Abstract
Dogs play many roles and their presence within people’s houses has increased. In rural settings dog faeces are not removed from the streets, representing an environmental pollution factor. Our aim was to evaluate the occurrence of environmental contamination with zoonotic intestinal parasites of three groups of dogs in Ponte de Lima, Portugal, with a particular emphasis on Echinococcus granulosus. We collected 592 dog faecal samples from the environment, farm and hunting dogs. Qualitative flotation coprological analysis was performed and the frequency in the positive samples ranged between 57.44% and 81.19% in different groups. We isolated up to four different parasites in one sample and detected seven intestinal parasitic species, genera or families overall. Ancylostomatidae was the most prevalent parasite, followed by Trichuris spp., Toxocara spp., Isospora spp., Dipylidium caninum, Taeniidae and Toxascaris leonina. Taeniidae eggs were analyzed with the PCR technique and revealed not to be from Echinococcus. The parasite prevalence and the diversity of zoonotic parasites found were high, which calls for a greater awareness of the problem among the population, especially hunters. Promoting research at the local level is important to plan control strategies. Health education should be developed with regard to farmers and hunters, and a closer collaboration between researchers, practitioners and public health authorities is needed.
1. Introduction
In human society, dogs play many roles such as pets, guarding, hunting and farming, and they are also used in therapeutic programs, life-saving actions, transport, and, last but not least, for fun and research [1]. There is evidence of the role of dogs in physical and psychological human well-being [2]. As communities become more urban, the presence of pets within houses has increased in popularity [3]. Nonetheless, dogs may represent a potential risk for human health due to the possibility of the transmission of zoonosis [4]. In urban settings, where the number of domestic animals has been increasing, dog feces represent an important pollution factor, as they are not regularly removed. Moreover, vehicular traffic, as well as the wind, can help spread viable pathogens present in dog feces, contaminating food which may later be a source of infection [5]. Parasite eggs can also be carried into human houses if adhered to shoes or animals’ paws [6]. Additionally, arthropods and other environmental factors, as the rain, may also play an important role in this context.
Current information on regional prevalence is essential to develop and modify of control measures in animal and public health [7,8]. In the Mediterranean countries, it is consensual that there is a lack of data on the prevalence of zoonotic diseases (both in animals and human), which prevents an effective epidemiological surveillance and leads to the failure in prevention. There is also an absence of intersectoral work and communication between human and animal health professionals, as well as a lack of knowledge of the human population [9]. Crucially, a relatively small number of reports have focused on the infection risk of populations living in rural or suburban settings [10], where the majority of dogs defecate in the countryside [11]. Many of these communities have large populations of free roaming domestic dogs and little access to veterinary care. These dogs have frequent contact with other animals, their feces, and a variety of refuse and foodstuffs that potentially contain zoonotic agents, which promotes infection with a variety of zoonotic agents and subsequent human exposure [12]. The aim of this study was to evaluate the occurrence of zoonotic intestinal parasites of dogs from different groups in the rural municipality of Ponte de Lima, in the northwest of Portugal, with a particular emphasis on Echinococcus granulosus.
2. Materials and Methods
2.1. Study Area
Most of the studies performed in Portugal concerning intestinal parasites of dogs have focused on the Centre and South of the country. The municipality of Ponte de Lima was selected for this study because it is in the North and, also, because it is a mainly rural one. The municipality belongs to the District of Viana do Castelo, and is located in the northwest of Portugal (Figure 1).
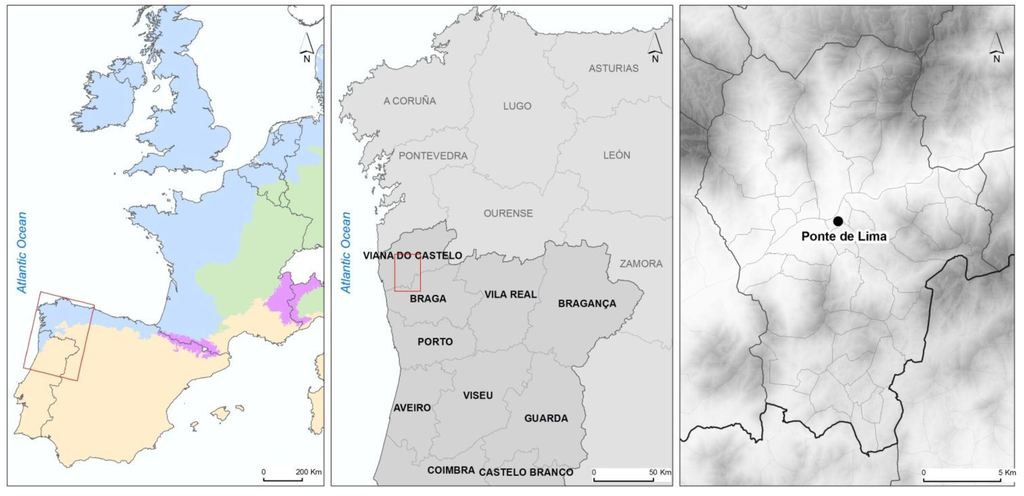
Figure 1.
Maps of the sampling area—Ponte de Lima in the northwest of Portugal.
The main town in this municipality is Ponte de Lima and the region has 43,498 inhabitants dispersed over 320.26 km2 and 51 civil parishes [13]. The main source of income in this rural municipality is agriculture and animal breeding, on relatively small, family size farms, producing mostly corn, potatoes, wine, dairy and meat cows, sheep, goats, pigs and poultry. The strong tourism development of the region has led to an increase in the human population, especially during the summer months, and, consequently, to an increase in the urbanization of rural areas, transforming the environment and creating favorable conditions for contacts between human beings and dogs. Dog ownership is very common in Ponte de Lima. In this municipality, in 2011, there were 796 ruminant farms and between 1100 and 1500 dogs registered (official data from veterinary services). Many owned dogs have free access to the countryside. In this area, many dogs are not officially registered, so the real size of dog population is unknown.
