Spatial Distribution of Lead in Sacramento, California, USA
Abstract
:1. Introduction
2. Methods
2.1. Sample Collection and Analysis Methods
2.2. Statistical Methods
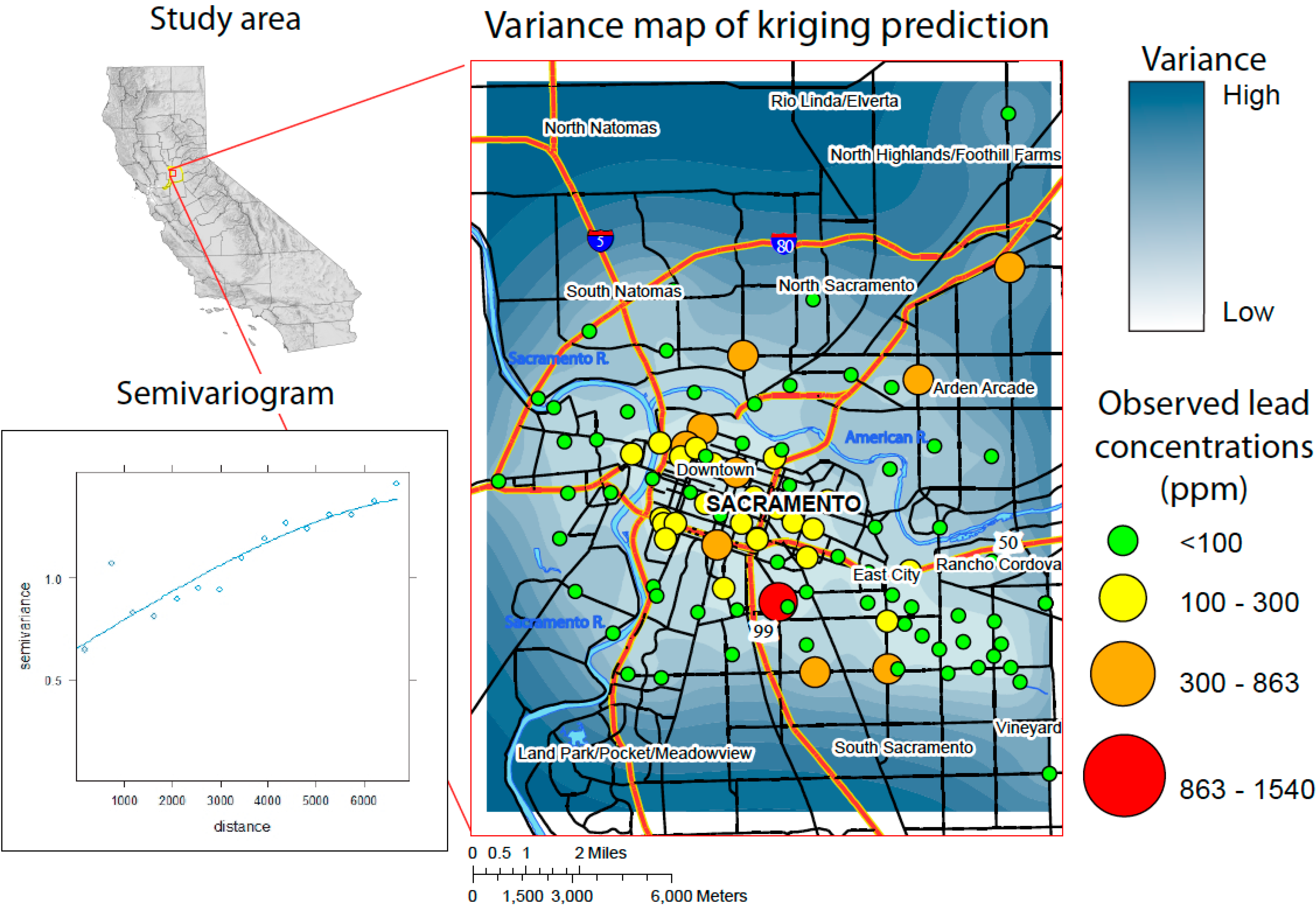
3. Results
3.1. Analytical Results
3.1.1. Elemental Analysis
| Analyte | Min. | Max. | Median | Mean | Standard Deviation | 25th percentile | 75th percentile | RPD |
|---|---|---|---|---|---|---|---|---|
| Ag (mg/kg) 2 | 0.03 | 0.81 | 0.11 | 0.14 | 0.09 | 0.09 | 0.14 | 18.7% |
| As (mg/kg) 1 | 2.7 | 27.9 | 7.5 | 8.9 | 4.7 | 6.0 | 10.0 | 5.7% |
| Bi (mg/kg) 2 | 0.07 | 0.67 | 0.15 | 0.16 | 0.09 | 0.12 | 0.18 | 21.3% |
| Cd (mg/kg) 1 | 0.08 | 2.63 | 0.34 | 0.49 | 0.47 | 0.23 | 0.56 | 11.4% |
| Cu (mg/kg) 1 | 14.7 | 104.5 | 39.3 | 41.9 | 15.7 | 32.3 | 50.4 | 11.2% |
| Mo (mg/kg) 2 | 0.57 | 4.13 | 1.48 | 1.58 | 0.66 | 1.2 | 1.8 | 40.5% |
| P (mg/kg) 1 | 230 | 1340 | 620 | 656 | 251 | 460 | 800 | 12.7% |
| Pb (mg/kg) 1 | 10.6 | 1540 | 52.6 | 128 | 205 | 25.4 | 146 | 10.4% |
| S (%) 1 | 0.01 | 0.13 | 0.03 | 0.03 | 0.02 | 0.02 | 0.04 | 18.1% |
| Sb (mg/kg) 1 | 0.6 | 8.2 | 1.2 | 1.5 | 1.2 | 0.9 | 1.7 | 8.9% |
| Sc (mg/kg) 1 | n.d. | 24.7 | 13.3 | 11.1 | 6.8 | 9.4 | 15.2 | 4.5% |
| Sn (mg/kg) 1 | 1.1 | 64.1 | 2.2 | 4.2 | 7.7 | 1.7 | 3.6 | 12.1% |
