Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Subjects
2.2. Test Products
2.3. Study Design and Protocol
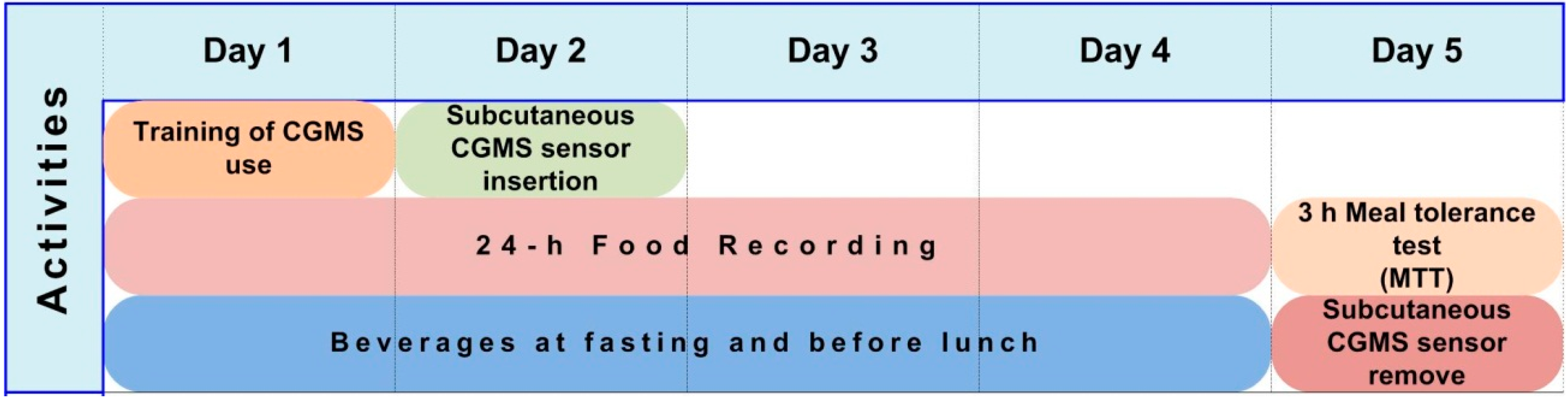
2.4. Treatments
2.5. Continuous Glucose Monitoring
2.6. Assessment of Glycemic Variability (GV)
2.7. Meal Tolerance Test
2.8. Biochemical Measurements
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Subjects
| Characteristic | Lean | Obese | p |
|---|---|---|---|
| N of subjects | 10 | 10 | -- |
| Gender (M/F) | 1/9 | 1/9 | -- |
| Age (years) | 26.4 ± 7.86 | 31.6 ± 4.97 | 0.094 |
| BMI (kg/m2) | 23.27 ± 1.22 | 30.73 ± 1.07 | <0.0001 |
| Glucose (mmol/L) | 4.25 ± 0.57 | 4.60 ± 0.31 | 0.105 |
| HbA1c (%) | 5.18 ± 0.51 | 5.03 ± 0.58 | 0.547 |
| Total cholesterol (mmol/L) | 4.48 ± 0.92 | 4.99 ± 0.75 | 0.192 |
| HDL-Cholesterol (mmol/L) | 1.27 ± 0.26 | 1.23 ± 0.07 | 0.629 |
| Triglycerides (mmol/L) | 1.36 ± 0.84 | 1.50 ± 0.18 | 0.620 |
3.2. Tolerability and Adverse Effects
3.3. Glycemic Excursions in 48-h CGM
| Characteristic | Lean | Obese | Lean and Obese | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DCS | NBS | p | DCS | NBS | p | DCS | NBS | p | |
| MBG (mmol/L) | 5.83 ± 0.95 | 5.15 ± 0.71 | <0.0001 | 6.28 ± 0.82 | 5.44 ± 0.63 | <0.0001 | 6.06 ± 0.89 | 5.29 ± 0.67 | <0.0001 |
| AUC (mmol.h/L) | 4.43 ± 0.49 | 3.97 ± 0.45 | 0.0329 | 5.85 ± 0.29 | 5.06 ± 0.20 | 0.0121 | 5.16 ± 0.32 | 4.51 ± 0.27 | 0.0007 |
| SD (mmol/L) | 1.00 ± 0.44 | 0.77 ± 0.25 | 0.1229 | 1.13 ± 0.39 | 1.07 ± 0.46 | 0.7651 | 1.07 ± 0.41 | 0.92 ± 0.40 | 0.2440 |