2.2. Fecal Sample Collection
Between December 2011 and November 2012, we collected dog feces from three different sample groups: environmental samples, farm and hunting dogs (Figure 2). Samples were randomly collected in public areas (preferably in areas attended by people, like green parks and others), ruminant farms and hunting dog packs, and are from different breeds and ages (not registered).
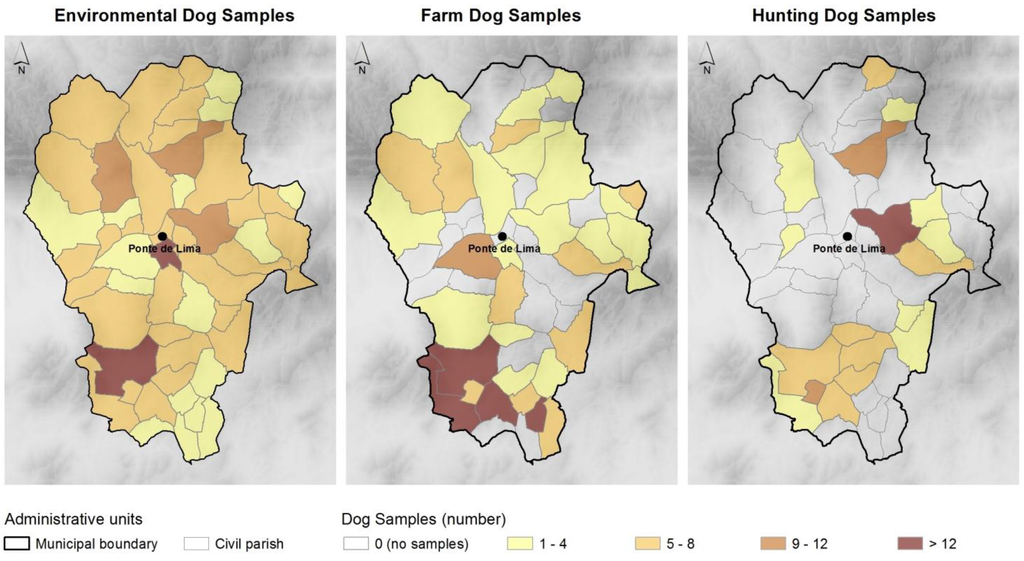
Figure 2.
Number of samples collected per group of dogs and civil parishes.
Fecal samples were collected fresh from the grounds in kennels, pastures or other non-specific sites. To collect environmental samples, we have walked across all the 51 civil parishes of this municipality. Three hundred ruminant farms were visited with the purpose of collecting farm dog samples, and we have collected samples in 141 of these farms. Whenever there was more than one dog in each farm, we collected more than one sample. The hunting dog samples were collected in wild boar hunting campaigns. We collected samples from 24 packs of hounds. Whenever possible, fecal samples were collected immediately after spontaneous elimination. These samples were placed in plastic containers, all individually identified, stored at 4 °C and processed within 48 h through coprological methods.
2.3. Coprological Methods Used
2.3.1. Qualitative Flotation Coprological Analysis
Each sample was first examined macroscopically for the possible detection of proglottids. Qualitative flotation coprological analysis was performed, as described in the literature [14]. Egg identification was based on morphological characteristics (shape and structure of shell) and measurements [14,15,16]. With the exception of Toxascaris leonina and Dipylidium caninum, isolated parasites were identified at the family/genus level. A dog was classified as positive if at least one dog parasite egg, oocyst, cyst or proglottid was observed, regardless of whether or not it was zoonotic.
2.3.2. Percoll Fractionation of Fecal Samples
Samples positive for Taeniidae eggs went through Percoll fractionation to render easier the molecular analysis to evaluate the presence of Echinococcus granulosus. Percoll (ref. 17-0891-01, GE Healthcare, Amersham Biosciences Limited, Amersham, UK) step gradients were used to perform a partial clean-up and concentration of parasite eggs. Percoll step gradients were based on the density of helminth eggs. The evaluation of Percoll fractionation was performed as described by Cardoso et al. [17].
2.3.3. Molecular Detection of Echinococcus granulosus
Fecal material isolated through Percoll feces gradient was used to further evaluate the presence of Echinococcus granulosus in samples positive for Taeniidae. To extract the total DNA, the QIAamp ADN Mini Kit (Qiagen, GmbH, Hilden, Germany) was used according to the protocol provided by the manufacturer for the purification of DNA from tissues. The presence of Echinococcus granulosus DNA in fecal samples was evaluated by using PCR. For each sample we performed PCR reactions for the amplification of an Echinococcus granulosus repeated sequence [18]. The result was analyzed using electrophoresis. DNA samples obtained from Echinococcus granulosus cysts and from feces positive for Echinococcus granulosus were used as positive controls. These controls were carried out using PCR followed by sequencing of amplified DNA fragments.
2.4. Data Analysis
Results were entered into a SPSS 22.0 database. We defined prevalence as the percentage of fecal samples positive for any parasite species, and the specific prevalence as the percentage of fecal samples positive for a given parasite species. Prevalence data from all the samples were stratified into three different groups of dogs defined above: environmental samples, farm and hunting dogs. The chi-square test was used to assess the differences in proportions. A Pareto analysis was performed to describe the relative importance of each parasite species in the overall samples and after stratification. The strata were specified according to the number of parasites found simultaneously in each sample: strata 1 for the samples with one parasitic form, strata 2 for the samples with two different parasites, and so on up to strata 4 with four different forms. To assess the possible role of each sample group in the level of parasitism (independent variable), a binomial logistic regression was used to calculate the odds (OR) of having there being or not a parasitic infection in dogs in each of the three sample group, with a confidence interval (CI) of 95%. For the purposes of the binomial regression, the response variable—level of parasitism—was categorized into two categories: no parasites in feces and parasite in feces. To go further and assess the influence of each of the three sample groups of the level of parasitism in dog feces (independent variable), a nominal logistic regression analysis was used to calculate the OR. The response variable was split into three levels: level zero with no parasitic forms, level one for samples showing one parasitic species and level two for fecal samples showing two of more parasitic species. A CI of 95% was used.
