1. Introduction
Mold allergies affect from 3% to 40% of the human population, and this level changes depending on the country, region, sex, age and other factors [
1]. So far, 78 allergens isolated from 18 mold species have been characterized in terms of chemical structure, nucleotide sequence and cellular function (International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub-committee). These 18 species belong to the following genera:
Alternaria, Cladosporium, Penicillium, Aspergillus, Curvularia, Epicoccum, Stachybotrys and
Trichophyton. Previous work found that
Alternaria alternata is the most allergenic species amongst those studied to date [
2,
3,
4,
5]. The high allergenicity of this species is hypothesized to be due to morphological changes in the respiratory epithelium, which is a result of the proteolytic enzymes produced by this mold. It is estimated that the presence of 100 spores of
Alternaria alternata in 1 m
3 of air is the threshold concentration, at which symptoms of allergy occur in patients who are sensitized to this species [
6].
The major allergen produced by
Alternaria alternata is the glycoprotein Alt a 1, which has a molecular weight of 30 kDa (dimer). This glycoprotein migrates as two separate bands of 16.4 and 15.3 kDa under reducing conditions on SDS-PAGE, suggesting that the monomers are linked by a disulfide bond. Alt a 1 is detected in the cytoplasm of mold spores and mycelia [
7]. Recent
X-ray crystallography revealed that the protein has a unique β-barrel fold that is cysteine-linked. Its biological function in cells is still unknown [
8]. Alt a 1 leads to IgE-mediated hypersensitivity in more than 95% of
Alternaria-sensitized patients [
5,
8,
9]. It should also be noted that the number of allergens in
A. alternate extracts may range from 10 to 30, and a few allergens were present in nearly all extracts studied [
10].
Elevated levels of airborne
A. alternata intensify rhinitis symptoms in allergic individuals [
3,
11]. They are the most common asthma-causing molds and also increase the severity of the disorder [
1,
12,
13,
14]. Long-term inhalation of antigens can cause allergic alveolitis (hypersensitivity pneumonitis (HP) [
15,
16]. As a source of allergens, molds of the
Alternaria genus may also be a factor in causing asthma in bakers [
17], and allergic alveolitis in carpenters and wood processors [
18]. Other professional groups exposed to
A. alternata allergens are workers in contact with either plant raw materials infested by these molds (grains, fruits, vegetables) or technical materials susceptible to microbial degradation (wood, paper, cloth). The high-risk groups that are in contact with the allergen include farmers, gardeners, employees of grain elevators, and food and herbal industries, forest service, monument conservators, librarians, museum personnel or those who deal with the storage and processing of municipal waste [
18,
19,
20,
21].
Occupational allergies associated with exposure to molds are poorly understood and difficult to detect. This is due to the multiplicity of factors arising from both occupational and non-occupational environments, which can cause symptoms similar to the disease [
21]. Phylogenetic classification of the
Alternaria genus is challenging. Recent molecular studies have revealed multiple non-monophyletic genera within the
Alternaria complex and
Alternaria species clades, which do not always correlate with morphological characteristic-based species groups [
22]. In addition, the sequences of DNA fragments of identified strains show high homology (99%–100%) to multiple reference sequences (deposited in databases (e.g., GenBank)) at the same time and those derived from molds belonging to different species. Furthermore, Balajee
et al. found that 20% of the ITS region sequence has come from erroneously identified mold species, and 14% of sequences derived from strains of the
Alternaria genus, are incorrectly described [
23].
The aim of this study was to evaluate the ability of environmental isolates of Alternaria, obtained from workplaces within libraries, a museum, a composting plant and a tannery, to produce the allergen, Alt a 1. The study also aimed to determine whether materials such as cellulose, compost and wet blue leather, that are stored/processed in the above working environments, stimulate or inhibit the amount of Alt a 1 generated.
The scope of the study included: (1) the isolation of Alternaria sp. strains from workplaces within libraries, a museum, a composting plant and a tannery; (2) determination of their percentages and frequencies of occurrence at each workplace; (3) morphological and genetic identification of the isolated Alternaria molds by analyzing nucleotide sequences of the ITS1/ITS2 regions, actin, calmodulin and Alt a 1 genes; (4) confirming the presence of the gene encoding the Alt a 1 allergenic protein; (5) evaluating Alt a 1 production using an immunoassay; and (6) comparing the amounts of Alt a 1 from control media and from those simulating the environments from which the tested strains were isolated.
