Prevalence of Virulence Determinants and Antimicrobial Resistance among Commensal Escherichia coli Derived from Dairy and Beef Cattle
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacterial Isolates Collection
2.2. Phylogenetic Analysis
2.3. Virulence Factors Identification
2.4. Analysis of Antimicrobial Susceptibility
2.5. Antimicrobial Resistance Genes Identification
2.6. Statistical Analysis
3. Results
3.1. Phylogenetic Grouping
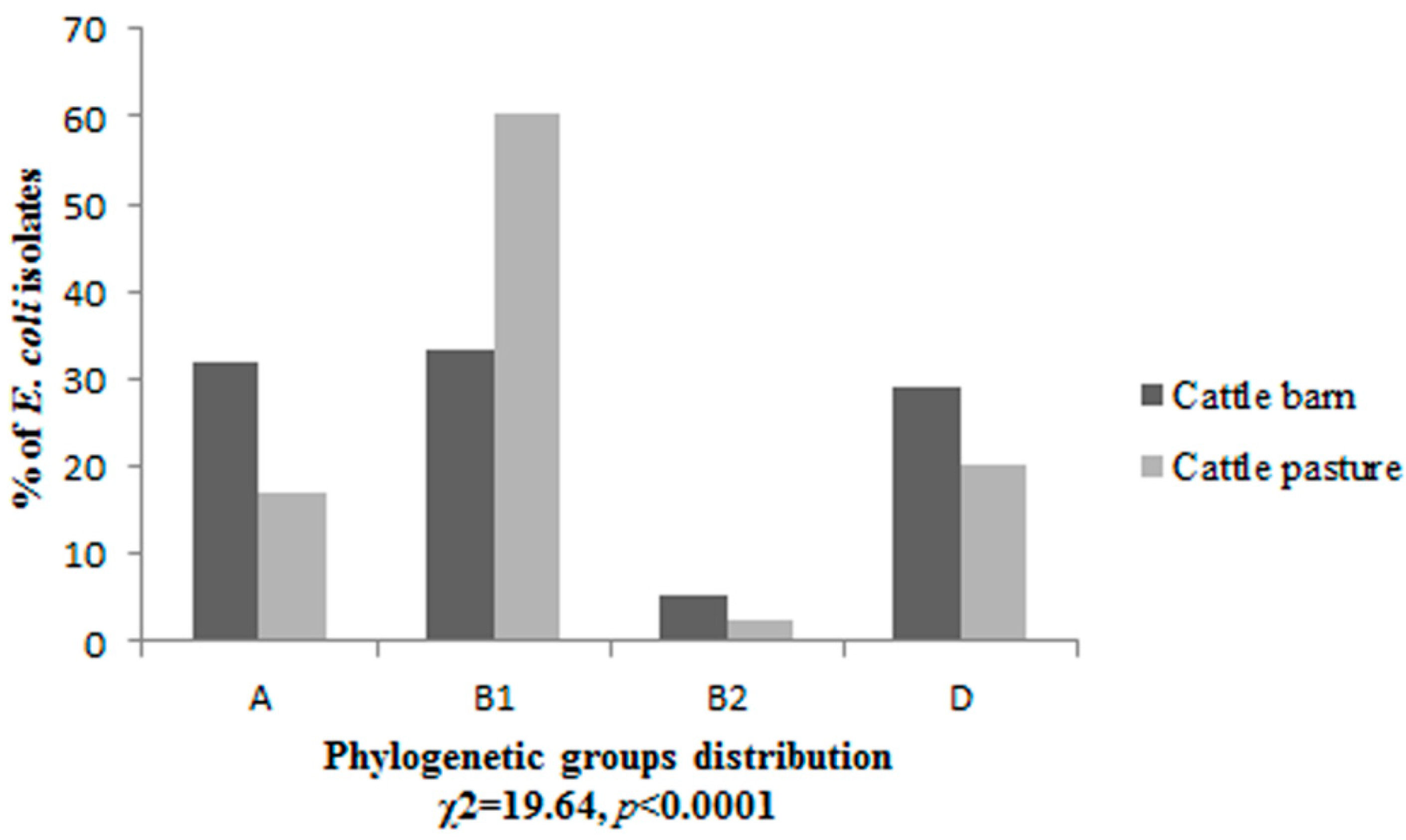
3.2. Prevalence of Virulence Factors
| (A) | ||||||||||
| VFs Profiles | Number of VFs-Positive Isolates in the Phylogenetic Groups | OR (95% CI) a | ||||||||
| Cattle Barn n = 147 | Cattle Pasture n = 118 | |||||||||
| A | B1 | B2 | D | A | B1 | B2 | D | |||
| escV | 1 | 2 | - | - | - | - | - | - | - | |
| stx1 | - | 1 | - | - | 1 | 1 | - | - | 1.63 (0.0035–762.296) b | |
| stx2 | - | 1 | 1 | 1 | 1 | 1 | - | 1 | 1.48 (0.293–7.512) b | |
| ehxA | - | - | - | 1 | 1 | - | - | - | 1.50 (0.089–25.307) b | |
| stx2, ehxA | - | - | - | - | - | 2 | - | - | - | |
| stx2, estI | - | - | - | - | 4 | - | - | - | - | |
| stx1, stx2, ehxA | - | - | - | - | - | - | - | 1 | - | |
| hlyA | - | - | - | - | - | 8 | - | 1 | - | |
| stx1, hlyA | - | 1 | - | - | - | 1 | - | 2 | 2.67 (0.214–33.283) b | |
| Total | 1 | 5 | 1 | 2 | 7 | 13 | - | 5 | ||
| Total no. (%) of VFs-positive isolates | 9 (6,1) | 25 (21,2) | 6.84 (1.036–45.220) * | |||||||
| No. (%) of STEC isolates stx1 and/or stx2 | 5 (3.4) | 15 (12.7) | 6.18 (1.041–36.672) * | |||||||
| a reference category—cattle barn; b the estimated OR, 95% CI refers to the sum of the VFs profiles of all the phylogroups; * statistically significant. | ||||||||||