| Sr (mg/kg) 1 | 106 | 378 | 232 | 236 | 48 | 206 | 261 | 2.3% |
| W (mg/kg) 2 | 0.7 | 2.9 | 1.4 | 1.4 | 0.4 | 1.2 | 1.6 | 23.1% |
| Zn (mg/kg) 1 | 52 | 6010 | 120 | 216 | 588 | 90.5 | 188 | 8.7% |
| Analyte | Min. | Max. | Median | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Pb (mg/kg) | n.d. | 750 | 152 | 171 | 141 |
| Zn (mg/kg) | 67 | 333 | 159 | 167 | 64 |
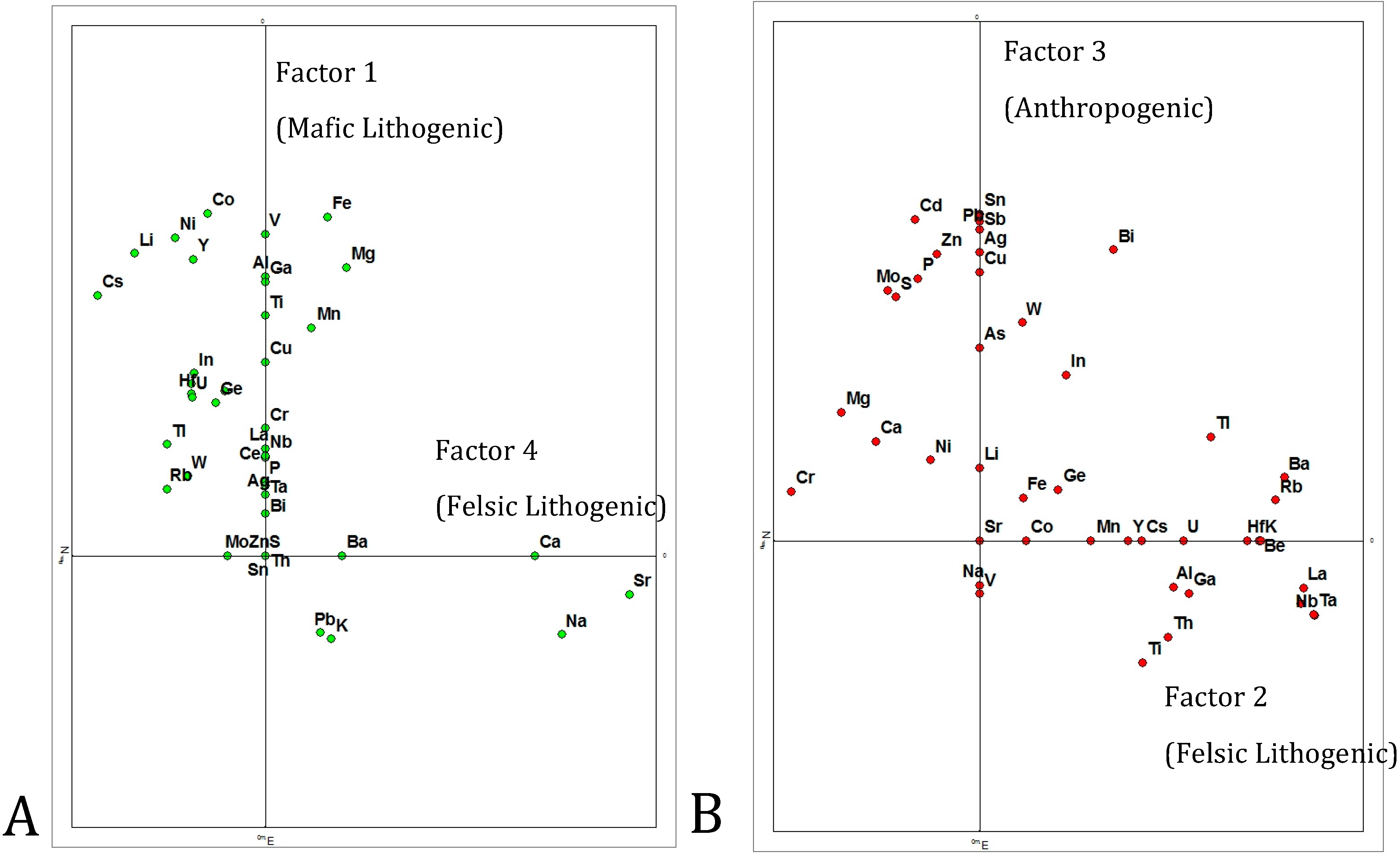
3.1.2. Intercorrelation of Metal Concentrations

3.2. Spatial Distribution of Metal Concentrations
3.2.1. Semivariance
| Analyte | Ag | As | Bi | Cd * | Cu | Mo | P | Pb * | S | Sb | Sc | Sn | W | Zn * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | 1 | 0.25 | 0.47 | 0.55 | 0.5 | 0.21 | 0.43 | 0.42 | 0.26 | 0.48 | 0.12 | 0.47 | 0.31 | 0.41 |
| As | 1 | 0.31 | 0.16 | 0.48 | 0.31 | 0.44 | 0.28 | 0.03 | 0.44 | 0.21 | 0.14 | 0.32 | 0.19 | |
| Bi | 1 | 0.46 | 0.50 | 0.34 | 0.39 | 0.59 | 0.22 | 0.63 | 0.15 | 0.69 | 0.44 | 0.43 | ||
| Cd | 1 | 0.56 | 0.53 | 0.51 | 0.79 | 0.58 | 0.53 | −0.1 | 0.61 | 0.38 | 0.82 | |||
| Cu | 1 | 0.49 | 0.51 | 0.46 | 0.37 | 0.63 | 0.22 | 0.58 | 0.52 | 0.51 | ||||
| Mo | 1 | 0.41 | 0.44 | 0.44 | 0.63 | 0.16 | 0.52 | 0.50 | 0.39 | |||||
| P | 1 | 0.35 | 0.37 | 0.39 | 0.10 | 0.33 | 0.31 | 0.48 | ||||||