3. Results
Air and surfaces in the studied workplaces were contaminated by molds to varying degrees. The highest numbers of airborne molds were recorded in Library A and the composting plant (5.3 × 10
3 CFU/m
3 and 1.7 × 10
3 CFU/m
3, respectively). The lowest airborne fungal contamination was detected in the Museum and Archive, and it ranged from 7.2 × 10
1 to 9.2 × 10
1 CFU/m
3. Fungal contamination of surfaces varied by institution, and was mainly dependent on the hygienic conditions of the stored objects and equipment. This concentration ranged from 1.5 × 10
1 CFU/100 cm
2 to 3.3 ×10
3 CFU/100 cm
2 (
Table 4).
Table 4.
Mold contamination of tested workplaces.
Table 4.
Mold contamination of tested workplaces.
| Working Environment | Level of Fungal Contamination at Workplaces | Alternaria sp. Concentration |
|---|
| Air [CFU/m3] | Surfaces [CFU/100cm2] | Number | Percentage [%] | Frequency [%] |
|---|
| Air [CFU/m3] | Surfaces [CFU/100cm2] | Air | Surfaces | Air | Surfaces |
|---|
| Archive | M: 9.2 × 101 | M: 3.3 × 103 | 6.1 × 100 | 0.0 | 6.6 | 0.0 | 14.3 | 0.0 |
| Min: 1.0 × 101 | Min: 5.0 × 100 |
| Max: 2.1 × 102 | Max: 1.2 × 104 |
| SD: 7.9 × 101 | SD: 1.2 × 103 |
| Tannery | M:7.3 × 102 | M:1.5 × 101 | 7.3 × 101 | 0.0 | 10.0 | 0.0 | 39.0 | 0.0 |
| Min:1.7 × 102 | Min 5.1 × 100 |
| Max:2.21 × 03 | Max:4.6 × 101 |
| SD:3.0 × 102 | SD:1.5 × 101 |
| Composting plant | M:1.7 × 103 | M:1.5 × 103 | 1.0 × 102 | 0.0 | 5.9 | 0.0 | 64.0 | 0.0 |
| Min:8.8 × 102 | Min:1.0 × 103 |
| Max:3.4 × 103 | Max:2.0 × 103 |
| SD:7.2 × 102 | SD:5.1 × 102 |
| Library A | M: 5.3 × 103 | M: 1.3 × 102 | 2.9 × 102 | 0.0 | 5.5 | 0.0 | 16.7 | 0.0 |
| Min: 2.2 × 103 | Min: 4.2 × 100 |
| Max: 1.1 × 104 | Max: 8.4 × 102 |
| SD: 2.9 × 103 | SD: 2.1 × 102 |
| Library B | M: 5.2 × 102 | M: 1.0 × 103 | 1.0 × 101 | 2.1 × 102 | 1.9 | 21.5 | 33.3 | 50.0 |
| Min: 2.5 × 102 | Min: 5.0 × 10° |
| Max: 1.3 × 103 | Max: 6.4 × 103 |
| SD: 4.4 × 102 | SD: 3.9 × 103 |
| Museum | M: 7.7 × 101 | M: 1.0 × 102 | 0.0 | 4.9 × 101 | 0.0 | 49.0 | 0.0 | 14.3 |
| Min.: 0.0 | Min.: 0.0 |
| Max.:2.4 × 102 | Maks.: 8.0 × 102 |
| SD: 5.8 × 101 | SD: 2.2 × 102 |
Molds of the
Alternaria genus were present in the air of the workplaces tested, (strains No. 1–7) at concentrations ranging from 6.1 × 10
0 CFU/m
3 to 2.9 × 10
2 CFU/m
3, accounting for 2%–10% of the fungal aerosols in the analyzed environments, and were detected using culture methods.
Alternaria strains were found on surfaces in Museum and Library B at concentrations of 4.9 × 10
1 CFU/100 cm
2 to 2.1 × 10
2 CFU/100 cm
2, representing 9%–49% of all molds.
Alternaria strains were isolated from the workplaces at a high incidence: 14%–64% in the air and 14%–50% on surfaces (
Table 4).
