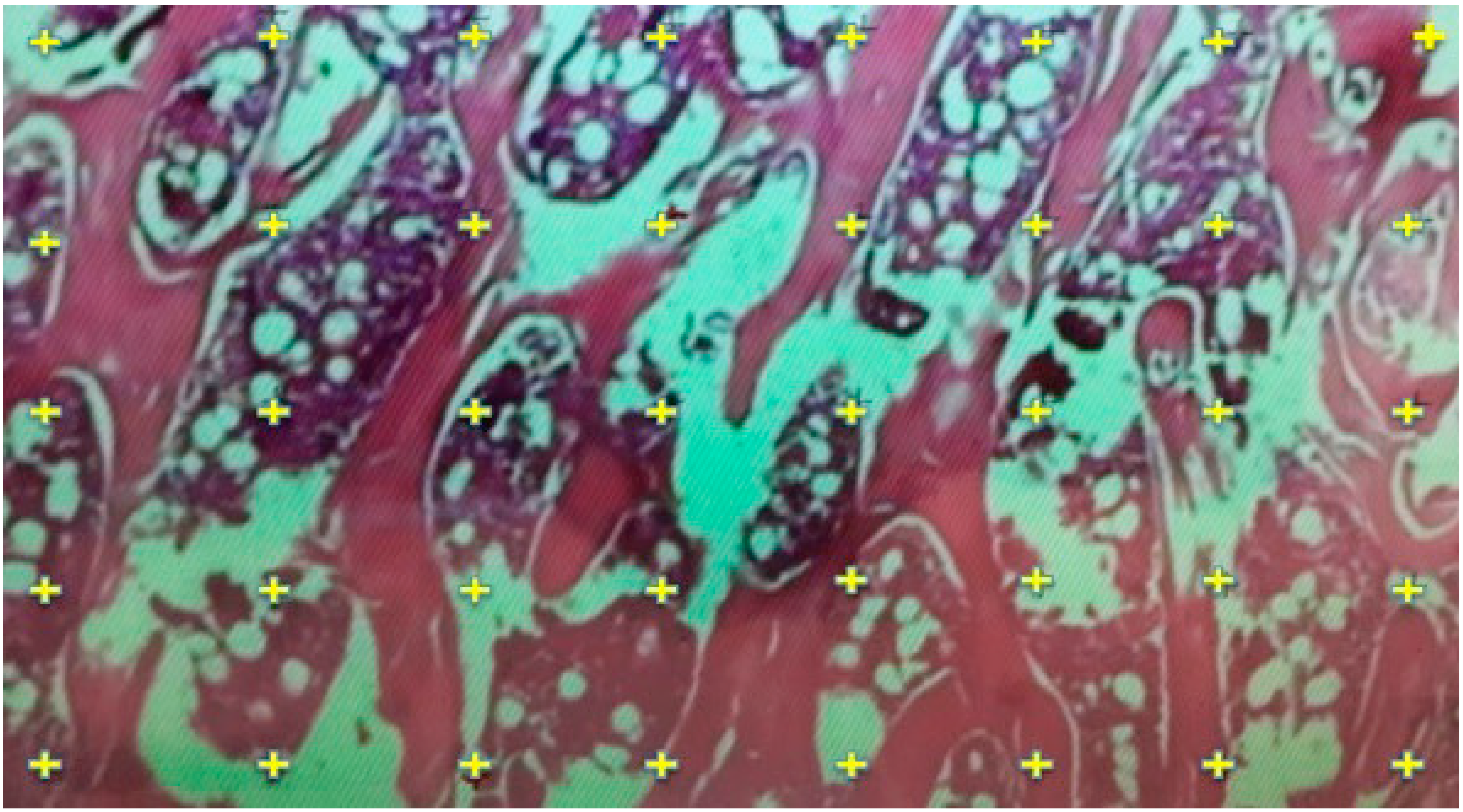
2.5. Histological Evaluation
2.6. Histomorphometric Evaluation for Trabecular Thickness and Trabeculae Number
2.7. TUNEL Analysis for Apoptosis Measurements
2.8. Statistical Analysis
3. Results
| Measured Variables | Group A | Group B | Group C | p Value |
|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ||
| (Min–Max) | (Min–Max) | (Min–Max) | ||
| Vitamin D (pg/mL) | 38.7 ± 20.2 | 97.5 ± 10.2 | 82.2 ± 13.8 | 0.002 |
| (17.4–73.2) | (78.6–109.7) | (64.6–95.7) | ||
| A. phosphatase (mIU/mL) | 1 ± 0.97 | 0.9 ± 0.2 | 0.8 ± 0.2 | n.s |
| (0.12–3.11) | (0.4–1.5) | (0.5–1) | ||
| Calcium ( mg/dL) | 8.9 ± 0.7 | 9 ± 0.2 | 9 ± 0.7 | n.s |
| (7.8–9.7) | (8.7–9.1) | (7.8–9.9) | ||
| Phosphorus (mg/dL) | 7.4 ± 0.8 | 7.1 ± 0.6 | 7.3 ± 1.3 | n.s |
| (6.4–8.5) | (6.3–7.7) | (6.1–9.6) | ||
| Cortical thickness (µm) | 156.9 ± 30.4 | 254.9 ± 27.7 | 293.7 ± 26.6 | 0.001 |
| (103.6–186.8) | (224–283.8) | (262.8–327.2) | ||
| Trabecular thickness (µm) | 39.7 ± 4.1 | 60.1 ± 8.7 | 61 ± 8.3 | 0.003 |
| (10.6–22.9) | (51.1–71.5) | (50.2–72.4) | ||
| Trabeculae n/mm2 | 4.4 ± 0.5 | 5.6 ± 0.3 | 5.6 ± 0.4 | 0.003 |
| (3.7–4.9) | (5.3–6) | (5.2–6.1) | ||
| Apoptotosis (%) | 41.1 ± 2.8 | 26.4 ± 4.7 | 4.1 ± 1.8 | 0.005 |
| (37.8–44.1) | (20.3–32.1) | (1.8–6.1) |
3.1. Biochemistry Analysis
3.2. Electron Microscopy and FESEM Results
3.3. Transmission Electron Microscopy Results
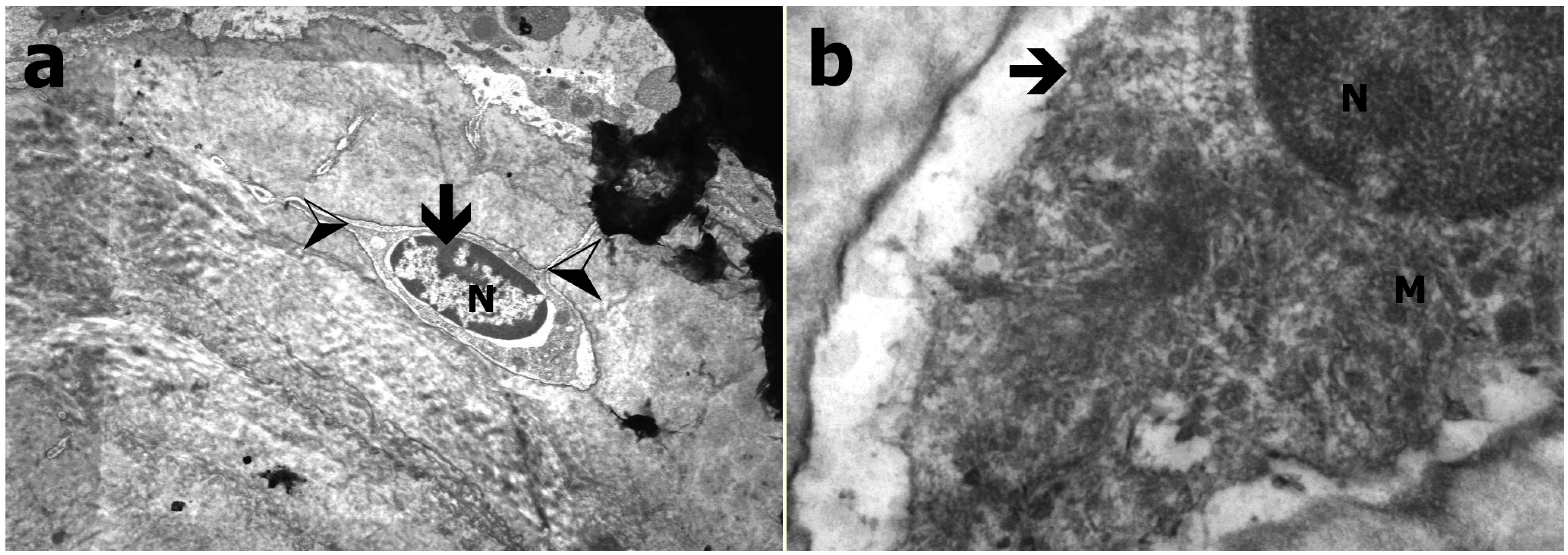
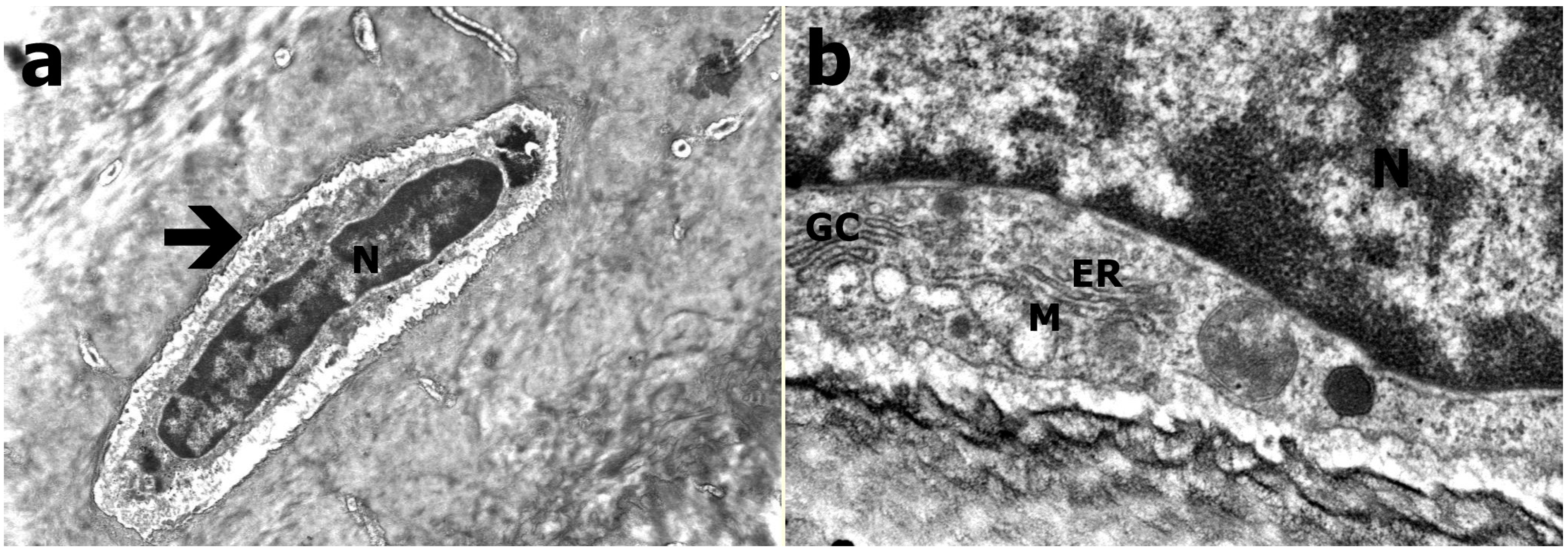
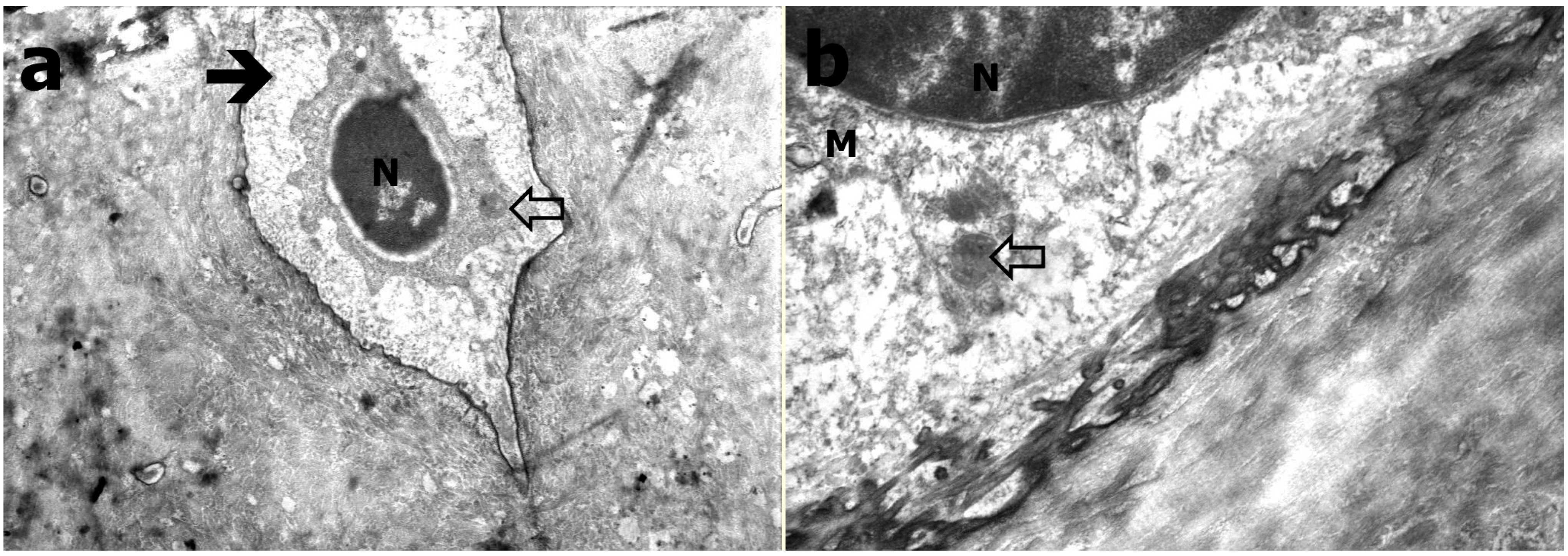
3.4. Results of Histological Evaluation
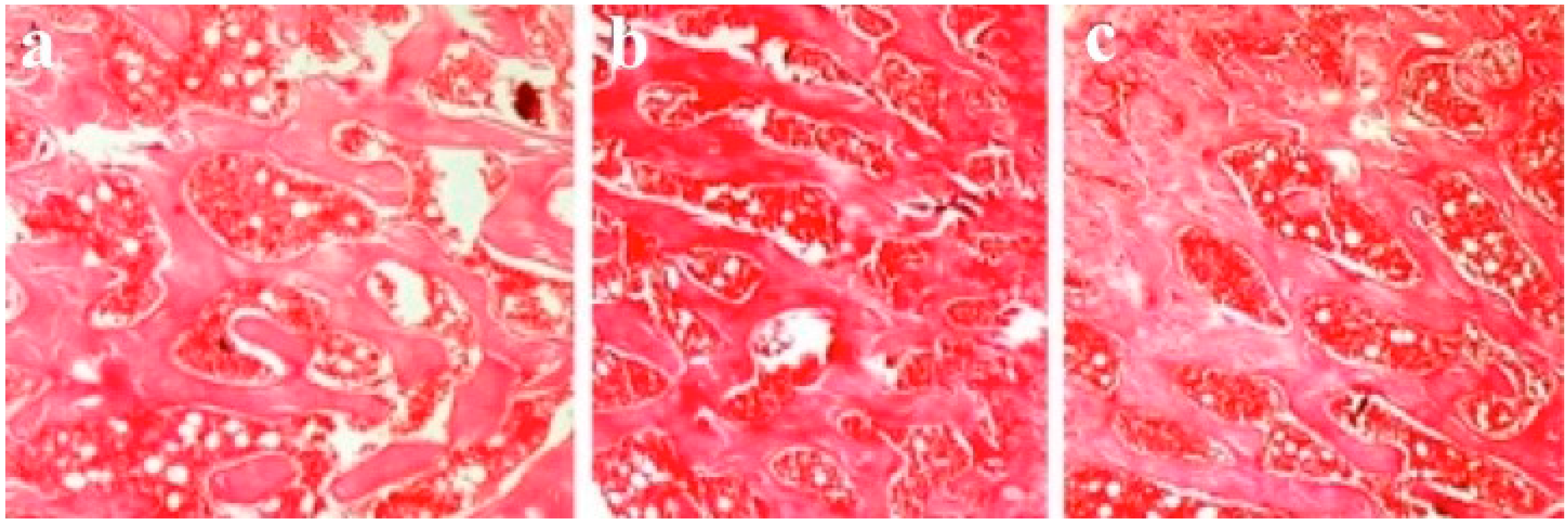
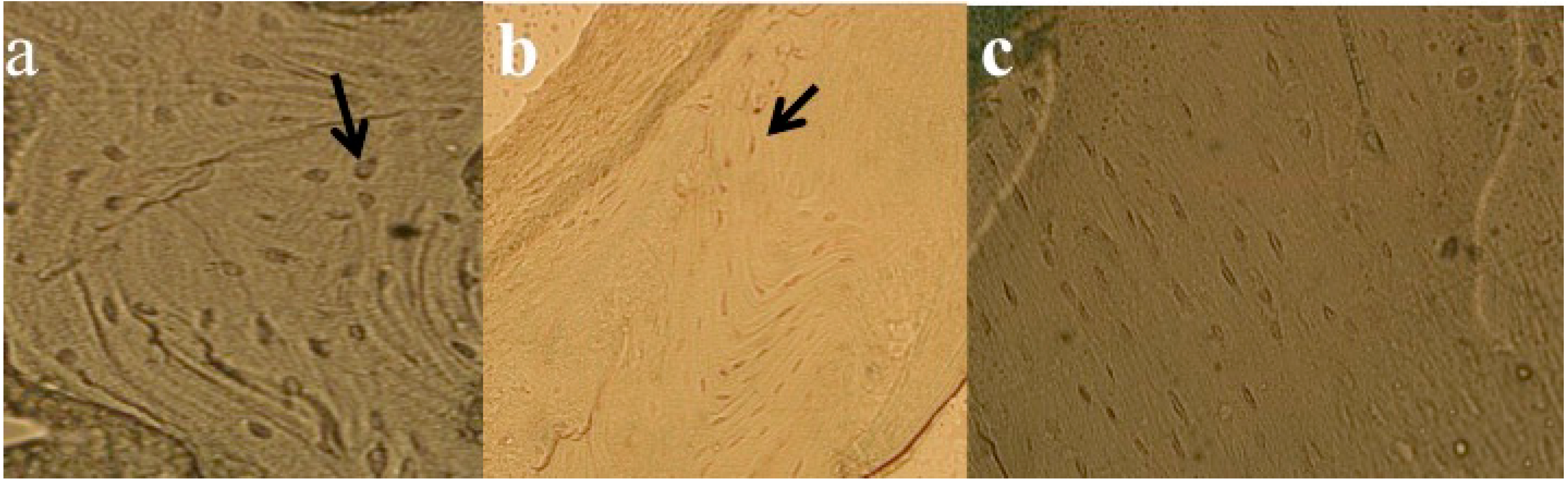