| (B) | ||||||||||
| VFs Profiles | Number of Animals with E. coli Positive for VFs Profiles | OR (95% CI) a | Test of Independence p-value | |||||||
| Cattle Barn n = 50 | Cattle Pasture n = 42 | |||||||||
| escV | 2 | 0 | - | - | ||||||
| stx1 | 1 | 2 | 3.07 (0.266–35.491) | 0.5622 | ||||||
| stx2 | 3 | 3 | 1.54 (0.289–8.155) | 0.680 | ||||||
| ehxA | 1 | 1 | 1.59 (0.096–26.539) | 1 | ||||||
| stx2, ehxA | 0 | 1 | - | - | ||||||
| stx2, estI | 0 | 2 | - | - | ||||||
| stx1, stx2, ehxA | 0 | 1 | - | - | ||||||
| hlyA | 0 | 6 | - | - | ||||||
| stx1, hlyA | 1 | 2 | 2.45 (0.214–28.009) | 0.590 | ||||||
| Total no. (%) of animals with E. coli positive for VFs | 7 (14) | 14 (33.3) | 3.07 (1.103–8.557) * | 0.044 * | ||||||
| No. (%) of animals with STEC isolates stx1 and/or stx2 | 4 (8) | 10 (23.8) | 3.59 (1.036–12.471) * | 0.044 * | ||||||
| a reference category—cattle barn; * statistically significant. | ||||||||||
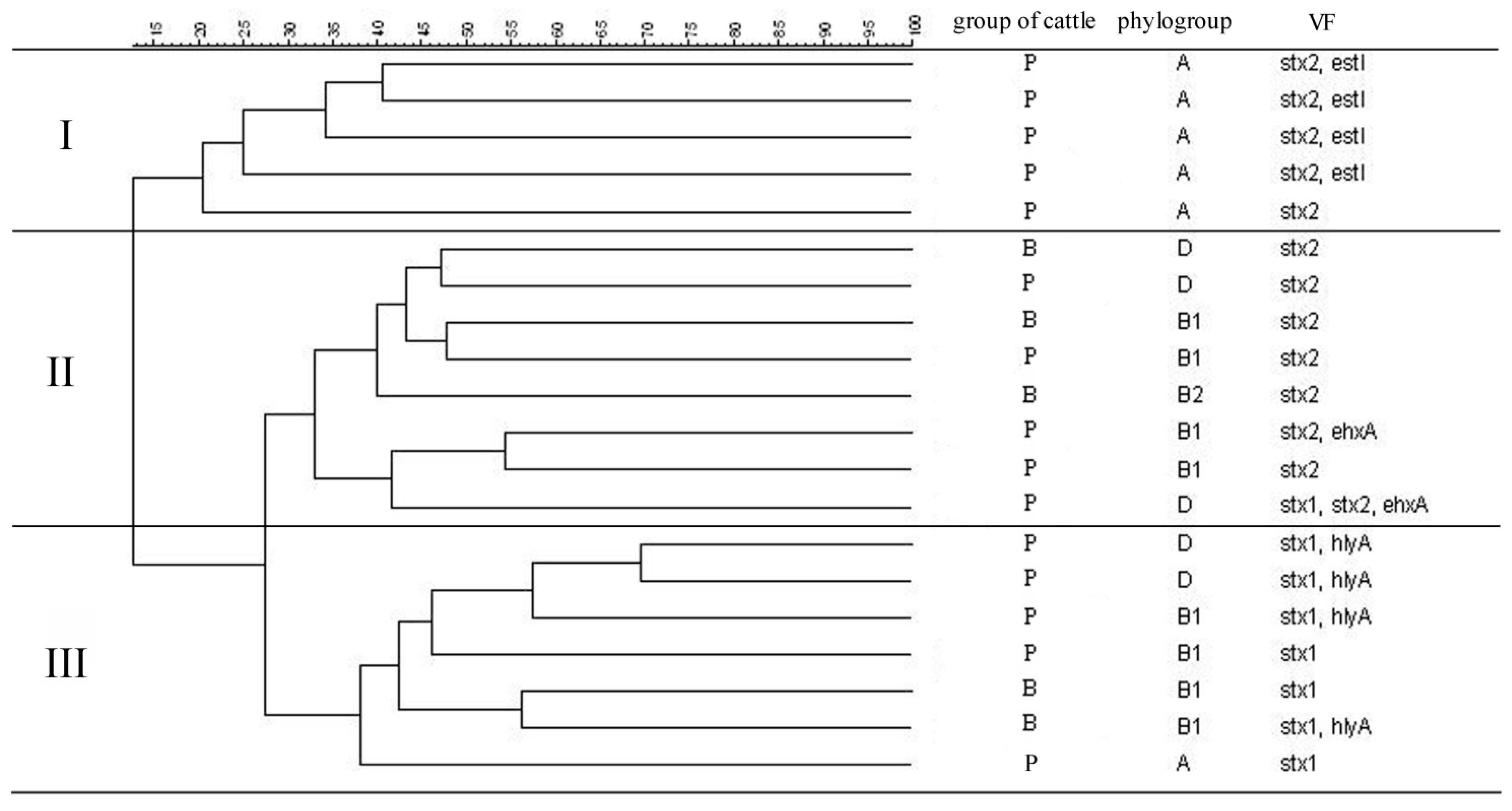
3.3. Similarity Analysis of STEC Isolates
3.4. Antimicrobial Resistance
| (A) | ||||
| Antimicrobial Agent | Number (%) of E. coli Isolates | OR (95% CI) a | ||
| Cattle Barn n = 147 | Cattle Pasture n = 118 | |||
| Ampicillin | 31 (21,1) | 8 (6,8) | 0.21 (0.07–0.625) * | |
| Cefuroxime | 39 (26,5) | 14 (11,9) | 0.34 (0.157–0.733) * | |
| Ceftazidim | 11 (7.5) | 1 (0.8) | 0.12 (0.013–0.831) * | |
| Streptomycin | 30 (20,4) | 14 (11,9) | 0.35 (0.123–1.020) | |
| Gentamicin | 17 (11,6) | 7 (5,9) | 0.44 (0.148–1.330) | |
| Neomycin | 75 (51) | 29 (24,6) | 0.24 (0.116–0.51) * | |
| Tetracycline | 35 (23,8) | 13 (11) | 0.31 (0.103–0.938) * | |