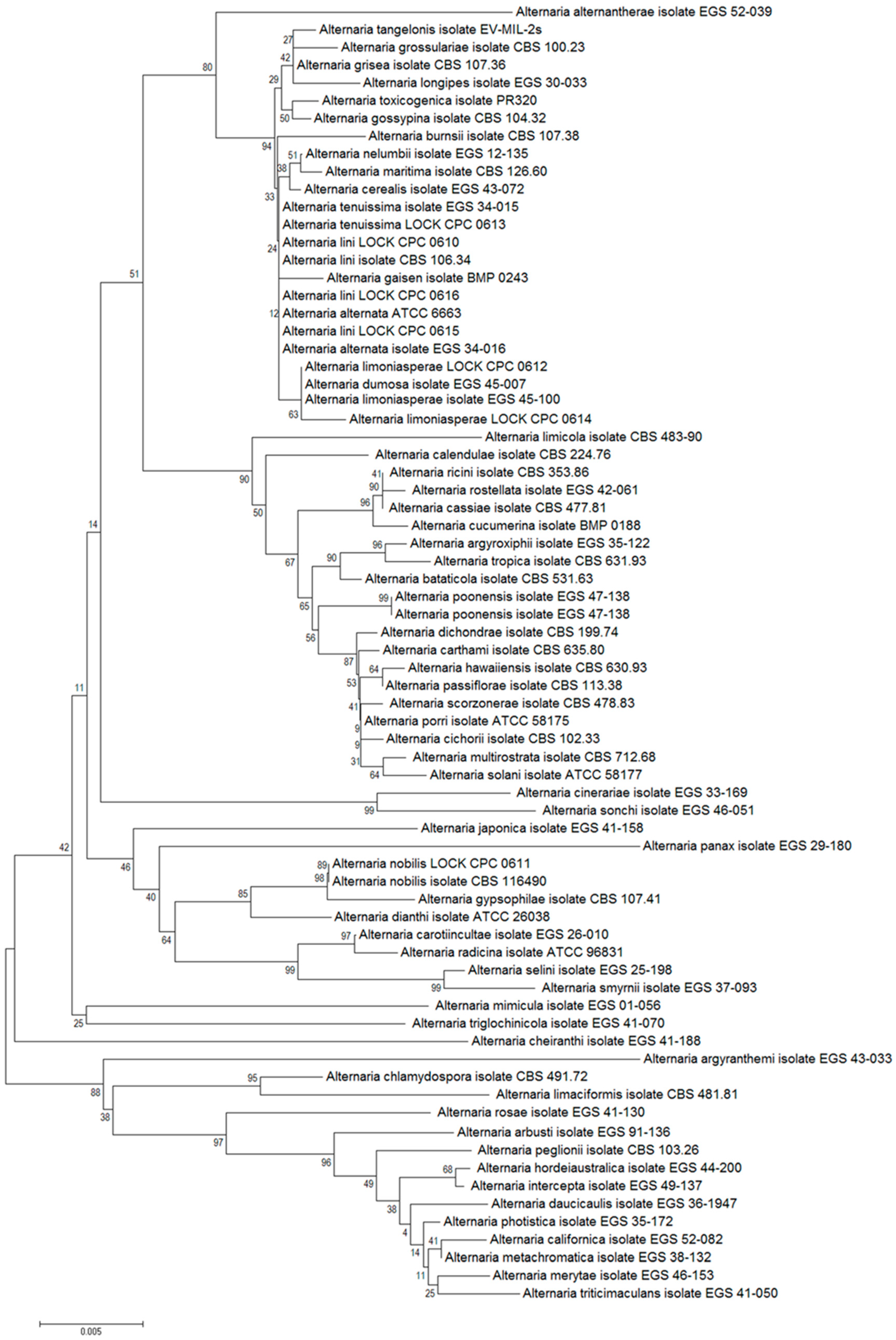
Genetic identification based on nucleotide sequence analyses of the ITS1/ITS2 regions, actin, calmodulin and the
Alt a 1 gene confirmed the phylogenetic affiliation of the three tested fungal strains with
Alternaria lini, two with
A. limoniasperae, while the remaining strains were affiliated with
A. tenuissima,
A. nobilis and
A. alternata. The degree of similarity of all analyzed nucleotide sequences ranged from 87% to 99% (
Table 5,
Figure 1).
