PM2.5 and Cardiovascular Diseases in the Elderly: An Overview
Abstract
:1. Introduction
2. Review of PM2.5
| Particulate | Particle Size (≤μm) | Influences on Human Health |
|---|---|---|
| PM100 | 100 | Persist in the air and no evidence of adverse effects on human health |
| PM10 | 10 | Enter the respiratory system, deposit in the respiratory tract and cause respiratory diseases |
| PM2.5 | 2.5 | Get into the alveoli through the respiratory tract and then enter into the blood circulation, causing various diseases. |
| PM0.1 | 0.1 |
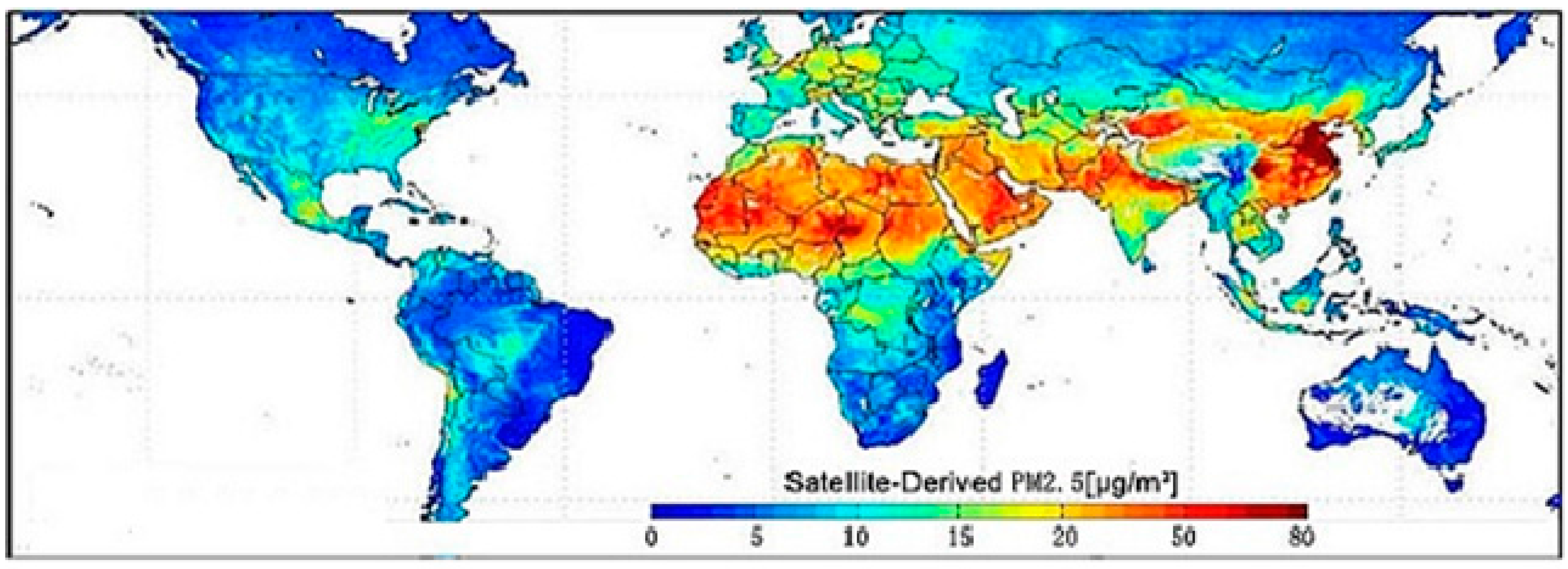
3. Plausible Pathophysiological Mechanisms Linking PM2.5 Exposure and CVD
3.1. Oxidative Stress and Inflammation
3.2. Abnormal Activation of the Haemostatic System
3.3. Disturbance of Autonomic Nervous System
3.4. Injury of Endothelial Cells
4. Epidemiological Studies Linking PM2.5 and CVD in the Elderly
5. Conclusions
- Walking or cycling in parks or country roads instead of busy streets during rush hours.
- Using more public transport rather than private cars and motorbikes.
- Avoiding outdoor exercise at times of high PM2.5 (especially those with cardiorespiratory disorders).
- Using an air purifier to optimize indoor air quality.
- Choosing appropriate respirators that fit snugly on the face if air pollution is severe.
- Reducing other known cardiovascular risk factors, such as smoking and alcohol.