3. Results
3.1. Risk of Infection by Strata
A total of 592 samples were collected from three different groups of fecal samples. Out of these dog fecal samples, 374 were positive for the presence of parasitic forms. The prevalence of parasites found in the three groups of dog fecal samples is presented in Table 1.

Table 1.
Prevalence of parasites found in dog fecal samples collected in Ponte de Lima, Portugal.
| Presence of parasite | Environmental Dog Samples (n = 296) | Farm Dog Samples (n = 195) | Hunting Dog Samples (n = 101) | Total (n = 592) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Negative | 119 | 40.20 | 83 | 42.56 | 19 | 18.81 | 218 | 36.82 |
| Positive | 177 | 59.80 | 112 | 57.44 | 82 | 81.19 | 374 | 63.17 |
3.2. Diversity of Parasites Found and Individual Prevalence
In the 592 samples, seven species/genera/families of intestinal parasites were detected from Classes Nematoda, Cestoda (although proglottides were not identified during these procedures) and Coccidia (Table 2).

Table 2.
Prevalence of parasitic species found in 592 dog fecal samples from three different groups.
| Parasite | Environmental Dog Samples (n = 296) (%) | Farm Dog Samples (n = 195) (%) | Hunting Dog Samples (n = 101) (%) |
|---|---|---|---|
| Ancylostomatidae | 44.59 | 31.28 | 70.30 |
| Trichuris spp. | 34.46 | 32.82 | 49.50 |
| Toxocara spp. | 7.43 | 11.28 | 10.89 |
| Toxascaris leonina | 0.68 | 0 | 0 |
| Dipylidium caninum | 0.68 | 1.02 | 0.99 |
| Taeniidae | 0.34 | 0.51 | 1.98 |
| Isospora spp. | 3.04 | 1.54 | 4.95 |
Taeniidae eggs were present in four samples. These samples were analyzed with the polymerase chain reaction technique, which revealed the eggs to be from Taenia spp. and not Echinococcus granulosus. These eggs were more frequent in hunting dog samples. The distribution of zoonotic (Ancylostomatidae, Trichuris spp., Toxocara spp., Dipylidium caninum and Taeniidae) and non-zoonotic parasites throughout the municipality is shown in Figure 3.
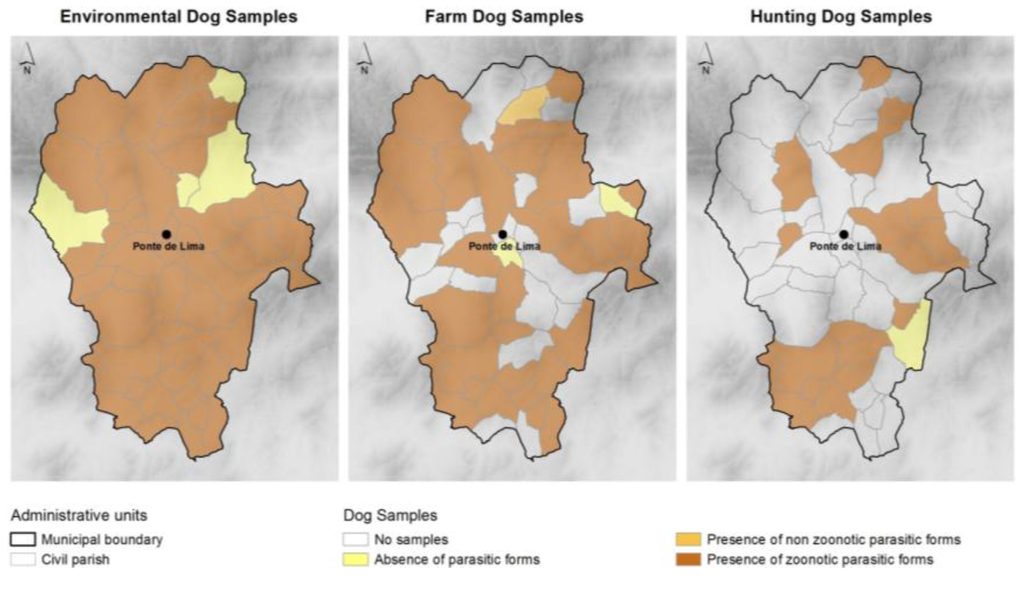
Figure 3.
Location of samples without and with (zoonotic and non-zoonotic) parasitic forms per dog group and civil parishes.
3.3. Parasite Associations and Infections
The presence of zero, one, two or more parasitic species in the fecal samples examined is summarized in Table 3.

Table 3.
Presence of zero, one and two or more parasitic species in 592 dog fecal samples examined.
| Number of Different Parasites | Environmental Dog Samples (n = 296) (%) | Farm Dog Samples (n = 195) (%) | Hunting Dog Samples (n = 101) (%) |
|---|---|---|---|
| 0 | 40.2 a | 42.6 a | 18.8 a |
| 1 | 33.4 a | 39.0 a | 30.7 b |
| >1 | 26.4 a | 18.5 b | 50.5 c |
a,b,c Each subscript letter indicates a subset of categories whose proportions on columns; did not differ significantly from each other at the, 0.05 level.
The parasites and association of parasites found in three different groups of dog fecal samples is presented in Table 4.

Table 4.