| CV (%) | 16.81 ± 5.88 | 15.14 ± 4.88 | 0.3904 | 17.74 ± 4.60 | 20.25 ± 9.64 | 0.5028 | 17.28 ± 5.16 | 17.70 ± 7.88 | 0.8374 |
| MAGE (mmol/L) | 2.32 ± 0.67 | 2.26 ± 0.76 | 0.8780 | 2.27 ± 1.07 | 1.77 ± 0.61 | 0.2100 | 2.30 ± 0.87 | 2.02 ± 0.72 | 0.2960 |
| MAG5 (mmol/L) | 0.12 ± 0.04 | 0.10 ± 0.02 | 0.2176 | 0.12 ± 0.03 | 0.15 ± 0.08 | 0.3095 | 0.12 ± 0.04 | 0.13 ± 0.07 | 0.7622 |
3.4. Excursions Exceeding Oral Glucose Tolerance Test (OGTT) Thresholds
3.5. Meal Tolerance Test (MTT)
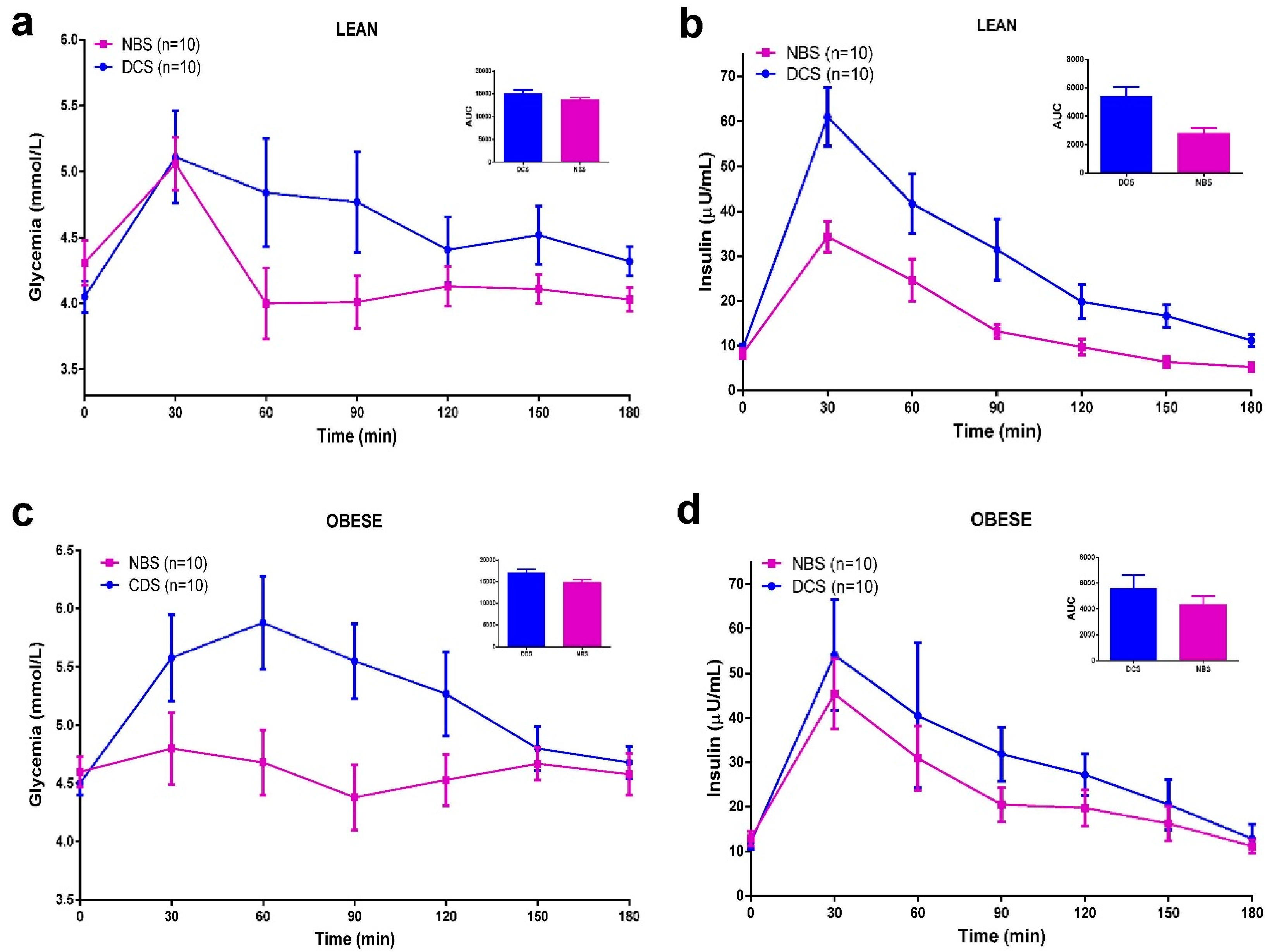
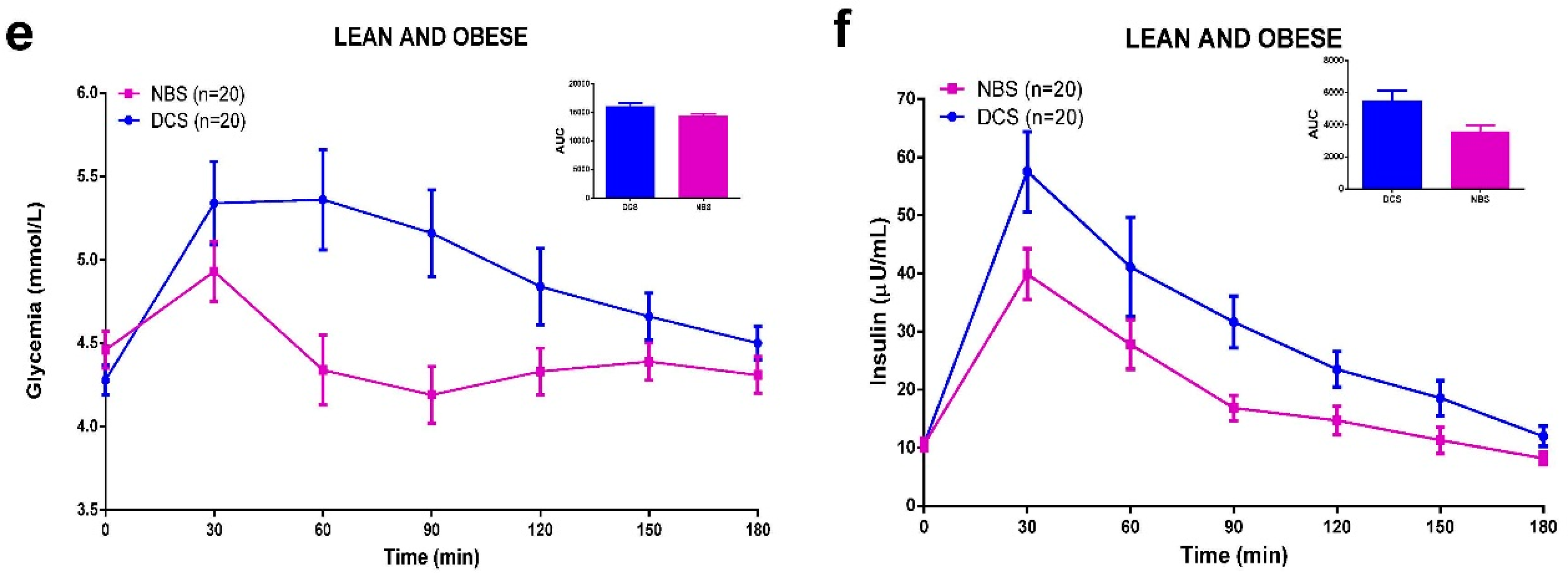
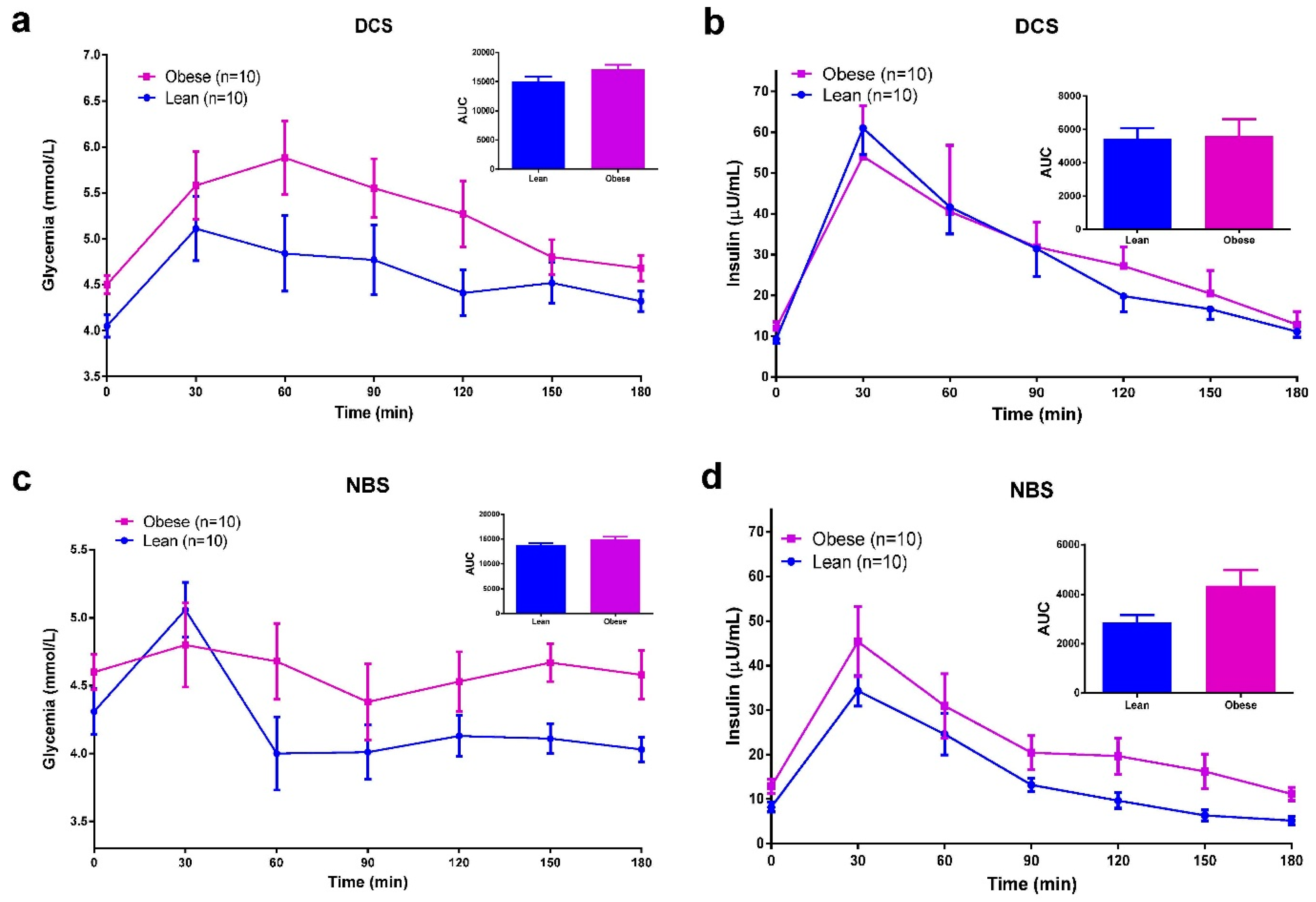
3.6. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Croup. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Postprandial Hyperglycemia and cardiovascular disease: Is the HEART2D study the answer? Diabetes Care 2009, 32, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Navigating the choices for diabetes prevention. N. Engl. J. Med. 2010, 362, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Jones, J. CODEX-aligned dietary fiber definitions help to bridge the “fiber gap”. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D. Dietary-resistant starch and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Pérez-Sánchez, E. Efectos del Consumo de una Formulación que Incluye Almidón Resistente de Plátano en la Glucemia Postprandial de Pacientes con Diabetes Mellitus Tipo 2. Tesis de Maestría en Ciencias Alimentarias; Universidad Juárez Autónoma de Tabasco: Villahermosa, Tabasco, México, 2007. [Google Scholar]