- Warranting more aggressive use of primary and secondary preventive therapies, including antiplatelet agents, lipid-lowering agents, and treatments for hypertension or diabetes.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Fact Sheet No. 317, March 2013. Available online: http://www.heart.org/idc/groups/heartpublic/@wcm/@sop/@smd/documents/downloadable/ucm_319574.pdf (accessed on 29 November 2014).
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.D.; Stanek, L.W.; Luben, T.J.; Johns, D.O.; Buckley, B.J.; Brown, J.S.; Ross, M. Particulate matter-induced health effects: Who is susceptible? Environ. Health Perspect. 2011, 119, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Franck, U.; Odeh, S.; Wiedensohler, A.; Wehner, B.; Herbarth, O. The effect of particle size on cardiovascular disorders—The smaller the worse. Sci. Total Environ. 2011, 409, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Amin, N.; Robinson, S.D.; Anand, A.; Davies, J.; Patel, D.; de la Fuente, J.M.; Cassee, F.R.; Boon, N.A.; Macnee, W.; et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006, 173, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Belenky, A.; Wang, B.H. High-throughput biopolymer desalting bysolid-phase extraction prior to mass spectrometric analysis. J. Chromatogr. A 2001, 921, 3–13. [Google Scholar] [CrossRef]
- Araujo, J.A.; Nel, A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Par. Fibre Toxicol. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kim, B.; Lee, K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.F. PM2.5 and mortality in long-term prospective cohort studies: Cause-effect or statistical associations? Environ. Health Perspect. 1998, 106, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Forsberg, B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, E.T. Air pollution and the London fog of December, 1952. J. R. Sanit. Inst. 1954, 74, 1–21. [Google Scholar] [PubMed]
- Logan, W.P. Mortality from fog in London. BMJ 1956, 1, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, D.G.; Liu, O.J.; Yue, X.; Pan, Z.; Ding, Y. Research on current pollution status and pollution characteristics of PM2.5 in China. Res. Environ. Sci. (Huan Jing Ke Xue Yan Jiu). 2000, 13, 1–5. (In Chinese) [Google Scholar]
- EPA. National Ambient Air Quality Standards (NAAQS). 2012. Available online: http://www.epa.gov/air/criteria.html (accessed on 16 February 2014). [Google Scholar]
- Franchini, M.; Mannucci, P.M. Air pollution and cardiovascular disease. Thromb. Res. 2012, 129, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011, 118, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Vanbilloen, H.; Hoylaerts, M.F.; Hoet, P.H.; Verbruggen, A.; Nemery, B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001, 164, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; Tager, I. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: A statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [PubMed]
- Quay, J.L.; Reed, W.; Samet, J.; Devlin, R.B. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappa B activation. Am. J. Respir. Cell Mol. Biol. 1998, 19, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, B.; Oortgiesen, M.; Carter, J.D.; Devlin, R.B. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol. Appl. Pharmacol. 1999, 154, 106–115. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.; Bauer, B.; Block, M.L.; Hong, J.S.; Miller, D.S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008, 22, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Törnqvist, H.; Mills, N.L.; Gonzalez, M.; Miller, M.R.; Robinson, S.D.; Megson, I.L.; Macnee, W.; Donaldson, K.; Söderberg, S.; Newby, D.E.; et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007, 176, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, N.; Olivieri, O.; Girelli, D. Air particulate matter and cardiovascular disease: A narrative review. Eur. J. Intern. Med. 2013, 24, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rückerl, R.; Greven, S.; Ljungman, P.; Aalto, P.; Antoniades, C.; Bellander, T.; Berglind, N.; Chrysohoou, C.; Forastiere, F.; Jacquemin, B.; et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007, 115, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Hansen, M.L.; Long, R.W.; Nielsen, K.R.; Eatough, N.L.; Wilson, W.E.; Eatough, D.J. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ. Health Perspect. 2004, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.; Soukup, J.M.; Silbajoris, R.; Devlin, R.B. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol. Appl. Pharmacol. 2005, 203, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Colicino, E.; Lin, X.; Mehta, A.; Kloog, I.; Zanobetti, A.; Byun, H.M.; Bind, M.A.; Cantone, L.; Prada, D.; et al. Cardiac autonomic dysfunction: Particulate air pollution effects are modulated by epigenetic immunoregulation of Toll-like Receptor 2 and dietary flavonoid Intake. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Seaton, A.; MacNee, W.; Donaldson, K.; Godden, D. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar] [CrossRef]