Parasites and association of parasites found in three different groups of dog fecal samples.
| Parasite | Environmental Dog Samples (n = 296) (%) | Farm Dog Samples (n = 195) (%) | Hunting Dog Samples (n = 101) (%) |
|---|---|---|---|
| Ancylostomatidae | 19.59 | 14.87 | 20.79 |
| Trichuris spp. | 11.49 | 16.41 | 5.94 |
| Toxocara spp. | 1.35 | 5.64 | 2.97 |
| Dipylidium caninum | 0.38 | 0 | 0.99 |
| Toxascaris leonina | 0.38 | 0 | 0 |
| Isospora spp. | 0.38 | 0 | 0 |
| Taeniidae | 0 | 0.51 | 0 |
| Ancylostomatidae + Trichuris spp. | 17.91 | 10.77 | 34.65 |
| Ancylostomatidae + Toxascaris leonina | 0 | 0 | 0.99 |
| Ancylostomatidae + Toxocara spp. | 2.03 | 2.56 | 2.97 |
| Trichuris spp. + Toxocara spp. | 1.01 | 2.56 | 0.99 |
| Ancylostomatidae + Dipylidium caninum | 0.38 | 0.51 | 0.99 |
| Ancylostomatidae + Isospora spp. | 0.38 | 0 | 0 |
| Ancylostomatidae + Trichuris spp. + Toxocara spp. | 1.69 | 0.51 | 1.98 |
| Ancylostomatidae + Trichuris spp. + Taeniidae | 0 | 0 | 0.99 |
| Ancylostomatidae + Toxocara spp. + Taeniidae | 0 | 0 | 0.99 |
| Ancylostomatidae + Trichuris spp. + Toxascaris leonina | 0 | 0 | 1.98 |
| Ancylostomatidae + Trichuris spp. + Isospora spp. | 1.35 | 1.54 | 1.98 |
| Ancylostomatidae + Isospora spp. + Toxocara spp. | 0.38 | 0 | 0 |
| Trichuris spp. + Toxocara spp. + Taeniidae | 0.38 | 0 | 0 |
| Dipylidiumcaninum + Toxocara spp. + Trichuris spp. | 0 | 0.51 | 0 |
| Ancylostomatidae + Isospora spp. + Toxocara spp. + Trichuris spp. | 0.38 | 0 | 0.99 |
| Ancylostomatidae + Isospora spp. + Toxocara spp. + Toxascaris leonina | 0.38 | 0 | 0.99 |
A Pareto analysis of the occurrence in samples of parasitic forms was performed and showed that 84.6% (479/566) of the parasitic forms were Ancylostomatidae and Trichuris spp (Figure 4).
Logistic regression analyses were performed. The binomial logistic regression showed that environmental and farm dog samples are not different with regard to risk factors for parasitism (p = 0.603). Nonetheless, the risk of parasitism occurrence is 2.9 times higher in hunting than in environmental dog fecal samples: OR 2.9 (1.7–5.0); p < 0.000. As for the nominal logistic regression analysis, hunting dogs was the reference category and two different levels of parasitism were assessed against the no parasite category. The risk of being infected with a single infection, does not differ between the three groups. Regarding the existence of multiple infections (two or more different parasites in the same sample), the risk is significantly higher (OR: 4.1) for hunting dogs (p < 0.000) than for environmental dog samples and also for hunting dogs (OR: 6.2) than for farm dogs (p < 0.000).
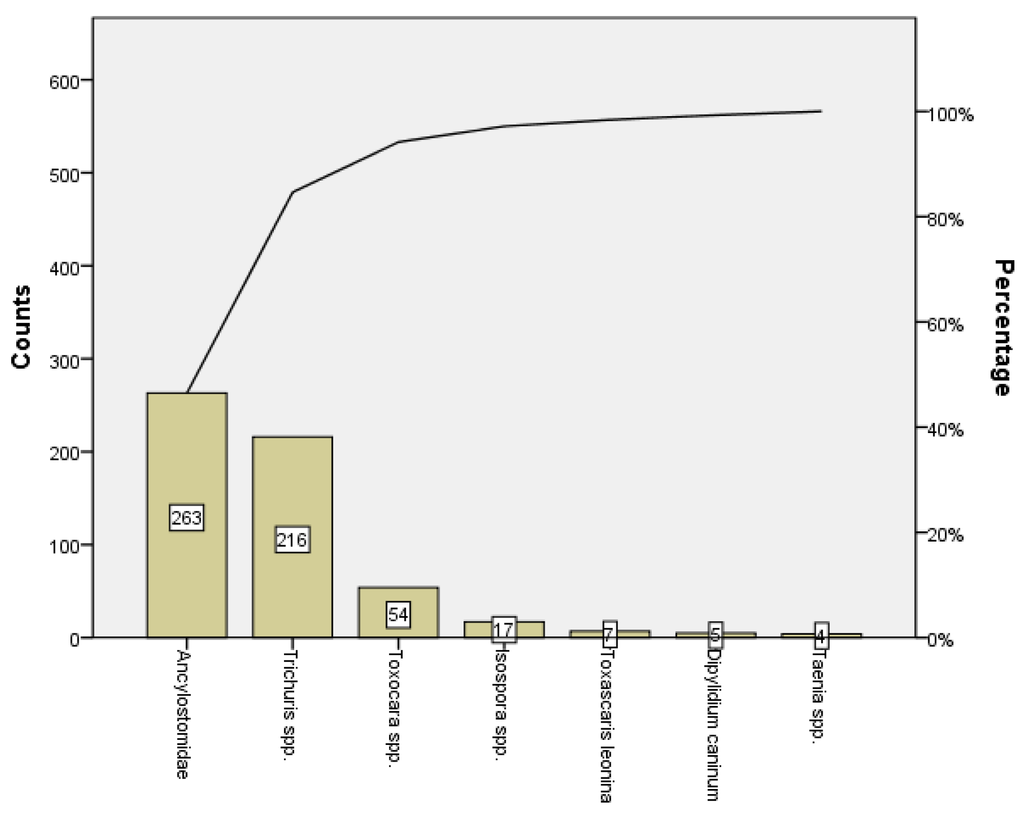
Figure 4.