| Pb | 1 | 0.41 | 0.57 | −0.2 | 0.54 | 0.23 | 0.75 | |||||||
| S | 1 | 0.34 | −0.2 | 0.34 | 0.35 | 0.61 | ||||||||
| Sb | 1 | 0.08 | 0.75 | 0.42 | 0.54 | |||||||||
| Sc | 1 | 0.08 | 0.27 | −0.1 | ||||||||||
| Sn | 1 | 0.47 | 0.54 | |||||||||||
| W | 1 | 0.44 | ||||||||||||
| Zn | 1 |
3.2.2. Prediction Map
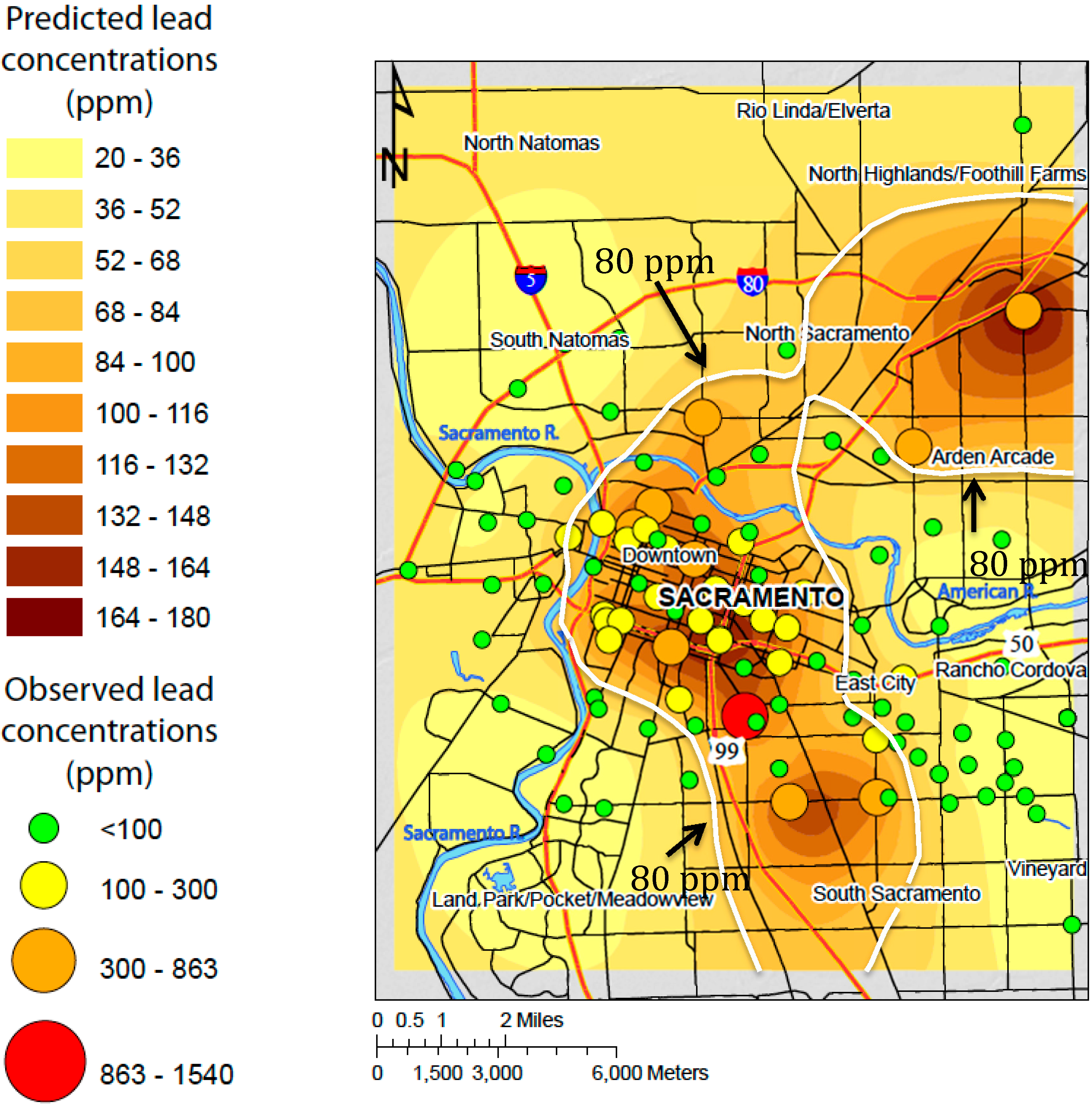
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Filippelli, G.M.; Laidlaw, M.A.S.; Latimer, J.C.; Raftis, R. Urban lead poisoning and medical geology: An unfinished story. GSA Today 2005, 15, 4–11. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M. Resuspension of urban soils as a persistent source of lead poisoning in children: A review and new directions. Appl. Geochem. 2008, 23, 2021–2039. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Matte, T.D.; Rogers, J.; Clickner, R.P.; Dietz, B.; Bornschein, R.L. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. Environ. Res. 1998, 79, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Bretsch, J.K. Soil lead and children’s blood lead levels in Syracuse, NY, USA. Environ. Geochem. Health 2002, 24, 375–385. [Google Scholar] [CrossRef]