Table 5.
Detection of Alt a 1 gene for tested Alternaria strains.
Table 5.
Detection of Alt a 1 gene for tested Alternaria strains.
| Strain No. | Strain | Presence of Alt a 1 gene | GenBank accession number of Alt a 1 gene * |
|---|
| 1 | Alternaria lini | + | KP341689 |
| 2 | Alternaria nobilis | + | KP341690 |
| 3 | Alternaria limoniasperae | + | KP341691 |
| 4 | Alternaria tenuissima | + | KP341692 |
| 5 | Alternaria limoniasperae | + | KP341693 |
| 6 | Alternaria lini | + | KP341694 |
| 7 | Alternaria lini | + | KP341695 |
| 8 | Alternaria alternata ATCC 6663 | + | KP341688 | |
Figure 1.
The phylogenetic tree constructed on the basis of actin gene sequences of reference and tested Alternaria strains. The tree was constructed using the Neighbor-Joining method and tested by bootstrapping (1000 replicates).
Figure 1.
The phylogenetic tree constructed on the basis of actin gene sequences of reference and tested Alternaria strains. The tree was constructed using the Neighbor-Joining method and tested by bootstrapping (1000 replicates).
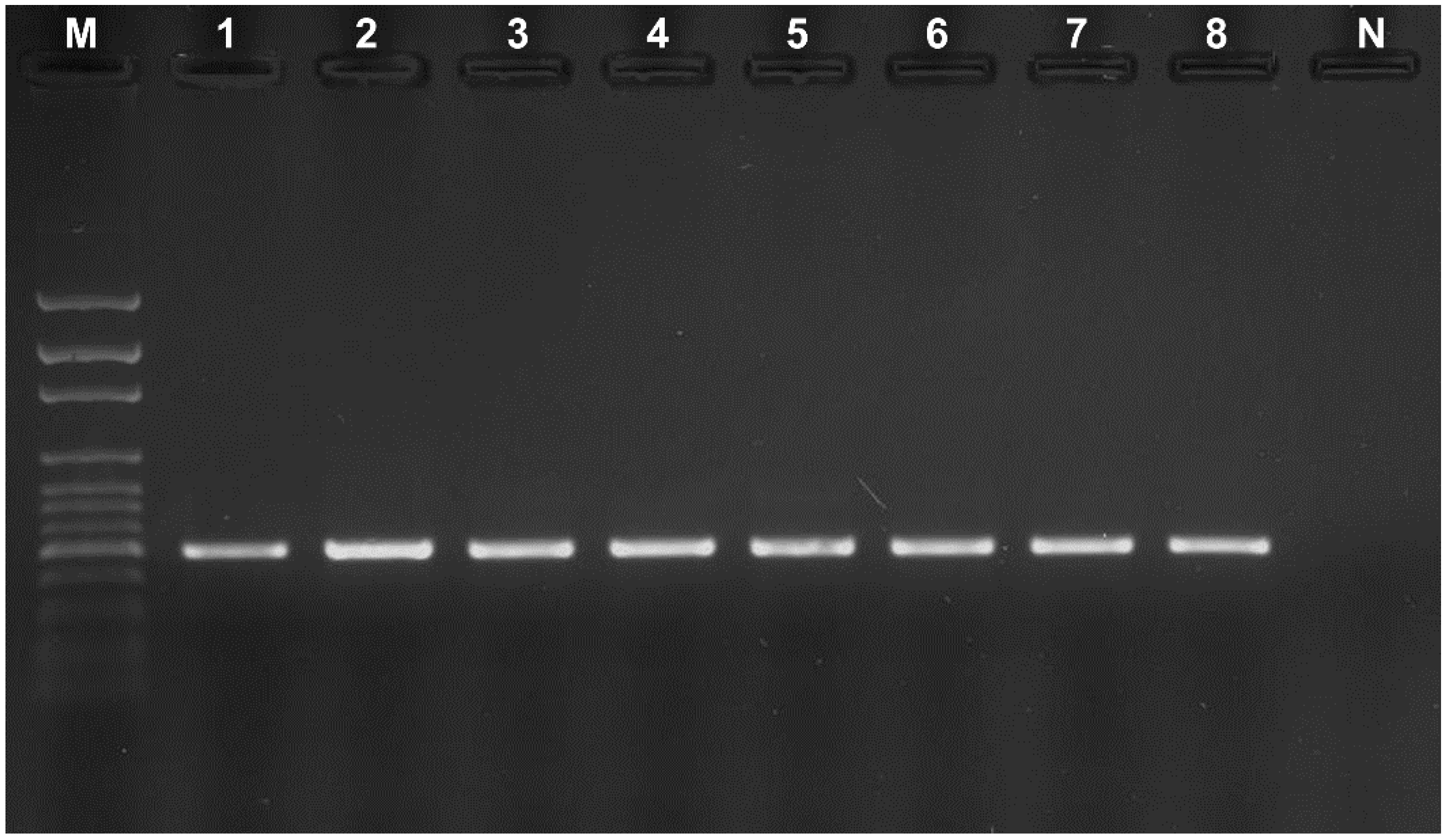
PCR amplification of the
Alt a 1 gene resulted in products that were approximately 510 bp in length. A gene encoding the allergenic protein Alt a 1 was identified in the tested strains, i.e. the isolates from the studied workplaces, and
Alternaria alternata from ATCC (
Figure 2).
