The Mediating Effect of Body Mass Index on the Relationship between Cigarette Smoking and Atopic Sensitization in Chinese Adults
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Information Collection
2.3. Definition of Smoking Categories
2.4. Body Mass Index (BMI) Calculation
2.5. Specific IgE Test and Definition of Atopic Sensitization
2.6. Statistical Analysis
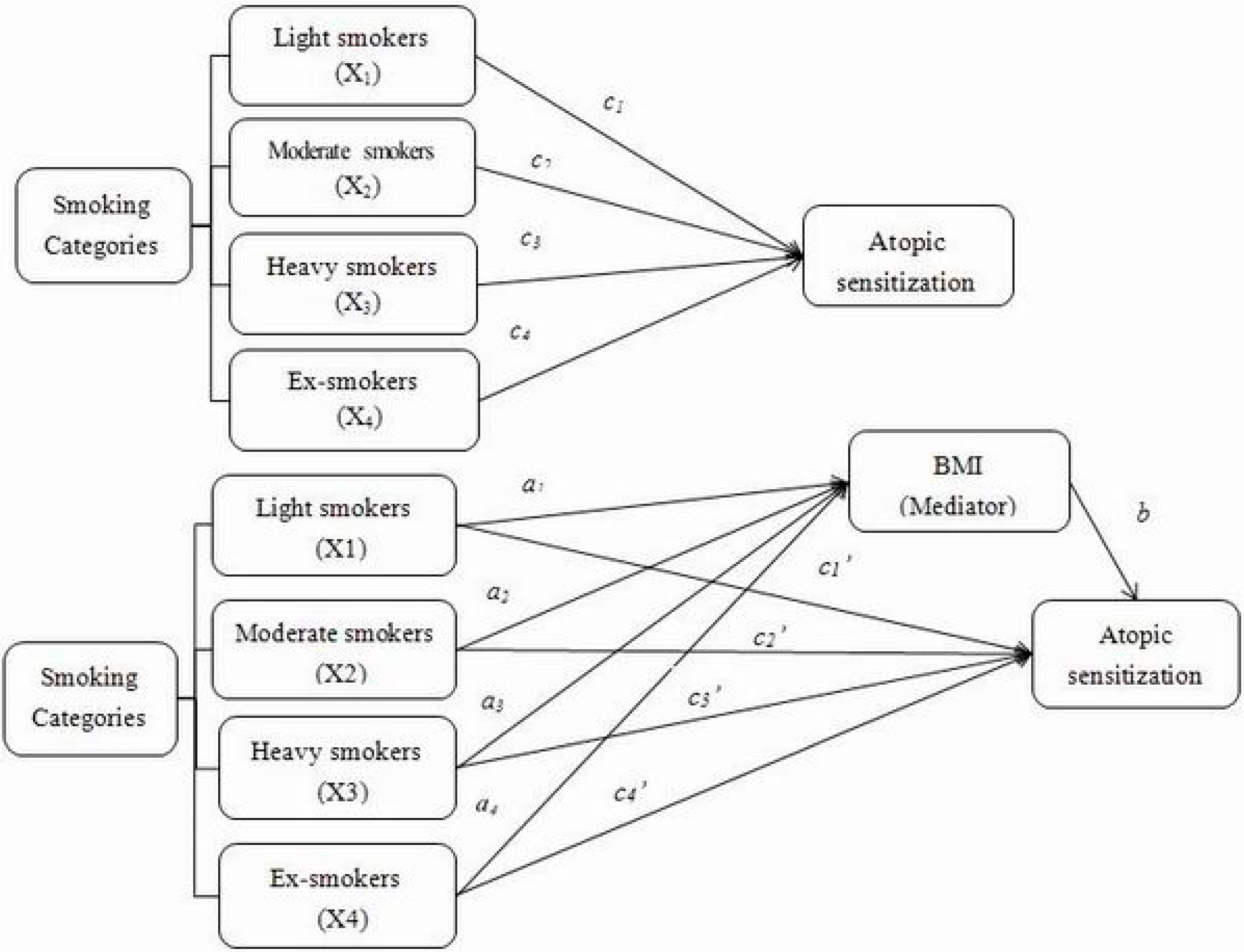
3. Results and Discussion
3.1. Result
3.1.1. Characteristics of Study Subjects
| Characteristics | Non-Smokers | Current Smokers, Average Numbers of Cigarettes Per Day | Former Smokers | p Value a | ||
|---|---|---|---|---|---|---|
| Light Smokers (1–9) | Moderate Smokers (10–20) | Heavy Smokers (≥21) | ||||
| N | 2942 | 184 | 249 | 53 | 129 | |
| Gender, %men | 16.76 | 55.43 | 72.29 | 84.91 | 75.97 | <0.0001 |
| Age group % | <0.0001 | |||||
| 18–39 | 53.13 | 55.43 | 42.17 | 22.64 | 25.58 | |
| 40–59 | 41.13 | 38.59 | 53.01 | 67.92 | 54.26 | |
| ≥60 | 5.74 | 5.98 | 4.82 | 9.43 | 20.16 | |
| Educational level % | <0.0001 | |||||
| 1st | 25.29 | 35.33 | 27.71 | 45.28 | 39.53 | |
| 2nd | 38.38 | 32.61 | 52.21 | 33.96 | 34.11 | |
| 3rd | 36.34 | 32.07 | 20.08 | 20.75 | 26.36 | |
| Family allergic disease history, %yes | 10.77 | 9.78 | 6.83 | 5.66 | 6.98 | 0.146 |
| Alcohol drinkers, %yes | 8.36 | 31.52 | 38.15 | 50.94 | 41.09 | <0.0001 |
| Atopy(allergic to any allergens), %yes | 23.05 | 16.85 | 15.26 | 22.64 | 20.93 | 0.023 |
| Allergic toinhalant Allergens only %yes | 18.41 | 12.57 | 12.08 | 18.00 | 13.56 | 0.030 |
| Allergictofood allergens only %yes | 2.96 | 2.55 | 1.86 | 2.38 | 3.77 | 0.870 |
| Allergic to both food andaeroallergens %yes | 4.15 | 3.16 | 2.31 | 4.65 | 6.42 | 0.453 |
| BMI,kg/m2mean(SD) | 22.81 (3.59) | 22.62 (3.50) | 23.61 (4.66) | 24.01 (3.32) | 24.12 (3.56) | <0.0001 |
| Atopic Sensitization to Different Types of Allergens | BMI (Mediator) | Atopic Sensitization (Y ) | ||||
|---|---|---|---|---|---|---|
| Path | β (SE) | p Value | Path | β (SE) | p Value | |
| Allergic to inhalant Allergens only | ||||||
| Non-smokers | Reference | Reference | ||||
| Light smokers(X1) | a1 | −0.906 (0.279) | 0.001 | c1 | −0.476 (0.239) | 0.046 |
| Moderate smokers(X2) | a2 | −0.338 (0.252) | 0.180 | c2 | −0.529 (0.216) | 0.014 |
| Heavy smokers(X3) | a3 | −0.397 (0.512) | 0.438 | c3 | −0.033 (0.383) | 0.932 |
| Former smokers (X4) | a4 | −0.156 (0.345) | 0.651 | c4 | −0.363 (0.286) | 0.204 |
| BMI, kg/m2 (M) | b | ---- | ---- | ---- | 0.029 (0.013) | 0.022 |
| Allergic to both food andinhalant allergens | ||||||
| Non-smokers | Reference | Reference | ||||
| Light smokers(X1) | a1 | −0.944 (0.289) | 0.001 | c1 | −0.729 (0.479) | 0.128 |
| Moderate smokers(X2) | a2 | −0.284 (0.261) | 0.278 | c2 | −1.165 (0.482) | 0.016 |
| Heavy smokers(X3) | a3 | 0.030 (0.541) | 0.056 | c3 | −0.528 (0.753) | 0.483 |
| Former smokers (X4) | a4 | −0.251 (0.354) | 0.478 | c4 | −0.095 (0.434) | 0.828 |
| BMI, kg/m2(M) | b | ---- | ---- | ---- | 0.013 (0.027) | 0.629 |