- Tsuji, J.S.; Serl, K.M. Current uses of the EPA lead model to assess health risk and action levels for soil. Environ. Geochem. Health 1996, 18, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M.J. Spatial and Temporal Relationships between Blood Lead and Soil Lead Concentrations In Detroit, Michigan. M.Sc. Thesis, Wayne State University, Detroit, MI, USA, 2010. [Google Scholar]
- Laidlaw, M.A.; Taylor, M.P. Potential for childhood lead poisoning in the inner cities of Australia due to exposure to lead in soil dust. Environ. Pollut. 2011, 159, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Laidlaw, M.A.; Gonzales, C.R. Estimation of leaded (Pb) gasoline’s continuing material and health impacts on 90 urbanized areas. Environ. Int. 2011, 37, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.; Mielke, H.W.; McElmurry, S.P.; Filippelli, G.M.; Laidlaw, M.A.S.; Taylor, M.P. Determining the relative importance of soil sample locations to predict risk of children’s lead (Pb) exposure. Environ. Int. 2013, 60, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.; Laidlaw, M.; McElmurry, S.; Filippelli, G.M.; Taylor, M. Linking source and effect: Resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan. Environ. Sci. Technol. 2013, 47, 2839–2345. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control (CDC). Blood lead levels among children in high-risk areas — California, 1987–1990. Morbid. Mortal. Weekly Rep. 1992, 41, 291–294. [Google Scholar]