3.5. Results of Histomorphometric Evaluation for Trabecular Thickness and Trabeculae Number
3.6. Results of TUNEL Analysis for Apoptosis Measurements
3.7. Correlations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hansson, M.K.; Alanko, T.; Decat, G.; Falsaperla, R.; Gryz, K.; Hietanen, M.; Karpowicz, J.; Rossi, P.; Sandström, M. Exposure of workers to electromagnetic fields. A review of open questions on exposure assessment techniques. Int. J. Occup. Saf. Ergon. 2009, 15, 3–33. [Google Scholar]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Lombardi, M.; Landini, L.; Simi, S. Biological effects and safety in magnetic resonance imaging: A review. Int. J. Environ. Res. Public Health 2009, 6, 1778–1798. [Google Scholar] [CrossRef] [PubMed]
- Karpowicz, J.; Gryz, K. Health risk assessment of occupational exposure to a magnetic field from magnetic resonance imaging devices. Int. J. Occup. Saf. Ergon. 2006, 12, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mc Robbie, D.W. Occupational exposure in MRI. Brit. J. Radiol. 2012, 85, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Riches, S.F.; Collins, D.J.; Charles-Edwards, G.D.; Shafford, J.C.; Cole, J.; Keevil, S.F.; Leach, M.O. Measurements of occupational exposure to switched gradient and spatially-varying magnetic fields in areas adjacent to 1.5 T clinical MRI systems. J. Magn. Reson. Imaging 2007, 26, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shang, P. A review of bio-effects of static magnetic field on rodent models. Prog. Biophys. Mol. Biol. 2014, 114, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, C.; Ren, L.; Zhou, Y.; Shang, P. The effects of static