- Olvera-Hernández, V. Almidón Resistente de Banano Enano Gigante (Cavendish AAA) en la Glicemia y Producción de Ácidos Grasos de Cadena Corta en Ratas con Síndrome Metabólico. Tesis de Maestría en Ciencias Alimentarias; Universidad Juárez Autónoma de Tabasco: Villahermosa, Tabasco, México, 2010. [Google Scholar]
- Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Francisco-Luria, M.U.; Córdova-Uscanga, R.; Rodríguez-Hernández, A.; Méndez, J.D.; Díaz-Zagoya, J.C. Effects of Native Banana Starch Supplementation on Body Weight and Insulin Sensitivity in Obese Type 2 Diabetics. Int. J. Environ. Res. Public Health 2010, 7, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Ble Castillo, J.; Aparicio Trápala, M.; Gómez Vázquez, A.; Rodríguez Hernández, A.; Mendez, J.; Juárez Rojop, I.; Aguilar Mariscal, H.; Ramón Frías, T.; Díaz Zagoya, J. Potential beneficial effects of native banana starch on glycemia and insulin resistance in obese nondiabetic women. Interciencia 2012, 37, 470–476. [Google Scholar]
- Flores-Gorosquera, E.; Garcia-Suarez, F.J.; Flores-Huicochea, E.; Nunez-Santiago, M.C.; Gonzalez-Soto, R.A.; Bello-Perez, L.A. Yield of starch extraction from plantain (Musa paradisiaca). Pilot plant study. Acta Cient. Venez. 2004, 55, 86–90. [Google Scholar] [PubMed]
- Goñi, I.; García-Diz, L.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [PubMed]