- Solt, M. Multivariate Analysis of Lead in Urban Soil in Sacramento, California. M.Sc. Thesis, California State University, Sacramento, CA, USA, 2010. [Google Scholar]
- Solt., M.; Deocampo, D.M. Multivariate analysis of lead in urban soil in Sacramento, California. Geol. Soc. Am. Abstr. Progr. 2010, 42, 615. [Google Scholar]
- Population of Sacramento County in 2010, State and County Quick Facts. Available online: http://quickfacts.census.gov/qfd/states/06/06067.html (accessed on 11 March 2015).
- Orr, W. Spatial Distribution of Lead Concentrations in Soils and Their Correlation with Reported Cases of Lead Poisoning, Sacramento, California. B.S. Thesis, California State University Sacramento, Sacramento, CA, USA, 2005. [Google Scholar]
- Deocampo, D.M.; Orr, W. Geochemistry of environmental lead in Sacramento, California. Geol. Soc. Am. Abstr. Progr. 2006, 38, 152. [Google Scholar]
- Childhood Lead Poisoning Data for California by County, Age, and Blood Lead Level for the Years 2007–2009; and Age of Housing Data for 2000. Available online: http://www.ehib.org/page.jsp?page_key=476&year=2009&pmn=EVENT%3DLEAD_TYPE%3DPRCT_AGE%3D0005_BLL%3D4594 (accessed on 9 March 2015).