Figure 2.
Detection of Alt a 1 gene in tested Alternaria strains. M—molecular weight marker from 100 bp to 3000 bp (Solis BioDyne, Tartu, Estonia), 1–7—tested Alternaria strains, 8—Alternaria alternata ATCC 6663 (positive control), N—negative control.
Figure 2.
Detection of Alt a 1 gene in tested Alternaria strains. M—molecular weight marker from 100 bp to 3000 bp (Solis BioDyne, Tartu, Estonia), 1–7—tested Alternaria strains, 8—Alternaria alternata ATCC 6663 (positive control), N—negative control.
Alt a 1 levels obtained on CYA culture medium were compared after culturing a selected environmental strain (
Alternaria lini) and
Alternaria alternata ATCC 6663, using different methods of sample preparation: filtration (F), filtration preceded by mycelium homogenization (HF) and sample concentration using ultrafiltration (HFU) (
Table 6). Alt a 1 levels increased from 0.446–0.902 ng/mL to 1.072–1.303 ng/mL following homogenization compared to its concentration in the post-culture filtrate. However, the highest (
p < 0.05) protein levels ranging from 8.932–44.071 ng/mL were obtained after using ultrafiltration for sample concentration (
Table 6).
Table 6.
Comparison of methods of Alt a 1 protein obtaining for selected strains of Alternaria.
Table 6.
Comparison of methods of Alt a 1 protein obtaining for selected strains of Alternaria.
| Species | Concentration of Alt a 1 in ELISA Test [ng/mL] |
|---|
| F | HF | HFU |
|---|
| Alternaria lini | 0.902 ± 0.039 | 1.303 ± 0.277 | 8.932 ± 0.362 *,$ |
| Alternaria alternata ATCC 6663 | 0.446 ± 0.022 | 1.072 ± 0.046 # | 44.071 ± 7.096 *,$ |
Strains isolated from workplaces produced Alt a 1 in concentrations ranging from 1.103 to 6.528 ng/mL, depending on the strain and culture medium—CYA, M
0 or supplemented M
0 (
Table 7).
Table 7.
Production Alt a 1 allergenic protein by tested Alternaria strains.
Table 7.
Production Alt a 1 allergenic protein by tested Alternaria strains.