- Gross, T.M.; Mastrototaro, J.J. Efficacy and reliability of the continuous glucose monitoring system. Diabetes Technol. Ther. 2000, 2, S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Hermanides, J.; Vriesendorp, T.M.; Bosman, R.J.; Zandstra, D.F.; Hoekstra, J.B.; Devries, J.H. Glucose variability is associated with intensive care unit mortality. Crit. Care Med. 2010, 38, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.V.; Muduli, S.K.; Avasthi, R. Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol. Ther. 2013, 15, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R.; Group, A.S. Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: The A1C-Derived Average Glucose (ADAG) study. Diabetologia 2010, 53, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Sulk, S.; Helbig, M.; Thomas, A.; Kohler, C. Differences in Glycemic Variability Between Normoglycemic and Prediabetic Subjects. J. Diabetes Sci. Technol. 2014, 8, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Fysekidis, M.; Cosson, E.; Banu, I.; Duteil, R.; Cyrille, C.; Valensi, P. Increased glycemic variability and decrease of the postprandial glucose contribution to HbA1c in obese subjects across the glycemic continuum from normal glycemia to first time diagnosed diabetes. Metabolism 2014, 63, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef] [PubMed]
- The DECODE Study Group on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and mortality: Comparison of WHO and American Diabetic Association diagnostic criteria. Lancet 1999, 354, 617–621. [Google Scholar]