| Doxycycline | 37 (25,2) | 16 (13,6) | 0.44 (0.184–1.027) | |
| Sulphamethoxazole | 19 (12,9) | 6 (5,1) | 0.36 (0.139–0.935) * | |
| Trimethoprim | 5 (3,4) | 6 (5,1) | 1.51 (0.441–5.190) | |
| Chloramphenicol | 8 (5,4) | 11 (9,3) | 1.79 (0.694–4.600) | |
| Nalidixic acid | 11 (7,5) | 11 (9.3) | 1.27 (0.531–3.044) | |
| Norfloxacin | 1 (0.7) | 3 (2.5) | 3.56 (0.251–50.652) | |
| Antimicrobial Susceptibility Characteristic | ||||
| R (“1”) | 121 (82.3) | 69 (58.5) | 0.28 (0.143–0.536) * | |
| S (“0”) | 26 (17.7) | 49 (41.5) | ||
| MDR (“1”) | 25 (17) | 10 (8.5) | 0.39 (0.147–1.018) | |
| NMDR (“0”) | 122 (83) | 108 (91.5) | ||
| R—resistant to at least one agent; S—susceptible; MDR—multidrug-resistant; NMDR—nonmultidrug-resistant; a reference category—cattle barn; * statistically significant. | ||||
| (B) | ||||
| Antimicrobial Agent | Number (%) of Animals with Resistant E. coli | OR (95% CI) a | Test of Independence p-value | |
| Cattle Barn n = 50 | Cattle Pasture n = 42 | |||
| Ampicillin | 21 (42) | 6 (14.3) | 0.23 (0.0821–0.645) * | 0.007 * |
| Cefuroxime | 24 (48) | 12 (28.6) | 0.43 ( 0.182–1.034) | 0.092 |
| Ceftazidim | 10 (20) | 1 (2.4) | 0.09 (0.012–0.797) * | 0.010 * |
| Streptomycin | 22 (44) | 9 (21.4) | 0.35 (0.138–0.875) * | 0.039 * |
| Gentamicin | 13 (26) | 6 (14.3) | 0.47 (0.163–1.384) | 0.261 |
| Neomycin | 38 (76) | 17 (40.5) | 0.21 (0.088–0.525) * | 0.001 * |
| Tetracycline | 20 (40) | 10 (23.8) | 0.47 (0.189–1.162) | 0.153 |
| Doxycycline | 23 (46) | 13 (31) | 0.53 (0.223–1.242) | 0.208 |
| Sulphamethoxazole | 17 (34) | 6 (14.3) | 0.32 (0.114–0.919) * | 0.033 * |