Pareto analysis of the occurrence of parasitic forms.
4. Discussion
4.1. Risk of Infection by Group
The prevalence of parasites found in dog fecal samples in Portugal and in other countries are presented in Table 5.

Table 5.
Prevalence of parasites found in dog fecal samples in Portugal and in other countries.
| Authors | Country | N | Dog Sample Origin | Overall Prevalence |
|---|---|---|---|---|
| Tarsitano et al. [5] | Italy | 152 | Environmental | 8.5% |
| Rinaldi et al. [19] | Italy | 415 | Environmental | 16.9% |
| Dubná et al. [20] | Czech Republic | 3780 | Environmental + Shelter Dogs | 17.6% |
| Martínez-Carrasco et al. [21] | Spain | 275 | Dogs presented to veterinary clinics + Shelter Dogs + Stray Dogs | 25.0% |
| Papazahariadou et al. [22] | Greece | 281 | Farm Dogs + Hunting Dogs | 26.0% |
| Gracenea et al. [23] | Spain | 505 | Shelter Dogs | 26.9% |
| Soriano et al. [24] | Argentina | 1944 | Environmental | 37.9% |
| Szabová et al. [1] | Slovak Republic | 752 | Environmental + Owned Dogs + Shelter Dogs | 45.7% |
| Balassiano et al. [25] | Brazil | 500 | Dogs presented to veterinary clinics | 46.4% |
| Fontanarrosa et al. [7] | Argentina | 2193 | Owned Dogs | 52.4% |
| Okoye et al. [26] | Nigeria | 413 | Stray Dogs | 52.6% |
| Benito et al. [27] | Spain | 1040 | Shelter Dogs | 53.6% |
| Katagiri and Oliveira-Sequeira [28] | Brazil | 254 | Owned Dogs | 54.3% |
| Beiromvand et al. [29] | Iran | 77 | Owned Dogs + Stray Dogs | 66.0% |
| Bajer et al. [30] | Poland | 108 | Sled Dogs | 68.0% |
| Ugbomoiko et al. [31] | Nigeria | 396 | Owned Dogs | 68.4% |
| Martínez-Moreno et al. [32] | Spain | 1800 | Shelter Dogs | 71.3% |
| Gingrich et al. [33] | Galapagos Island | 97 | Owned Dogs | 71.4% |
| Eguía-Aguilar et al. [34] | México | 120 | Stray Dogs | 85.0% |
| Mandarino-Pereira et al. [35] | Brazil | 81 | Environmental | 92.6% |
| Crespo and Jorge [36] | Portugal | 576 | Environmental | 17.9% |
| Cruz et al. [37] | Portugal | 49 | Environmental | 18.4% |
| Neves et al. [38] | Portugal | 368 | Dogs presented to veterinary clinics | 20.6% |
| Mateus et al. [39] | Portugal | 100 | Shelter Dogs | 41.0% |
| Crespo et al. [40] | Portugal | 548 | Environmental | 50.0% |
| Cardoso et al. [17] | Portugal | 301 | Farm Dogs | 58.8% |
The diversity of results obtained from different studies highlights the importance of promoting research at the local level to plan control strategies [7]. The differences reflected in Table 5 may be partially explained by the origin of the dog samples. Animals placed in shelters are dewormed by veterinarians [41], but the high densities observed frequently in shelters may contribute to the propagation of these parasites [42]. There is also a wide disparity with regard to the sampling collection methods (many of the studies included in Table 5 involved collection of samples per rectum or in necropsy), the number of samples collected and the coprological method used, making it difficult to compare results. There is no unique technique capable to do the parasitological diagnosis of all kinds of parasitic species that may be present in feces [43]. Although compared to post-mortem fecal examination fecal flotation is less sensitive in the detection of parasites [21,44], faecal flotation is considered a very valuable method for the assessment of the majority of dog parasites [45]. The frequency found in the positive samples is high, and the risk of parasitism and occurrence of multiple infections is higher in hunting dogs. This highlights the need of health education in a specific target group: hunters and their families.
A much lower parasite prevalence in hunting dog samples than in the present study has been previously reported [22]. Hunting dogs are at the highest risk of worm infections and are therefore responsible for most environmental contamination and human disease [46]. Helminth zoonoses transmitted among dogs, wildlife, and people have been discussed by Jenkins et al. [47]. In Portugal the impact of wildlife on public health is unknown [17], however, zoonotic helminths in wild carnivores fecal samples have been found in footpaths of a protected area in Ponte de Lima, where people, domestic and wild animals coexist [48].
Access to soil, the hygiene of the environment, illness, the owners’ level of education, and veterinary care, are all associated with intestinal parasite infections in dogs [25]. Concerning the farm and hunting dogs that were sampled, no clinical signs were observed, this is in agreement with Neves et al. [38]. In a study about dog owners’ awareness conducted in this municipality it was concluded that the practices used to deworm dogs, if any, were not correct, and few owners referred fecal elimination after deworming [49], which is in agreement with other surveys [6,22]. Szabová et al. [1] found that the environmental samples had the lowest prevalence recorded. In our study, some of the environmental samples were not as fresh as those from the farm or hunting dogs, so it was somehow expected to find lower parasite prevalence in these samples. On the other hand, supposing that environmental samples correspond to stray dogs, these usually do not undergo deworming, and therefore they are possible carriers of many parasites [32,35]. Our results could be explained by our environmental samples in fact not being from homeless dogs, but from owned dogs that have free outside access, as Ponte de Lima is mainly a rural municipality.