3.1.2. Associations between Each Smoking Category, BMI and Atopic Sensitization to Different Types of Allergens
3.1.3. Indirect and Direct Effect, through Potential Mediator, of Smoking Categories on Atopic Sensitizationto Different Types of Allergens
| Atopic Sensitization to Different Types of Allergens | Indirect Effect of Smoking on Atopic Sensitization to Different Types of Allergens (ab paths) | Direct Effect of Smoking on Atopic Sensitization to Different Types of Allergens (c’ paths) | ||||
|---|---|---|---|---|---|---|
| Path | Point Estimate (SE) | BC 95% CI | Path | Point Estimate (SE) | BC 95% CI | |
| Only allergic to aeroallergens | ||||||
| Non-smokers | Reference | Reference | ||||
| Light smokers (X1) | a1b | −0.026 (0.015) | −0.062 to −0.004 | c’1 | −0.452 (0.239) | −0.921 to 0.018 |
| Moderate smokers (X2) | a2b | −0.010 (0.013) | −0.043 to 0.008 | c’2 | −0.525 (0.217) | −0.950 to −0.101 |
| Heavy smokers (X3) | a3b | −0.011 (0.017) | −0.059 to 0.014 | c’3 | −0.021 (0.384) | −0.773 to 0.731 |
| Former smokers (X4) | a4b | −0.005 (0.011) | −0.034 to 0.013 | c’4 | −0.361 (0.286) | −0.921 to 0.200 |
| Allergic to both food and aeroallergens | ||||||
| Non-smokers | Reference | Reference | ||||
| Light smokers (X1) | a1b | −0.012 (0.029) | −0.071 to 0.043 | c’1 | −0.716 (0.480) | −1.657 to 0.227 |
| Moderate smokers (X2) | a2b | −0.004 (0.015) | −0.051 to 0.015 | c’2 | −1.160 (0.482) | −2.105 to −0.215 |
| Heavy smokers (X3) | a3b | 0.0004 (0.017) | −0.031 to 0.040 | c’3 | −0.524 (0.753) | −1.999 to 0.951 |
| Former smokers (X4) | a4b | −0.003 (0.014) | −0.048 to 0.014 | c’4 | −0.092 (0.434) | −0.942 to 0.758 |
3.2. Discussion
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurukulaaratchy, R.J.; Matthews, S.; Arshad, S.H. Does environment mediate earlier onset of the persistent childhood asthma phenotype? Pediatrics 2004, 113, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Settipane, R.J.; Hagy, G.W.; Settipane, G.A. Long-term risk factors for developing asthma and allergic rhinitis: A 23-year follow-up study of college students. Allergy Proc.: Off. J. Reg. State Allergy Soc. 1994, 15, 21–25. [Google Scholar] [CrossRef]
- Mishra, N.C.; Rir-Sima-Ah, J.; Langley, R.J.; Singh, S.P.; Pena-Philippides, J.C.; Koga, T.; Razani-Boroujerdi, S.; Hutt, J.; Campen, M.; Kim, K.C.; et al. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J. Immunol. 2008, 180, 7655–7663. [Google Scholar] [CrossRef]
- Hancox, R.J.; Welch, D.; Poulton, R.; Taylor, D.R.; McLachlan, C.R.; Greene, J.M.; Sears, M.R. Cigarette smoking and allergic sensitization: A 32-year population-based cohort study. J. Allergy Clin. Immunol. 2008, 121, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Nielsen, N.H.; Madsen, F.; Frolund, L.; Dirksen, A.; Jorgensen, T. Smoking and the development of allergic sensitization to aeroallergens in adults: A prospective population-based study. The Copenhagen allergy study. Allergy 2001, 56, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Nielsen, N.H.; Madsen, F.; Frolund, L.; Dirksen, A.; Jorgensen, T. Factors related to allergic sensitization to aeroallergens in a cross-sectional study in adults: The Copenhagen allergy study. Clin. Exp. Allergy: J. Brit. Soc. Allergy Clin. Immunol. 2001, 31, 1409–1417. [Google Scholar] [CrossRef]