magnetic fields on bone. Prog. Biophys. Mol. Biol. 2014, 114, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Hadjiargyrou, M.; Di Masi, E.; Meng, Y.; Simon, M.; Tan, Z.; Rafailovich, M.H. The role of moderate static magnetic fields on biomineralization of osteoblasts on sulfonated polystyrene films. Biomaterials 2011, 32, 7831–7838. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.H.; Ou, K.L.; Lee, S.Y.; Lin, C.T.; Chang, W.J.; Chen, C.C.; Huang, H.M. Static magnetic fields promote osteoblast-like cells differentiation via increasing the membrane rigidity. Ann. Biomed. Eng. 2007, 35, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Marcacci, M.; Tampieri, A. Evaluation of the effects of a moderate intensity static magnetic field application on human osteoblast-like cells. Am. J. Biomed. Eng. 2012, 2, 263–268. [Google Scholar] [CrossRef]
- Jing, D.; Cai, J.; Shen, G.; Huang, J.; Li, F.; Li, J.; Lu, L.; Luo, E.; Xu, Q. The preventive effects of pulsed electromagnetic fields on diabetic bone loss in streptozotocin-treated rats. Osteoporos. Int. 2011, 22, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Cai, J.; Wu, Y.; Shen, G.; Li, F.; Xu, Q.; Xie, K.; Tang, C.; Liu, J.; Guo, W.; Wu, X.; Jiang, M.; Luo, E. Pulsed electromagnetic fields partially preserve bone mass, micro-architecture, and strength by promoting bone formation in hind limb-Suspended Rats. J. Bone Miner. Res. 2014, 29, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, S.; Cho, Y.; Leesungbok, R. The effect of magnetic fields produced by a neodymium magnet (inserted in the implant abutment) on the osteoblast activity. Bone 2009, 44, 302–303. [Google