| Trimethoprim | 5 (10) | 6 (14.3) | 1.50 (0.423–5.315) | 0.540 |
| Chloramphenicol | 8 (16) | 9 (21.4) | 1.43 (0.498–4.116) | 0.690 |
| Nalidixic acid | 10 (20) | 9 (21.4) | 1.09 (0.397–3.000) | 1 |
| Norfloxacin | 1 (2) | 2 (4.8) | 2.45 (0.214–28.008) | 0.590 |
| a reference category—cattle barn; * statistically significant. | ||||
3.5. Frequency of Antimicrobial Resistance Genes
| Antimicrobial Agent | Resistance Gene | Number (%) of Isolates with Resistance Genes | Number (%) of Animals with E. coli Positive for Resistance Genes |
|---|---|---|---|
| Cattle Barn | |||
| Ampicillin | n = 31 | n = 21 | |
| blaTEM | 6 (19.4) | 4 (19) | |
| blaSHV | 4 (12.9) | 3 (14.3) | |
| Streptomycin | n = 30 | n = 22 | |
| aadA1 | 10 (33.3) | 6 (27.3) | |
| Tetracycline | n = 35 | n = 20 | |
| tetA | 9 (25.7) | 5 (25) | |
| tetB | 1 (2.9) | 1 (5) | |
| tetC | 1 (2.9) | 1 (5) | |
3.6. Associations between Virulence Factors and Antimicrobial Resistance
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lawrence, J.G. Common themes in the genome strategies of pathogens. Curr. Opin. Genet. Dev. 2005, 15, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [PubMed]
- Rhoades, J.R.; Duffy, G.; Koutsoumanis, K. Prevalence and concentration of verocytotoxigenic Escherichia coli, Salmonella enterica and Listeria monocytogenes in the beef production chain: A review. Food Microbiol. 2009, 26, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Etcheverría, A.I.; Padola, N.L. Shiga toxin-producing Escherichia coli: Factors involved in virulence and cattle colonization. Virulence 2013, 4, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Courvalin, P. Modes of antimicrobial action and mechanisms of bacterial resistance: Chapter 1. In Antimicrobial Resistance in Bacteria of Animal Origin; Aarestrup, F.M., Ed.; ASM Press: Washington, DC, USA, 2006; pp. 1–18. [Google Scholar]