4.2. Diversity of Parasites Found and Individual Prevalence
In the examined samples, seven parasitic species were isolated. A similar number has been recorded in other studies [17,21,41], although several refer a higher [1,7,20,22,23,24,25,28,29,34,43,50,51] or lower [26,30,31,33,35,36,52] number of parasitic species. In addition to the number of parasitic species, there is a great variability in isolated species and specific prevalence which indicates the necessity of being cautious when extrapolating conclusions from data from one location to another.
There is also variety concerning the most prevalent intestinal parasite found in different studies: Ancylostomatidae or Ancylostoma caninum [7,17,26,28,30,32,33,34,35,36,43,50,52], Toxocara spp. or Toxocara canis [1,5,22,24,51,53], Isospora canis [38], Giardia spp. [41,54] and Cryptosporidium spp. [25]. Once again this discrepancy may be due to most of the variables previously mentioned, namely the different methodologies used.
A Pareto analysis showed that most of the parasitic forms were Ancylostomatidae and Trichuris spp. Interestingly Rubel et al. [55] reported that the highest prevalence of eggs of both these parasite was detected in areas with lower socio-economic level, and Szabová et al. [1] pointed to the contamination of the environment in which animals move.
For Papazahariadou et al. [22], Ancylostomatidae were more frequent in farm dogs rather than hunting dogs, unlike in our study.This may be associated, in our study, with the local habit of keeping hunting dogs in kennels where they defecate, maintaining a highly infective habitat [11]. Dogs are hosts to hookworms that may cause zoonotic diseases, most notably cutaneous larva migrans [56].
Trichuris spp. were the most frequent intestinal parasites found in hunting dogs. Most of the other studies have reported a much lower prevalence [1,17,20,24,26,38,53,54]. The eggs may remain viable and infective in the environment for years, leading to high infection rates in dogs [57]. Humans can be infected by Trichuris vulpis some cases have already been reported [58]. Toxocara eggs were more commonly found in farm dogs. According to Cardoso et al. [17], the domestic slaughtering of pigs and small ruminants showed a statistical association with Toxocara infections, and this practice is usual in farm dog owners in this municipality. Human infection is caused by direct contact with contaminated soil or dog hair [59]. Visceral larva migrans, ocular larva migrans [60], and severe diseases affecting the central nervous system and/or the eye can occur [61]. We found infective Toxocara spp. eggs in a few environmental samples. In most rural and urban resource-limited communities, children are considered the highest risk group [12], however in farms, adults and children are equally susceptible to soil-transmitted infections [62]. Very high Toxocara canis seroprevalence has been found in farmers, veterinarians, slaughterhouse staff and hunters [63]. Health education to raise public awareness is therefore strongly encouraged [26].
Toxascaris leonina is an ascarid less frequent than others, as our and other studies confirm [20,24,38,41,51,53]. In contrast, Beiromvand et al. [29] found it to be the most frequent. Dipylidium caninum has been usually considered the most frequent Cestoda in dogs [27,32,50], as reported in our study. Humans can become infected and very young children are the ones most often affected [64]. Symptoms are usually absent, although abdominal discomfort, diarrhea and pruritus may be present [65].
The detection of Taeniidae eggs in fecal samples by routine microscopy suffers from low sensitivity [6]. A cross-sectional survey in Germany and other European countries have detected these eggs only in 0.25% of the samples [66]. In Portugal, owning cattle was found to be a significant risk factor for Taenia spp. presence in dogs [17] however, in our study, the higher prevalence of positive samples was not in farm dogs, but in hunting dogs. The coat of the foxes can be contaminated with Taeniidae eggs [67], so hunters—human and dog—can be directly exposed to these immediately infective eggs [6]. Echinococcus spp. eggs are morphologically indistinguishable from Taenia spp. Echinococcosis is one of the five most important zoonoses in the Mediterranean region [68], nonetheless, it remains a neglected zoonosis [69]. Despite the low sensitivity of fecal based methods to Echinococcus granulosus egg detection, it is revelant for public health to remark the absence of these parasites in our study.
Unlike in some studies [25,38,41,54], helminth eggs were more commonly identified than protozoan. The trend of reducing helminthic and increasing protozoan infection has been attributed to the knowledge of dog owners about potential zoonotic transmission of these agents and how to control them [70]. These facts stand in contrast with the results of our study, in this may be explained by limited awareness in Ponte de Lima. Although the methods used are not appropriate for protozoan diagnosis we did find Isospora spp. and these parasites were more prevalent in hunting dog samples.
Undoubtedly, the present study revealed poor hygiene conditions and animal housing, similarly to Cardoso et al. [17]. Furthermore, a wide diversity of zoonotic parasites has been detected, and well distributed throughout the municipality.
4.3. Parasite Associations and Infections
The majority of the dogs were infected by only one species of parasite. Similar results have been reached by other studies, but with a distinct number of different parasites found: up to two [38], up to three [21,26,30,32,35,36,41], up to six [51] and up to seven [1]. Hunting dogs had a significantly larger risk of being infected with multiple parasitic species.The most frequent association observed between parasites was Ancylostomatidae and Trichuris spp. A subsequent Pareto analysis, carried out by strata confirmed the predominance of these two parasites. Interestingly, the same association has also been found between human whipworms and hookworms [71]. It is still not clear what drives this affinity between parasites. Answers to this question are essential to adequately understand the epidemiology and control of these diseases. These findings warrant further studies to understand whether there are biological factors influencing such co-existence [57].
4.4. Limitations of the Study
Our study design had some necessary limitations: the prevalence of infection in dogs could not be determined in environmental samples. Consequently although we knew the background of each individual dog from the other groups, we did not plan to trace back the samples to the animal of origin, as our aim was not to do that. We did not record breeds or ages, and with regard to environmental samples, multiple samples may have originated from a single dog, although we tried to avoid this from taking place. In our survey, the size of the dog population is expected to be large enough to draw reliable conclusions.