| Strain No. | Species | Concentration of Alt a 1 in ELISA test [ng/mL] |
|---|
| CYA | M0 | Suplemented M0 |
|---|
| 1 | Alternaria lini | 6.528 ± 2.219 | 1.938 ± 0.187 *↓ | C: 1.627 ± 0.082 |
| 2 | Alternaria nobilis | 1.890 ± 0.269 | 1.103 ± 0.484 | W: 2.444 ± 1.411 |
| 3 | Alternaria limoniasperae | 1.500 ± 0.201 | 3.066 ± 0.937 *↑ | CE: 2.051 ± 0.835 |
| 4 | Alternaria tenuissima | 2.010 ± 0.373 | 4.110 ± 0.638 *↑ | C: 2.508 ± 0.800 |
| 5 | Alternaria limoniasperae | 2.598 ± 1.694 | 5.164 ± 3.231 | C: 1.584 ± 0.644 |
| 6 | Alternaria lini | 3.260 ± 0.437 | 1.680 ± 0.494 *↓ | C: 1.126 ± 0.677 |
| 7 | Alternaria lini | 1.485 ± 1.010 | 2.296 ± 0.995 | C: 1.572 ± 0.401 |
| 8 | Alternaria alternata ATCC 6663 | 0.902 ± 0.299 | 0.824 ± 0.280 | C: 0.598 ± 0.077 |
| W: 0.975 ± 0.430 |
| CE: 0.551 ± 0.102 |
There was no statistically significant relationship between the type of medium and the concentration of Alt a 1 produced by the tested strains on CYA medium and mineral M
0 medium. In the case of two strains (
A. limoniasperae isolated from the composting plant and
A. tenuissima from the library), mineral M
0 medium stimulated the molds to produce more allergen compared to CYA medium. A different situation was observed for two strains of
Alternaria lini isolated from the archive and the library. A lower, statistically significant, Alt a 1 level was achieved on M
0 medium compared to its concentration on CYA medium. There were no statistically significant differences in Alt a 1 levels obtained on mineral M
0 medium and on the medium simulating environmental conditions. It was shown that the addition of material (cellulose, compost, wet-blue leather) to M
0 culture medium did not affect the concentration of Alt a 1. The culture collection strain
Alternaria alternata ATCC 6663 produced smaller amounts of the allergen (0.551–0.975 ng/mL), compared to the environmental isolates (
Table 7).
4. Discussion
The highest concentration of airborne molds amongst the workplaces was found in the composting plant, slightly lower in the libraries and the tannery, and lowest in the museum and the archive. However, fungal contamination levels in all studied workplaces (from 7.7 × 10
1 to 5.3 × 10
3 CFU/m
3) were lower than the threshold values of occupational exposure specified by the Polish Committee (for the Highest Permissible Concentrations and Intensities of Noxious Agents at the Workplace: 5.0 × 10
3/5.0 × 10
4 CFU/m
3 for the total fungal count in public facilities/organic dust contaminated workplaces) [
36]. However, there are no (national or international) legal regulations establishing permissible workplace concentrations of number of microorganisms [
37]. Evaluation of the level of airborne microbiological contamination in working environments is currently carried out on the basis of recommendations from the literature including “threshold limit value”, “acceptable maximum value”; “maximum allowable concentration”
etc. [
38,
39]. What is worth emphasizing a series of studies including various fungal species suggest that respiratory symptoms, airway inflammation, and lung function impairment begin to related with exposure levels of approximately 10
5 spores/m
3 [
40]. This contamination level is probably too high if spores from mycotoxin-producing and/or opportunistic pathogenic species are prevalent. Moreover, people with asthma and sensitized to fungal allergens are more susceptible than working population in general. It is estimated that
Alternaria alternata spores in concentration 100/m
3 in the air can cause allergy symptoms in sensitized people [
6]. In 2 from 6 tested working environment (composting plant and Library A) this limit has been exceeded in case of
Alternaria strains. Employees of these workplaces are the most exposed to allergic diseases.
The present study shows that molds of the genus
Alternaria, producing the allergenic protein Alt a 1, represent a significant component of the ecosystem of the tested workplaces. This is based on their relative proportions (2%–49%), and their frequencies of isolation from the pool of all detected molds (14%–64%). Species of the genus
Alternaria were already isolated from workplaces such as libraries, archives and museums in previous studies [
41,
42]. Grisoni
et al. detected high levels of
Alternaria alternata in the vicinity of a composting plant and a wastewater treatment plant throughout the year, and this concentration decreased with increasing distance from the plants [
43].
The present study indicates that persons employed in museums, archives, libraries, composting plants and tanneries involve a risk of mould exposure of the
Alternaria genus. The ability of the isolates to produce Alt a 1 protein has been confirmed. Other authors have already pointed to the allergenic effects of
Alternaria alternata in the home and workplace environments, especially on people exposed to moldy wood dust, as well as on bakers and museum personnel [
17,
21,
44].