- Strachan, D.P.; Harkins, L.S.; Johnston, I.D.; Anderson, H.R. Childhood antecedents of allergic sensitization in young British adults. J. Allergy Clin. Immunol. 1997, 99, 6–12. [Google Scholar]
- Omenaas, E.; Bakke, P.; Elsayed, S.; Hanoa, R.; Gulsvik, A. Total and specific serum IgE levels in adults: Relationship to sex, age and environmental factors. Clin. Exp. Allergy 1994, 24, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.; Chinn, S.; Luczynska, C.; Burney, P. The association of smoking with sensitization to common environmental allergens: Results from the European Community Respiratory Health Survey. J. Allergy Clin. Immunol. 1999, 104, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, B.; Schindler, C.; Medici, T.C.; Zellweger, J.P.; Leuenberger, P. IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) team. Int. Arch. Allergy Immunol. 1996, 111, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Barbee, R.A.; Kaltenborn, W.; Lebowitz, M.D.; Burrows, B. Longitudinal changes in allergen skin test reactivity in a community population sample. J. Allergy Clin. Immunol. 1987, 79, 16–24. [Google Scholar] [CrossRef]
- Kulig, M.; Luck, W.; Lau, S.; Niggemann, B.; Bergmann, R.; Klettke, U.; Guggenmoos-Holzmann, I.; Wahn, U. Effect of pre- and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life. Multicenter Allergy Study Group, Germany. Allergy 1999, 54, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Mener, D.J.; Garcia-Esquinas, E.; Navas-Acien, A.; Dietert, R.R.; Shargorodsky, J.; Lin, S.Y. Lead exposure and increased food allergic sensitization in U.S. children and adults. Int.Forum Allergy Rhinol. 2014, 5. [Google Scholar] [CrossRef]
- Shiue, I. Self and environmental exposures to drinking, smoking, gambling or video game addiction are associated with adult hypertension, heart and cerebrovascular diseases, allergy, self-rated health and happiness: Japanese General Social Survey, 2010. Int.J. Cardiol. 2014, 181, 403–412. [Google Scholar] [CrossRef]
- Hjern, A.; Hedberg, A.; Haglund, B.; Rosen, M. Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents. Clin. Exp. Allergy 2001, 31, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P. Obesity and atopy. Clin. Exp. Allergy 2015, 45, 75–86. [Google Scholar] [CrossRef]
- Zhou, B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults—Study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed.Environ. Sci. 2002, 15, 83–96. [Google Scholar] [PubMed]