4.5. “One Health” Approach Is Required
A close collaboration between veterinary and public health professionals in a “One Health” approach is required [5,6,11,72]. In Portugal, veterinary practitioners acting as information sources about zoonoses transmitted by canids are needed, but there are basic priorities that should be considered first. So far, data is limited to only a few urban areas and no information is available for large territories of the country with more suitable socioeconomic and environmental conditions for parasitic transmission. Multidisciplinary approaches will lead to a more complete understanding of the actual epidemiological situation in the country. Few studies have been undertaken to determine the prevalence of these organisms and/or their associated diseases in people from the same community. Many physicians are not knowledgeable about these infections [73], and they feel that a collaborative relationship with a veterinarian who possesses specialty training in zoonoses would be valuable to their practice [74]. Several studies have shown that physicians delegate to veterinarians the responsibility to do health education of communities concerning zoonoses [46,74,75], and suggest that veterinarians should be involved not only in controlling zoonotic disease in animals, but also in providing information for patients and physicians [75]. Nonetheless, communication among veterinarians, physicians, and dogs owners or human patients seems to be insufficient [76]. Although changing in human behavior is an extremely difficult challenge [46], this is essential for the success of the control and prevention of these diseases. Effective campaigns and education programs could be instituted to prevent of zoonotic infections associated with household pets and address issues surrounding poorly prepared or cooked food [76].
5. Conclusions
Zoonoses involving dog parasites are both common and important, with some causing serious diseases. Understanding the epidemiology of zoonotic parasitic infections is important to minimize of the risk to humans. The epidemiological research conducted in Ponte de Lima revealed a considerable environmental contamination with zoonotic parasites. The two most prevalent parasites are Ancylostomatidae and Trichuris spp. The high level of environmental zoonotic contamination found calls for raising the awareness of the problem among the population. Hunting dogs were at higher risk of harboring zoonotic parasites showing higher prevalence and a higher number of multiple infections. Health education and risk communication should therefore be developed to and target children and families, farmers, and, especially, hunters. Also, closer collaboration is needed between researchers and practitioners (human and veterinary medical professionals), as well as with public health authorities. The prevention of zoonoses requires a global commitment, however, the main task still regards local populations and requires changes to human behavior. Population awareness is a pressing need.
Acknowledgments
The authors would like to thank to Sónia Santos for the kind design of the relevant maps. This work was supported by the Portuguese Science and Technology Foundation (FCT) under the Project PEst-OE/AGR/UI0772/2014.
Author Contributions
Teresa Letra Mateus with all co-authors carried out the study design. Teresa Mateus performed the search and drafted the manuscript. António Castro participated in the percoll fractionation of fecal samples and molecular detection of Echinococcus granulosus. João Niza Ribeiro participated in data analysis. João Niza Ribeiro and Madalena Vieira Pinto revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szabova, E.; Juris, P.; Miterpakova, M.; Antolova, D.; Papajova, I.; Sefcikova, H. Prevalence of important zoonotic parasites in dog populations from the Slovak Republic. Helminthologia 2007, 44, 170–176. [Google Scholar]
- Beck, A.M.; Meyers, N.M. Health enhancement and companion animal ownership. Annu. Rev. Public Health 1996, 17, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.M.; van Zutphen, L.; Hoek, D.; Yaya, F.O.; Roelfsema, J.; Pinelli, E.; van Knapen, F.; Kortbeek, L.M. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet. Parasitol. 2009, 163, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Ben, S. Zoonoses in the bedroom. Emerg. Infect. Dis. 2011, 17, 167–172. [Google Scholar] [CrossRef]
- Tarsitano, E.; Greco, G.; Decaro, N.; Nicassio, F.; Lucente, M.S.; Buonavoglia, C.; Tempesta, M. Environmental monitoring and analysis of faecal contamination in an urban setting in the city of Bari (Apulia Region, Italy): Health and hygiene implications. Int. J. Environ. Res. Public Health 2010, 7, 3972–3986. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; van Knapen, F.; Schweiger, A.; Overgaauw, P.A.M. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet. Parasitol. 2011, 182, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Fontanarrosa, M.F.; Vezzani, D.; Basabe, J.; Eiras, D.F. An epidemiological study of gastrointestinal parasites of dogs from Southern Greater Buenos Aires (Argentina): Age, gender, breed, mixed infections, and seasonal and spatial patterns. Vet. Parasitol. 2006, 136, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Sowemimo, O.A. The prevalence and intensity of gastrointestinal parasites of dogs in ile-ife, Nigeria. J. Helminthol. 2009, 83, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Seimenis, A. Overview of the epidemiological situation on echinococcosis in the Mediterranean region. Acta Trop. 2003, 85, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Blaszkowska, J.; Kurnatowski, P.; Damiecka, P. Contamination of the soil by eggs of geohelminths in rural areas of Lodz District (Poland). Helminthologia 2011, 48, 67–76. [Google Scholar] [CrossRef]