Also should be mentioned that
Alternaria like other molds is a potential sorce of β-D-glucan (a polymer of N-acetyl-β-D-glucosamine, chitin). β-D-Glucan is a component of the fungal cell wall, which is a potent activator of the immune system, causing a non-allergic respiratory disease. The effects of exposure to glucan can lead to fatigue, headaches and other neurological symptoms in exposed workers [
45,
46]. Occupational exposure to β-D-glucan has been most commonly described in the municipal waste industry, wastewater treatment plants and in different branches of agriculture [
47,
48,
49].
In the future, the sensitivity of employees within tested environments to Alt a 1 should be confirmed by medical examination. A occupational risk assessment at the workplace is the responsibility of the employer. Risk assessment should be done if there is a suspicion that the employee’s health problems are associated with exposure to a biological agent at the workplace. The suspicion that the disease results from the employee’s exposure to mold must be confirmed by an occupational physician. Diagnostics of the allergy include skin prick tests and determination of IgE specific for mold allergens of employees in the serum. Many authors claim that allergen extracts produced by molds, show a clear lack of homogeneity for a number of reasons, including strain identification and variability, culture conditions or production methods [
50,
51,
52].
In this study, a gene encoding the major allergen Alt a 1 was detected not only in
Alternaria alternata strains, but also in
A. lini,
A. limoniasperae,
A. tenuissima and
A. nobilis. Hong
et al. found the
Alt a 1 gene and its homologs in
A. alternata A. limoniasperae, A. tenuissima and others species of the genus
Alternaria [
31]. Moreover, a comparison between Alt a 1 homologs of several
Alternaria species revealed greater sequence divergence than that found in similar comparisons of other ribosomal and protein-coding genes. Therefore it is used as a marker to identify strains belonging to this species.
Sáenz-de-Santamaría
et al., suggested a strong cross-reactivity with other species affiliated with
Alternaria alternata, indicating that it may also concern the genera
Stemphyllium,
Ulocladium or
Curvularia [
52]. This fact is very importand in assesment of allery risk in working environments. It should be be taken into account that detection of Alt a 1 sensivity in workers is not always related to
Alternaria alternata sensitiveness.
In the present study, allergen concentrations obtained from the culture filtrates of
Alternaria strains ranged from 0.598 to 6.528 ng/mL, which confirm Alt a 1 production variability. Sáenz-de-Santamaría
et al., demonstrated that various species of the
Alternaria genus produce different amounts of Alt a 1, e.g.,
A. tenuisima FMR5813 produces over four times more Alt a 1 than
A. alternata IMMS 93039 [
52]. Martínez
et al., studied various strains of the
A. alternata species, and Alt a 1 levels in post-culture extracts ranged from 0.5 to 16.1 ng/mL [
53]. It is also important to note that environmental strains of
Alternaria alternata produced higher levels of Alt a 1 (1.103 to 6.528 ng/mL) than the strain obtained from the ATCC collection (0.551 to 0.975 ng/mL).
So far, little is known about the impact that technical materials, present in the natural environments of molds, have on allergen production. This study could not demonstrate an effect of technical materials on the production of Alt a 1. For all strains tested, Alt a 1 levels in the mineral medium containing cellulose, compost or wet-blue leather, were statistically comparable to those obtained from the control medium (without additives).
Different results were obtained by Gutarowska
et al., who studied molds isolated from home environments [
54]. They found that more allergenic proteins were produced on building materials (wallpaper, carton-gypsum board), compared to laboratory medium M
0. Other studies also reported the effect of different culture media on the amount of allergens produced by molds belonging to the
Aspergillus,
Cladosporium and
Penicillium genera [
55,
56].
Alt a 1 levels are significantly affected by the method used to obtain proteins from a mold culture. In the present study, the allergenic protein Alt a 1 was extracted from a culture filtrate. Other studies also showed that Alt a 1 accumulates in culture media, and therefore culture filtrates constitute the optimum source of this protein [
57,
58,
59]. Furthermore, the present study has demonstrated that the homogenization of the mycelium increases Alt a 1 levels by 44%–140%, while ultrafiltration increases it by 10%–98%. The results show that homogenization and ultrafiltration seem to be suitable methods for the industrial production of Alt a 1, for example, for immunotherapeutic purposes. Lizaso
et al. found strong reactivity of
Alternaria alternata extracts, prepared using
in vivo ultrafiltration, in skin prick tests (SPT) [
59].