Scholar] [CrossRef]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Russo, A.; Sartori, M.; Giavaresi, G.; Sandri, M.; Fini, M.; Maltarello, M.C.; Shelyakova, T.; Ortolani, A.; Visani, A.; et al. Modifying bone scaffold architecture in vivo with permanent magnets to facilitate fixation of magnetic scaffolds. Bone 2013, 56, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Terashima, S.; Miyakoshi, J. Enhanced secretion of prostaglandin E2 from osteoblasts by exposure to a strong static magnetic field. Bioelectromagnetics 2008, 29, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shafieisarvestani, Z.; Bighamsadegh, A. Bone healing enhancement with bone marrow transplantation and static magnetic field in rabbit model. Bone 2011, 48. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kanai, S. Efficacy of static magnetic field for locomotor activity of experimental osteopenia. Evid. Based Compl. Alt. 2007, 4, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kanai, S.; Kawamoto, M.; Endo, H.; Higashino, H. Study on application of static magnetic field for adjuvant arthritis rats. Evid. Based Compl. Alt. 2004, 1, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Okano, H.; Tomita, N.; Ikada, Y. Recovery effects of a 180 mT static magnetic field on bone mineral density of osteoporotic lumbar vertebrae in ovariectomized rats. Evid. Based Compl. Alt. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Jagt, O.P.; van der Linden, J.C.; Waarsing, J.H.; Verhaar, J.A.; Weinans, H. Electromagnetic fields do not affect bone micro-architecture in osteoporotic rats. Bone Joint Res. 2014, 3, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.C.; Tomita, N.; Ikada, Y. Effects of static magnetic field on bone formation of rat femurs. Med. Eng. Phys. 1998, 20, 397–402. [Google Scholar] [CrossRef]