- Mackay, D.F.; Gray, L.; Pell, J.P. Impact of smoking and smoking cessation on overweight and obesity: Scotland-wide, cross-sectional study on 40,036 participants. BMC Public Health 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Clair, C.; Chiolero, A.; Faeh, D.; Cornuz, J.; Marques-Vidal, P.; Paccaud, F.; Mooser, V.; Waeber, G.; Vollenweider, P. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: Cross-sectional data from a population-based survey. BMC Public Health 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Wareham, N.; Luben, R.; Welch, A.; Bingham, S.; Day, N.; Khaw, K.T. Cigarette smoking and fat distribution in 21,828 British men and women: A population-based study. Obes.Res. 2005, 13, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rennie, D.; Cormier, Y.; Dosman, J. Association between obesity and atopy in adults. Int. Arch. Allergy Immunol. 2010, 153, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, Y.; Wang, Z.; Zhou, X.H.; Zhao, J.; Suo, J.; Dong, X.; Liu, M. Effect modification by gender and smoking status on the association between obesity and atopic sensitization in Chinese adults: A hospital-based case-control study. BMC Public Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Chiolero, A.; Jacot-Sadowski, I.; Faeh, D.; Paccaud, F.; Cornuz, J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity 2007, 15, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Hanke, M.; Rumpf, H.J.; Thyrian, J.R. Smoking status, cigarettes per day, and their relationship to overweight and obesity among former and current smokers in a national adult general population sample. Int. J. Obes. 2005, 29, 1289–1294. [Google Scholar] [CrossRef]
- Ali, S.M.; Lindstrom, M. Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: Differing patterns for underweight and overweight/obesity. Eur. J. Public Health 2006, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Conduct of Tobacco Smoking Surveys for the General Population; World Health Organization: Geneva, Switzerland, 1983; Document WHO/SMO/83. [Google Scholar]
- WHO. Report of a WHO Expert Committee Technical Report Series, Physical Status: The Use and Interpretation of Anthropometry; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Husemoen, L.L.; Glumer, C.; Lau, C.; Pisinger, C.; Morch, L.S.; Linneberg, A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy 2008, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, A.; Schutz, Y.; Jequier, E.; Wahren, J. Increased 24-h energy expenditure in cigarette smokers. N. Engl. J. Med. 1986, 314, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.C.; Cornelius, M.F.; Vogel, R.L.; Walker, J.F.; Stamford, B.A. Effect of caffeine and/or cigarette smoking on resting energy expenditure. Int.J. Obes. Relat. Metab. Disord. 1994, 18, 551–556. [Google Scholar] [PubMed]
- Dallosso, H.M.; James, W.P. The role of smoking in the regulation of energy balance. Int. J. Obes. 1984, 8, 365–375. [Google Scholar] [PubMed]