- Bonzini, M.; Tripodi, A.; Artoni, A.; Tarantini, L.; Marinelli, B.; Bertazzi, P.A.; Apostoli, P.; Baccarelli, A. Effects of inhalable particulate matter on blood coagulation. J. ThrombHaemost. 2010, 8, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Fröhlich, M.; Döring, A.; Immervoll, T.; Wichmann, H.E.; Hutchinson, W.L.; Pepys, M.B.; Koenig, W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur. Heart J. 2001, 22, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, C.R.; Wellenius, G.A.; Ghelfi, E.; Lawrence, J.; González-Flecha, B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim. Biophys. Acta 2005, 1725, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Duan, Y.; Whitsel, E.A.; Zheng, Z.J.; Heiss, G.; Chinchilli, V.M.; Lin, H.M. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: A population-based study. Am. J. Epidemiol. 2004, 159, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pieters, N.; Plusquin, M.; Cox, B.; Kicinski, M.; Vangronsveld, J.; Nawrot, T.S. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: A meta-analysis. Heart 2012, 98, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.B.; Ghio, A.J.; Kehrl, H.; Sanders, G.; Cascio, W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur. Respir. J. 2003, 40, 76s–80s. [Google Scholar] [CrossRef]
- Montiel-Dávalos, A.; Ibarra-Sánchez Mde, J.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; López-Marure, R. Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban particulate matter. Toxicol. In Vitro. 2010, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.M.; Jia, Y.P.; Li, G.X.; Liu, L.Q.; Mo, Y.Z.; Jin, X.B.; Pan, X.C. Relationship between ambient fine particles and ventricular repolarization changes and heart rate variability of elderly people with heart disease in Beijing, China. Biomed. Environ. Sci. 2013, 26, 629–637. [Google Scholar] [PubMed]
- Park, S.K.; O’Neill, M.S.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Effects of air pollution on heart rate variability: The VA normative aging study. Environ. Health Perspect. 2005, 113, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Liberda, E.N.; Qu, S.; Guo, X.; Li, X.; Zhang, J.; Meng, J.; Yan, B.; Li, N.; Zhong, M.; et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Ito, K.; Hwang, J.S.; Maciejczyk, P.; Chen, L.C. Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 2006, 114, 1662–1669. [Google Scholar] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M.L. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D. Cardiovascular effects of air pollution. Clin. Sci. (Lond) 2008, 115, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Lifetips. Who’s at Risk for Heart Disease? Heart Disease Tips. 2015. Available online: http://heartdisease.lifetips.com//cat/63924/who-s-at-risk-for-heart-disease/index.html (accessed on 16 February 2015).
- Johnston, F.H.; Hanigan, I.C.; Henderson, S.B.; Morgan, G.G. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: Retrospective analysis of daily mortality, 1994–2007. BMJ 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- Kunzli, N.; Jerrett, M.; Garcia-Esteban, R.; Basagana, X.; Beckermann, B.; Gilliland, F.; Medina, M.; Peters, J.; Hodis, H.N.; Mack, W.J. Ambient air pollution and the progression of atherosclerosis in adults. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Hoffmann, B.; Moebus, S.; Mohlenkamp, S.; Stang, A.; Lehmann, N.; Dragano, N.; Schmermund, A.; Memmesheimer, M.; Mann, K.; Erbel, R.; Jockel, K.H.; Heinz Nixdorf Recall Study Investigative Group. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 2007, 116, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yu, I.T.; Wang, X.; Tian, L.; Tse, L.A.; Wong, T.W. Differential effects of fine and coarse particles on daily emergency cardiovascular hospitalizations in Hong Kong. Atmos. Environ. 2013, 64, 296–302. [Google Scholar] [CrossRef]