- Gul, O.; Atik, O.S.; Erdogan, D.; Goktas, G.; Elmas, C. Transmission and scanning electron microscopy confirm that bone microstructure is similar in osteopenic and osteoporotic patients. Eklem Hast. Cerrahisi 2013, 24, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Yang, J.C.; Lee, S.Y.; Chen, C.A.; Lin, C.T.; Chen, C.C.; Huang, H.M. The role of the calmodulin-dependent pathway in static magnetic field induced mechanotransduction. Bioelectromagnetics 2010, 31, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.M.; Lee, S.Y.; Yao, W.C.; Lin, C.T.; Yeh, C.Y. Static magnetic fields up-regulate osteoblast maturity by affecting local differentiation factors. Clin. Orthop. Relat. R. 2006, 447, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Tian, Z.; Qian, A.; Li, J.; Wu, J.; Wang, Z.; Zhang, D.; Yin, D.; Brandi, M.L.; Shang, P. Large gradient high magnetic field affects FLG29.1 cells differentiation to form osteoclast-like cells. Int. J. Radiat. Biol. 2012, 88, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Aydin, N.; Bezer, M. The effect of an intramedullary implant with a static magnetic field on the healing of the osteotomised rabbit femur. Int. Orthop. 2011, 35, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tomita, N.; Ohata, R.; Yan, Q.; Ikada, Y. Static magnetic field effects on bone formation of rats with an ischemic bone model. Biomed. Mater. Eng. 2001, 11, 257–263. [Google Scholar] [PubMed]