- Kaesbohrer, A.; Schroeter, A.; Tenhagen, B.A.; Alt, K.; Guerra, B.; Appel, B. Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance. Zoonoses Public Health 2012, 59 (Suppl. 2), 158–165. [Google Scholar] [CrossRef]
- Martínez, J.L.; Baquero, F. Interactions among strategies associated with bacterial infection: Pathogenicity, epidemicity, and antibiotic resistance. Clin. Microbiol. Rev. 2002, 15, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Versalovic, J.; Schneider, M.; de Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.A.; Wu, X.Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Hagedorn, P.; Brast, S.; Heusipp, G.; Bielaszewska, M.; Friedrich, A.W.; Karch, H.; Schmidt, M.A. Rapid identification and differentiation of clinical isolates of enteropathogenic Escherichia coli (EPEC), atypical EPEC, and Shiga toxin-producing Escherichia coli by a one-step multiplex PCR method. J. Clin. Microbiol. 2006, 44, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 8th ed.; Document M7-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Frech, G.; Kehrenberg, C.; Schwarz, C.S. Resistance phenotypes and genotypes of multiresistant Salmonella enterica subsp. enterica serovar Typhimurium var. Copenhagen isolates from animal sources. J. Antimicrob. Chemother. 2003, 51, 180–182. [Google Scholar]
- Kozak, G.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 10 October 2014).