- Cruz, A.M.; Sarmento, S.; Almeida, S.M.; Silva, A.V.; Alves, C.; Freitas, M.C.; Wolterbeek, H. Association between atmospheric pollutants and hospital admissions in Lisbon. Environ. Sci. Pollut. Res. Int. 2015, 22, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; He, G.; Fan, M.; Wang, Z.; Liu, Y.; Ma, J.; Ma, Z.; Liu, J.; Liu, Y.; Wang, L.; Liu, Y. Smog episodes, fine particulate pollution and mortality in China. Environ. Res. 2015, 136, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Siponen, T.; Yli-Tuomi, T.; Aurela, M.; Dufva, H.; Hillamo, R.; Hirvonen, M.R.; Huttunen, K.; Pekkanen, J.; Pennanen, A.; Salonen, I.; et al. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup. Environ. Med. 2015, 72, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.S.; Chang, C.C.; Liou, S.H.; Yang, C.Y. The effects of fine particulate air pollution on daily mortality: A case-crossover study in a subtropical city, Taipei, Taiwan. Int. J. Environ. Res. Public Health. 2014, 11, 5081–5093. [Google Scholar] [CrossRef] [PubMed]
- Wellenius, G.A.; Schwartz, J.; Mittleman, M.A. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am. J. Cardiol. 2006, 97, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Chen, B.; Zhao, N.; London, S.J.; Song, G.; Chen, G.; Zhang, Y.; Jiang, L.; HEI Health Review Committee Part 1. A time-series study of ambient air pollution and daily mortality in Shanghai, China. Res. Rep. Health Eff. Inst. 2010, 154, 17–78. [Google Scholar] [PubMed]
- Kan, H.; London, S.J.; Chen, G.; Zhang, Y.; Song, G.; Zhao, N.; Jiang, L.; Chen, B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008, 116, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Wilker, E.H.; Mittleman, M.A.; Coull, B.A.; Gryparis, A.; Bots, M.L.; Schwartz, J.; Sparrow, D. Long-term exposure to black carbon and carotid intima-media thickness: The normative aging study. Environ. Health Perspect. 2013, 121, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.; Ishikawa, N.; Sheppard, L.; Siscovick, D.; Checkoway, H.; Kaufman, J. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am. J. Epidemiol. 2003, 157, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Zanobetti, A.; Nordio, F.; Coull, B.A.; Baccarelli, A.A.; Schwartz, J. Effects of airborne fine particles (PM 2.5) on deep vein thrombosis admissions in the northeastern United States. J. Thromb. Haemost. 2015, 13, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.L.; Miller, B.G.; Dick, S.; Dennekamp, M.; Ford, I.; Hillis, G.S.; Ayres, J.G.; Seaton, A. A panel study of air pollution in subjects with heart failure: Negative results in treated patients. Occup. Environ. Med. 2009, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Fang, J.; Mittleman, M.A.; Kapral, M.K.; Wellenius, G.A. Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology 2011, 22, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Willocks, L.J.; Bhaskar, A.; Ramsay, C.N.; Lee, D.; Brewster, D.H.; Fischbacher, C.M.; Chalmers, J.; Morris, G.; Scott, E.M. Cardiovascular disease and air pollution in Scotland: No association or insufficient data and study design. BMC Public Health 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Hoffmann, B.; Fischer, P.; Houthuijs, D.; Nieuwenhuijsen, M.; Weinmayr, G.; et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: Results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014, 66, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Hartog, J.J.; Boogaard, H.; Nijland, H.; Hoek, G. Do the health benefits of cycling outweigh the risks? Cien. Saude Colet 2011, 16, 4731–4744. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tu, Y.; Yu, Z.; Lu, R. PM2.5 and Cardiovascular Diseases in the Elderly: An Overview. Int. J. Environ. Res. Public Health 2015, 12, 8187-8197. https://doi.org/10.3390/ijerph120708187
Wang C, Tu Y, Yu Z, Lu R. PM2.5 and Cardiovascular Diseases in the Elderly: An Overview. International Journal of Environmental Research and Public Health. 2015; 12(7):8187-8197. https://doi.org/10.3390/ijerph120708187
Chicago/Turabian StyleWang, Chenchen, Yifan Tu, Zongliang Yu, and Rongzhu Lu. 2015. "PM2.5 and Cardiovascular Diseases in the Elderly: An Overview" International Journal of Environmental Research and Public Health 12, no. 7: 8187-8197. https://doi.org/10.3390/ijerph120708187
APA StyleWang, C., Tu, Y., Yu, Z., & Lu, R. (2015). PM2.5 and Cardiovascular Diseases in the Elderly: An Overview. International Journal of Environmental Research and Public Health, 12(7), 8187-8197. https://doi.org/10.3390/ijerph120708187