- Aïda, L.; Frédéric, L.; Soumaya, G.; Philippe, H.; Mohsen, S.; Hafedh, A. Static magnetic field induced hypovitaminosis D in rat. J. Vet. Med. Sci. 2013, 75, 1181–1185. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Anderson, F.H.; Patel, S.; Sahota, O.; van Staa, T.P. Calcium and vitamin D in the prevention of osteoporotic fractures. QJM 2006, 99, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F.; Suda, T.; Cousins, R.J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 1971, 10, 2799–2804. [Google Scholar] [PubMed]
- Holick, M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 2006, 81, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Balk, E.M.; Brendel, M.; Ip, S.; Lau, J.; Lee, J.; Lichtenstein, A.; Patel, K.; Raman, G.; Tatsioni, A.; et al. Vitamin D and calcium: A systematic review of health outcomes. Evid. Rep. Technol. Assess. 2009, 183, 1–420. [Google Scholar]
- Bell, T.D.; Demay, M.B.; Burnett-Bowie, S.A. The biology and pathology of vitamin D control in bone. J. Cell. Biochem. 2010, 111, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gungor, H.R.; Ok, N.; Akkaya, S.; Akkaya, N. Are there any adverse effects of static magnetic field from magnetic resonance imaging devices on bone health of workers? Eklem Hast. Cerrahisi 2014, 25, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Atherton, K.; Berry, D.J.; Parsons, T.; Macfarlane, G.J.; Power, C.; Hyppönen, E. Vitamin D and chronic widespread pain in a white middle-aged British population: Evidence from a cross-sectional population survey. Ann. Rheum. Dis. 2009, 68, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Straube, S.; Moore, A.R.; Derry, S.; McQuay, H.J. Vitamin D and chronic pain. Pain 2009, 141, 10–13. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gungor, H.R.; Akkaya, S.; Ok, N.; Yorukoglu, A.; Yorukoglu, C.; Kiter, E.; Oguz, E.O.; Keskin, N.; Mete, G.A. Chronic Exposure to Static Magnetic Fields from Magnetic Resonance Imaging Devices Deserves Screening for Osteoporosis and Vitamin D Levels: A Rat Model. Int. J. Environ. Res. Public Health 2015, 12, 8919-8932. https://doi.org/10.3390/ijerph120808919
Gungor HR, Akkaya S, Ok N, Yorukoglu A, Yorukoglu C, Kiter E, Oguz EO, Keskin N, Mete GA. Chronic Exposure to Static Magnetic Fields from Magnetic Resonance Imaging Devices Deserves Screening for Osteoporosis and Vitamin D Levels: A Rat Model. International Journal of Environmental Research and Public Health. 2015; 12(8):8919-8932. https://doi.org/10.3390/ijerph120808919
Chicago/Turabian StyleGungor, Harun R., Semih Akkaya, Nusret Ok, Aygun Yorukoglu, Cagdas Yorukoglu, Esat Kiter, Emin O. Oguz, Nazan Keskin, and Gulcin A. Mete. 2015. "Chronic Exposure to Static Magnetic Fields from Magnetic Resonance Imaging Devices Deserves Screening for Osteoporosis and Vitamin D Levels: A Rat Model" International Journal of Environmental Research and Public Health 12, no. 8: 8919-8932. https://doi.org/10.3390/ijerph120808919
APA StyleGungor, H. R., Akkaya, S., Ok, N., Yorukoglu, A., Yorukoglu, C., Kiter, E., Oguz, E. O., Keskin, N., & Mete, G. A. (2015). Chronic Exposure to Static Magnetic Fields from Magnetic Resonance Imaging Devices Deserves Screening for Osteoporosis and Vitamin D Levels: A Rat Model. International Journal of Environmental Research and Public Health, 12(8), 8919-8932. https://doi.org/10.3390/ijerph120808919