- Schierack, P.; Rödiger, S.; Kuhl, C.; Hiemann, R.; Roggenbuck, D.; Li, G.; Weinreich, J.; Berger, E.; Nolan, L.K.; Nicholson, B.; et al. Porcine E. coli: Virulence-associated genes, resistance genes and adhesion and probiotic activity tested by a new screening method. PLoS One 2013, 26. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Budzynska, A.; Gospodarek, E. Prevalence of genes encoding virulence factors among Escherichia coli with K1 antigen and non-K1 E. coli strains. J. Med. Microbiol. 2012, 61, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Junker, E.; Schroeter, A.; Malorny, B.; Lehmann, S.; Helmuth, R. Phenotypic and genotypic characterisation of antimicrobial resistance in German Escherichia coli isolates from cattle, swine and poultry. J. Antimicrob. Chemother. 2003, 52, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Mellmann, A.; Harmsen, D.; Cummings, C.A.; Zentz, E.B.; Leopold, S.R.; Rico, A.; Prior, K.; Szczepanowski, R.; Ji, Y.; Zhang, W.; et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Han, D.; Jang, J.; Lee, S.N.; Ko, G.; Choi, H.Y.; Kim, J.H.; Sadowsky, M.J.; Hur, H.G. Absence of Escherichia coli phylogenetic group B2 strains in humans and domesticated animals from Jeonnam Province, Republic of Korea. Appl. Environ. Microbiol. 2009, 75, 5659–5666. [Google Scholar] [CrossRef] [PubMed]
- Carlos, C.; Pires, M.M.; Stoppe, N.C.; Hachich, E.M.; Sato, M.I.; Gomes, T.A.; Amaral, L.A.; Ottoboni, L.M. Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; Cowling, A. The distribution and genetic structure of Escherichia coli in Australian vertebrates: Host and geographic effects. Microbiology 2003, 149, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Baldy-Chudzik, K.; Mackiewicz, P.; Stosik, M. Phylogenetic background, virulence gene profiles, and genomic diversity in commensal Escherichia coli isolated from ten mammal species living in one zoo. Vet. Microbiol. 2008, 131, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hosokawa, Y.; Makita, K.; Noda, J.; Ueno, H.; Muramatsu, Y.; Ueno, H; Mukai, T.; Yamamoto, H.; Ito, M.; et al. Factors associated with antimicrobial-resistant Escherichia coli in zoo animals. Res. Vet. Sci. 2012, 93, 574–580. [Google Scholar] [CrossRef]
- Hussein, H.S. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 2007, 85, E63–E72. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Murakami, M.; Maruyama, N.; Yamamoto, K.; Haruna, M.; Ito, K.; Yamada, Y. Comparison of the prevalence of shiga toxin-producing Escherichia coli strains O157 and O26 between beef and dairy cattle in Japan. J. Vet. Med. Sci. 2013, 75, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Łoś, J.M.; Łoś, M.; Węgrzyn, A.; Węgrzyn, G. Altruism of Shiga toxin-producing Escherichia coli: Recent hypothesis versus experimental results. Front. Cell. Infect. Microbiol. 2013, 2. [Google Scholar] [CrossRef]
- Mauro, S.A.; Koudelka, G.B. Shiga toxin: Expression, distribution, and its role in the environment. Toxins (Basel) 2011, 3, 608–625. [Google Scholar] [CrossRef]
- Call, D.R.; Davis, M.A.; Sawant, A.A. Antimicrobial resistance in beef and dairy cattle production. Anim. Health. Res. Rev. 2008, 9, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, J.; Pusz, P.; Bok, E.; Stosik, M.; Baldy-Chudzik, K. The phenotypic and genotypic characteristics of antibiotic resistance in Escherichia coli populations isolated from farm animals with different exposure to antimicrobial agents. Pol. J. Microbiol. 2013, 62, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.C.; Moore, D.A.; Sischo, W.M. Field trial evaluating the influence of prophylactic and therapeutic antimicrobial administration on antimicrobial resistance of fecal Escherichia coli in dairy calves. Appl. Environ. Microbiol. 2006, 72, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Petrovic, O. Antibiotic resistance of commensal Escherichia coli of food-producing animals from three Vojvodinian farms, Serbia. Int. J. Antimicrob. Agents 2008, 31, 360–363. [Google Scholar] [CrossRef] [PubMed]