- Tugel, A.J. Soil Survey of Sacramento County, California; Natural Resources Conservation Service: Sacramento, CA, USA, 1993. [Google Scholar]
- Alpers, C.N.; Antweiler, R.C.; Taylor, H.E.; Dileanis, P.D.; Domagalski, J.L. Metals Transport in the Sacramento River, California, 1996–1997, Volume 2: Interpretation of Metal Loads; Water-Resources Investigations Report 99-4286; U.S. Geological Survey: Sacramento, CA, USA, 2000. [Google Scholar]
- Goldhaber, M.B.; Morrison, J.M.; Holloway, J.M.; Wanty, R.B.; Hesel, D.R.; Smith, D.B. A regional soil and sediment geochemical study in Northern California. Appl. Geochem. 2009, 24, 1482–1499. [Google Scholar] [CrossRef]
- Dunlap, C.E.; Alpers, C.N.; Bouse, R.; Taylor, H.E.; Unruh, D.M.; Flegal, A.R. The persistence of lead form past gasoline emissions and mining drainage in a large riparian system: Evidence from lead isotopes in the Sacramento River, California, USA. Geochem. Cosmochim. Acta 2008, 72, 5935–5948. [Google Scholar] [CrossRef]
- Carlisle, J. Revised California Human Health Screening Levels for Lead; Office of Environmental Health Hazard Assessment, CalEPA: Sacramento, CA, USA, 2009. [Google Scholar]
- Diawara, M.M.; Litt, J.S.; Unis, D.; Alfonso, N.; Martinez, L.; Crock, J.G. Arsenic, cadmium, lead, and mercury in surface soils Pueblo, Colorado: Implications for population health risk. Environ. Geochem. Health 2006, 28, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F.M.; Brown, S.E.; Ander, E.L.; Rawlins, B.G.; O’Donnell, K.E. GSUE: Urban geochemical mapping in Great Britain. Geochem. Explor. Environ. Anal. 2005, 5, 325–336. [Google Scholar] [CrossRef]
- McGrath, D.; Zhang, C.; Carton, O.T. Geostatistical analyses and hazard assessment on soil lead in Silvermines Area, Ireland. Environ. Pollut. 2004, 127, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Nagourney, S.; Tummilo, N.J., Jr.; Birri, J.; Peist, K.; Macdonald, B.; Kane, J.S. Industrial sludge reference material for the analysis of acid-extractable metals. Talanta 1999, 50, 25–34. [Google Scholar] [CrossRef] [PubMed]
- R version 2.8.1.; The R Foundation for Statistical Computing: Vienna, Austria, 2008.
- Environmental Protection Agency (EPA). Standard Operating Procedure 906, Guidelines for Data Review of Contract Laboratory Program Analytical Services Inorganic Data Packages; EPA: Washington, DC, USA, 2005. [Google Scholar]
- Brown, R.W.; Gonzalez, C.; Hooper, M.J.; Bayat, A.C.; Fornerette, A.M.; McBride, T.J. Soil Lead (Pb) in residential transects through Lubbock, Texas: A preliminary assessment. Environ. Geochem. Health 2008, 30, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Reagan, P.L. Soil as an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Qin, Y.; Sang, S.X. Accumulation and sources of heavy metals in urban topsoils: A case study from the city of Xuzhou, China. Environ. Geol. 2005, 48, 101–107. [Google Scholar]
- Revised California Human Health Screening Level for Lead, 2009. Available online: http://oehha.ca.gov/risk/pdf/LeadCHHSL51809.pdf (accessed on 1 January 2015).