- Chen, H.; Hansen, M.J.; Jones, J.E.; Vlahos, R.; Anderson, G.P.; Morris, M.J. Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res. 2008, 1228, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Visness, C.M.; London, S.J.; Daniels, J.L.; Kaufman, J.S.; Yeatts, K.B.; Siega-Riz, A.M.; Liu, A.H.; Calatroni, A.; Zeldin, D.C. Association of obesity with IgE levels and allergy symptoms in children and adolescents: Results from the National Health and Nutrition Examination Survey 2005–2006. J.Allergy Clin. Immunol. 2009, 123, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Sood, A. Obesity, adipokines, and lung disease. J. Appl. Physiol. 2010, 108, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Linneberg, A. The link between the epidemics of obesity and allergic diseases: Does obesity induce decreased immune tolerance? Allergy 2007, 62, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hsia, J.; Yang, G. Prevalence of smoking in China in 2010. N. Engl. J. Med. 2011, 364, 2469–2470. [Google Scholar] [CrossRef] [PubMed]
- Saules, K.K.; Pomerleau, C.S.; Snedecor, S.M.; Mehringer, A.M.; Shadle, M.B.; Kurth, C.; Krahn, D.D. Relationship of onset of cigarette smoking during college to alcohol use, dieting concerns, and depressed mood: Results from the Young Women’s Health Survey. Add.Behav. 2004, 29, 893–899. [Google Scholar] [CrossRef]
- Wee, C.C.; Rigotti, N.A.; Davis, R.B.; Phillips, R.S. Relationship between smoking and weight control efforts among adults in the United States. Arch. Internal Med. 2001, 161, 546–550. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, K.W.; Yoon, Y.S.; Lee, S.Y.; Kim, S.S.; Oh, S.W. Cigarette smoking increases abdominal and visceral obesity but not overall fatness: An observational study. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wang, Y.; Wang, Z.; Cai, F.; Xie, B.; Qu, S.; Liu, M. The Mediating Effect of Body Mass Index on the Relationship between Cigarette Smoking and Atopic Sensitization in Chinese Adults. Int. J. Environ. Res. Public Health 2015, 12, 3381-3394. https://doi.org/10.3390/ijerph120303381
Luo X, Wang Y, Wang Z, Cai F, Xie B, Qu S, Liu M. The Mediating Effect of Body Mass Index on the Relationship between Cigarette Smoking and Atopic Sensitization in Chinese Adults. International Journal of Environmental Research and Public Health. 2015; 12(3):3381-3394. https://doi.org/10.3390/ijerph120303381
Chicago/Turabian StyleLuo, Xiao, Yupeng Wang, Zhiqiang Wang, Fuwen Cai, Biao Xie, Siyang Qu, and Meina Liu. 2015. "The Mediating Effect of Body Mass Index on the Relationship between Cigarette Smoking and Atopic Sensitization in Chinese Adults" International Journal of Environmental Research and Public Health 12, no. 3: 3381-3394. https://doi.org/10.3390/ijerph120303381
APA StyleLuo, X., Wang, Y., Wang, Z., Cai, F., Xie, B., Qu, S., & Liu, M. (2015). The Mediating Effect of Body Mass Index on the Relationship between Cigarette Smoking and Atopic Sensitization in Chinese Adults. International Journal of Environmental Research and Public Health, 12(3), 3381-3394. https://doi.org/10.3390/ijerph120303381