- De Verdier, K.; Nyman, A.; Greko, C.; Bengtsson, B. Antimicrobial resistance and virulence factors in Escherichia coli from Swedish dairy calves. Acta Vet. Scand. 2012, 54. [Google Scholar] [CrossRef]
- Enne, V.I.; Cassar, C.; Sprigings, K.; Woodward, M.J.; Bennett, P.M. A high prevalence of antimicrobial resistant Escherichia coli isolated from pigs and a low prevalence of antimicrobial resistant E. coli from cattle and sheep in Great Britain at slaughter. FEMS Microbiol. Lett. 2008, 278, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, A.R.; Hancock, D.D.; Besser, T.E.; Call, D.R. Role of calf-adapted Escherichia coli in maintenance of antimicrobial drug resistance in dairy calves. Appl. Environ. Microbiol. 2004, 70, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, A.R.; Besser, T.E.; Hancock, D.D.; Call, D.R. Use of a nonmedicated dietary supplement correlates with increased prevalence of streptomycin-sulfa-tetracycline-resistant Escherichia coli on a dairy farm. Appl. Environ. Microbiol. 2006, 72, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, M.; Walsh, C.; Slowey, R.; Leonard, N.; Fanning, S. Molecular characterization of multidrug-resistant Escherichia coli isolates from Irish cattle farms. Appl. Environ. Microbiol. 2011, 77, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ehricht, R.; Mafura, M.; Stokes, M.; Smith, N.; Pritchard, G.C.; Woodward, M.J. Escherichia coli isolates from extraintestinal organs of livestock animals harbour diverse virulence genes and belong to multiple genetic lineages. Vet. Microbiol. 2012, 160, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Bukh, A.S.; Schønheyder, H.C.; Emmersen, J.M.; Søgaard, M.; Bastholm, S.; Roslev, P. Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: A 10 year population-based study in Denmark. J. Antimicrob. Chemother. 2009, 64, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Girardeau, J.P.; Dalmasso, A.; Bertin, Y.; Ducrot, C.; Bord, S.; Livrelli, V.; Vernozy-Rozand, C.; Martin, C. Association of virulence genotype with phylogenetic background in comparison to different seropathotypes of Shiga toxin-producing Escherichia coli isolates. J. Clin. Microbiol. 2005, 43, 6098–6107. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Meyer, K.P.; Sadowsky, M.J. Relationship between phylogenetic groups, genotypic clusters, and virulence gene profiles of Escherichia coli strains from diverse human and animal sources. Appl. Environ. Microbiol. 2007, 73, 5703–5710. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, E.; Mazurek, J.; Stosik, M.; Wojciech, M.; Baldy-Chudzik, K. Prevalence of Virulence Determinants and Antimicrobial Resistance among Commensal Escherichia coli Derived from Dairy and Beef Cattle. Int. J. Environ. Res. Public Health 2015, 12, 970-985. https://doi.org/10.3390/ijerph120100970
Bok E, Mazurek J, Stosik M, Wojciech M, Baldy-Chudzik K. Prevalence of Virulence Determinants and Antimicrobial Resistance among Commensal Escherichia coli Derived from Dairy and Beef Cattle. International Journal of Environmental Research and Public Health. 2015; 12(1):970-985. https://doi.org/10.3390/ijerph120100970
Chicago/Turabian StyleBok, Ewa, Justyna Mazurek, Michał Stosik, Magdalena Wojciech, and Katarzyna Baldy-Chudzik. 2015. "Prevalence of Virulence Determinants and Antimicrobial Resistance among Commensal Escherichia coli Derived from Dairy and Beef Cattle" International Journal of Environmental Research and Public Health 12, no. 1: 970-985. https://doi.org/10.3390/ijerph120100970
APA StyleBok, E., Mazurek, J., Stosik, M., Wojciech, M., & Baldy-Chudzik, K. (2015). Prevalence of Virulence Determinants and Antimicrobial Resistance among Commensal Escherichia coli Derived from Dairy and Beef Cattle. International Journal of Environmental Research and Public Health, 12(1), 970-985. https://doi.org/10.3390/ijerph120100970