- Pyorala, K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: Results from two population studies in Finland. Diabetes Care 1979, 2, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Lamarche, B.; Mauriège, P.; Cantin, B.; Dagenais, G.R.; Moorjani, S.; Lupien, P.-J. Hyperinsulinemia as an Independent Risk Factor for Ischemic Heart Disease. New Engl. J. Med. 1996, 334, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Point: Postprandial glucose levels are a clinically important treatment target. Diabetes Care 2010, 33, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Chinese Diabetes Society. Chinese clinical guideline for continuous glucose monitoring (2012). Chin. Med. J. (Engl.) 2012, 125, 4167–4174. [Google Scholar]
- Ma, C.M.; Yin, F.Z.; Wang, R.; Qin, C.M.; Liu, B.; Lou, D.H.; Lu, Q. Glycemic variability in abdominally obese men with normal glucose tolerance as assessed by continuous glucose monitoring system. Obesity (Silver Spring) 2011, 19, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch–A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Tappenden, K.A.; McBurney, M.I. Systemic short-chain fatty acids rapidly alter gastrointestinal structure, function, and expression of early response genes. Dig. Dis. Sci. 1998, 43, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Wright, J.W.; Loizon, E.; Debard, C.; Vidal, H.; Shojaee-Moradie, F.; Russell-Jones, D.; Umpleby, A.M. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Domínguez, G.; Ble-Castillo, J.L.; Aparicio-Trápala, M.A.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Ble-Castillo, D.J.; García-Vázquez, C.; Olvera-Hernández, V.; Pérez-Pimienta, B.; Diaz-Zagoya, J.C.; et al. Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects. Int. J. Environ. Res. Public Health 2015, 12, 7491-7505. https://doi.org/10.3390/ijerph120707491
Jiménez-Domínguez G, Ble-Castillo JL, Aparicio-Trápala MA, Juárez-Rojop IE, Tovilla-Zárate CA, Ble-Castillo DJ, García-Vázquez C, Olvera-Hernández V, Pérez-Pimienta B, Diaz-Zagoya JC, et al. Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects. International Journal of Environmental Research and Public Health. 2015; 12(7):7491-7505. https://doi.org/10.3390/ijerph120707491
Chicago/Turabian StyleJiménez-Domínguez, Guadalupe, Jorge L. Ble-Castillo, María A. Aparicio-Trápala, Isela E. Juárez-Rojop, Carlos A. Tovilla-Zárate, Deysi J. Ble-Castillo, Carlos García-Vázquez, Viridiana Olvera-Hernández, Bedelia Pérez-Pimienta, Juan C. Diaz-Zagoya, and et al. 2015. "Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects" International Journal of Environmental Research and Public Health 12, no. 7: 7491-7505. https://doi.org/10.3390/ijerph120707491
APA StyleJiménez-Domínguez, G., Ble-Castillo, J. L., Aparicio-Trápala, M. A., Juárez-Rojop, I. E., Tovilla-Zárate, C. A., Ble-Castillo, D. J., García-Vázquez, C., Olvera-Hernández, V., Pérez-Pimienta, B., Diaz-Zagoya, J. C., & Mendez, J. D. (2015). Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects. International Journal of Environmental Research and Public Health, 12(7), 7491-7505. https://doi.org/10.3390/ijerph120707491