- Garcia, R.; Millan, E. Assessment of Cd, Pb, and Zn contamination in roadside soils and grasses from Gipuzkoa (Spain). Chemosphere 1998, 37, 1615–1625. [Google Scholar] [CrossRef]
- Wu, J.; Edwards, R.; He, X.E.; Liue, Z.; Kleinman, M. Spatial analysis of bioavailable soil lead concentrations in Los Angeles, California. Environ. Res. 2010, 110, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.E.; Wixson, B.G. Use of factor analysis to differentiate pollutants from other trace metals in surface soils of the mineralized area of Madison County, Missouri, USA. Water Air Soil Pollut. 1987, 33, 339–348. [Google Scholar] [CrossRef]
- Cicchella, D.; de Vivo, B.; Lima, A.; Albanese, S.; McGill, R.A.R.; Parrish, R.R. Heavy metal pollution and Pb isotopes in urban soils of Napoli, Italy. Geochem. Explor. Environ. Anal. 2008, 8, 103–112. [Google Scholar] [CrossRef]
- Ratha, D.S.; Sahu, B.K. Source and distribution of metals in urban soil of Bombay, India, using multivariate statistical techniques. Environ. Geol. 1993, 22, 276–285. [Google Scholar] [CrossRef]
- Korre, A. Statistical and spatial assessment of soil heavy metal contamination in areas of poorly recorded, complex sources of pollution-Part 1: Factor analysis for contamination assessment. Stochast. Environ. Res. Risk Assess. 1999, 13, 260–287. [Google Scholar] [CrossRef]
- Sutton, P.M.; Athanasoulis, M.; Flessel, P.; Guirguis, G.; Haan, M.; Schlag, R.; Goldman, L.R. Lead levels in the household environment of children in three high-risk communities in California. Environ. Res. 1995, 68, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Zhang, Z.; Dai, J. Factorial kriging and stepwise regression approach to identify environmental factors influencing spatial multi-scale variability of heavy metals in soils. J. Hazard. Mater. 2013, 261, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Zhang, Z.; Dai, B. Multivariate geostatistical analyses of heavy metals in soils: Spatial multi-scale variations in Wulian, Eastern China. Ecotox. Environ. Safe. 2014, 107, 140–147. [Google Scholar] [CrossRef]
- Mielke, H.W.; Laidlaw, M.A.S.; Gonzales, C.R. Lead (Pb) legacy from vehicle traffic in eight California urbanized areas: Continuing influence of lead dust on children’s health. Sci. Total Environ. 2010, 19, 3965–3975. [Google Scholar] [CrossRef]
- Gould, E. Childhood lead poisoning: Conservative estimates of the social and economic benefits of lead hazard control. Environ. Health Persp. 2009, 117, 1162–1167. [Google Scholar] [CrossRef]
- CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. Available online: http://www.cdc.gov/nceh/lead/acclpp/CDC_Response_Lead_Exposure_Recs.pdf. (accessed on 9 March 2015).
- Surkan, P.J.; Zhang, A.; Trachtenberg, F.; Daniel, D.B.; McKinlay, S.; Bellinger, D.C. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology 2007, 28, 1170–1177. [Google Scholar]
- Menke, A.; Muntner, P.; Batuman, V.; Silbergeld, E.K.; Guallar, E. Blood lead below 0.48 µmol/L (10µg/dL) and mortality among US adults. Circulation 2006, 114, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, G.M.; Laidlaw, M.A.S. The elephant in the playground, confronting lead-contaminated soils as an important source of lead burdens to urban populations. Persp. Biol. Med. 2010, 53, 31–45. [Google Scholar] [CrossRef]
- Bellinger, D.C. The protean toxicities of lead: New chapters in a familiar story. Int. J. Environ. Res. Public Health 2011, 8, 2593–2628. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solt, M.J.; Deocampo, D.M.; Norris, M. Spatial Distribution of Lead in Sacramento, California, USA. Int. J. Environ. Res. Public Health 2015, 12, 3174-3187. https://doi.org/10.3390/ijerph120303174
Solt MJ, Deocampo DM, Norris M. Spatial Distribution of Lead in Sacramento, California, USA. International Journal of Environmental Research and Public Health. 2015; 12(3):3174-3187. https://doi.org/10.3390/ijerph120303174
Chicago/Turabian StyleSolt, Michael J., Daniel M. Deocampo, and Michelle Norris. 2015. "Spatial Distribution of Lead in Sacramento, California, USA" International Journal of Environmental Research and Public Health 12, no. 3: 3174-3187. https://doi.org/10.3390/ijerph120303174
APA StyleSolt, M. J., Deocampo, D. M., & Norris, M. (2015). Spatial Distribution of Lead in Sacramento, California, USA. International Journal of Environmental Research and Public Health, 12(3), 3174-3187. https://doi.org/10.3390/ijerph120303174