- Habluetzel, A.; Traldi, G.; Ruggieri, S.; Attili, A.R.; Scuppa, P.; Marchetti, R.; Menghini, G.; Esposito, F. An estimation of toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet. Parasitol. 2003, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Himsworth, C.G.; Skinner, S.; Chaban, B.; Jenkins, E.; Wagner, B.A.; Harms, N.J.; Leighton, F.A.; Thompson, R.C.A.; Hill, J.E. Short report: Multiple zoonotic pathogens identified in canine feces collected from a remote canadian indigenous community. Am. J. Trop. Med. Hyg. 2010, 83, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Estatísticas Demográficas; National Statistics Institute—Portugal Statistics: Lisboa, Portugal, 2011. [Google Scholar]
- Foreyt, W.J. Veterinary Parasitology; Blackwell Publishing: Iowa, USA, 2001; p. 246. [Google Scholar]
- Zajac, A.M.; Conboy, G.A. Veterinary Clinical Parasitology, 8th ed.; Wiley Blackwell: West Sussex, UK, 2012; p. 368. [Google Scholar]
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.J. Diagnosing Helminthiasis by Coprological Examination. Coprological Examination, 2nd ed.; Janssen Research Foundation: Beerse, Belgium, 1986; p. 205. [Google Scholar]
- Cardoso, A.S.; Costa, I.M.H.; Figueiredo, C.; Castro, A.; Conceicao, M.A.P. The occurrence of zoonotic parasites in rural dog populations from Northern Portugal. J. Helminthol. 2014, 88, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, I.; Branzburg, A.; Campos-Ponce, M.; Abdel Hafez, S.K.; Raoul, F.; Craig, P.S.; Hamburger, J. Copro-diagnosis of echinococcus granulosus infection in dogs by amplification of a newly identified repeated DNA sequence. Am. J. Trop. Med. Hyg. 2003, 69, 324–330. [Google Scholar] [PubMed]
- Rinaldi, L.; Biggeri, A.; Carbone, S.; Musella, V.; Catelan, D.; Veneziano, V.; Cringoli, G. Canine faecal contamination and parasitic risk in the city of Naples (southern Italy). BMC Vet. Res. 2006, 2, 29–29. [Google Scholar] [CrossRef] [PubMed]
- Dubna, S.; Langrova, I.; Napravnik, J.; Jankovska, I.; Vadlejch, J.; Pekar, S.; Fechtner, J. The prevalence of intestinal parasites in dogs from Prague, rural areas, and shelters of the Czech Republic. Vet. Parasitol. 2007, 145, 120–128. [Google Scholar] [CrossRef]
- Martinez-Carrasco, C.; Berriatua, E.; Garijo, M.; Martinez, J.; Alonso, F.D.; de Ybanez, R.R. Epidemiological study of non-systemic parasitism in dogs in Southeast Mediterranean Spain assessed by coprological and post-mortem examination. Zoonoses Public Health 2007, 54, 195–203. [Google Scholar]
- Papazahariadou, A.; Founta, A.; Papadopoulos, E.; Chliounakis, S.; Antoniadou-Sotiriadou, K.; Theodorides, Y. Gastrointestinal parasites of shepherd and hunting dogs in the serres prefecture, northern Greece. Vet. Parasitol. 2007, 148, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Gracenea, M.; Soledad Gomez, M.; Torres, J. Prevalence of intestinal parasites in shelter dogs and cats in the metropolitan area of Barcelona (Spain). Acta Parasitol. 2009, 54, 73–77. [Google Scholar] [CrossRef]
- Soriano, S.V.; Pierangeli, N.B.; Pianciola, L.; Mazzeo, M.; Lazzarini, L.E.; Saiz, M.S.; Kossman, A.V.; Bergagna, H.F.J.; Chartier, K.; Basualdo, J.A. Molecular characterization of echinococcus isolates indicates goats as reservoir for Echinococcus Canadensis G6 genotype in Neuquen, Patagonia Argentina. Parasitol. Int. 2010, 59, 626–628. [Google Scholar] [CrossRef]
- Cramer Balassiano, B.C.; Campos, M.R.; Alves Alcantara de Menezes, R.D.C.; Salim Pereira, M.J. Factors associated with gastrointestinal parasite infection in dogs in Rio De Janeiro, Brazil. Prev. Vet. Med. 2009, 91, 234–240. [Google Scholar]
- Okoye, I.C.; Obiezue, N.R.; Okorie, C.E.; Ofoezie, I.E. Epidemiology of intestinal helminth parasites in stray dogs from markets in south-eastern Nigeria. J. Helminthol. 2011, 85, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.; Carmena, D.; Postigo, I.; Estibalez, J.J.; Martinez, J.I.; Guisantes, J.A. Intestinal helminths in dogs in alava, north of Spain. Rev. Ibér. Parasitol. 2003, 63, 121–126. [Google Scholar]
- Katagiri, S.; Oliveira-Sequeira, T.C.G. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in sao paulo state, Brazil. Zoonoses Public Health 2008, 55, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Beiromvand, M.; Akhlaghi, L.; Massom, S.H.F.; Meamar, A.R.; Motevalian, A.; Oormazdi, H.; Razmjou, E. Prevalence of zoonotic intestinal parasites in domestic and stray dogs in a rural area of Iran. Prev. Vet. Med. 2013, 109, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Bajer, A.; Bednarska, M.; Rodo, A. Risk factors and control of intestinal parasite infections in sled dogs in Poland. Vet. Parasitol. 2011, 175, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ugbomoiko, U.S.; Ariza, L.; Heukelbach, J. Parasites of importance for human health in Nigerian dogs: High prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008, 4. [Google Scholar] [CrossRef]
- Martinez-Moreno, F.J.; Hernandez, S.; Lopez-Cobos, E.; Becerra, C.; Acosta, I.; Martinez-Moreno, A. Estimation of canine intestinal parasites in Cordoba (Spain) and their risk to public health. Vet. Parasitol. 2007, 143, 7–13. [Google Scholar]
- Gingrich, E.N.; Scorza, A.V.; Clifford, E.L.; Olea-Popelka, F.J.; Lappin, M.R. Intestinal parasites of dogs on the Galapagos Islands. Vet. Parasitol. 2010, 169, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Eguia-Aguilar, P.; Cruz-Reyes, A.; Maninez-Maya, J.J. Ecological analysis and description of the intestinal helminths present in dogs in Mexico City. Vet. Parasitol. 2005, 127, 139–146. [Google Scholar]
- Mandarino-Pereira, A.; de Souza, F.S.; Lopes, C.W.G.; Pereira, M.J.S. Prevalence of parasites in soil and dog feces according to diagnostic tests. Vet. Parasitol. 2010, 170, 176–181. [Google Scholar]
- Crespo, M.V.M.; Jorge, A.T. Contaminação parasitária por ovos de helmintes de alguns jardins e parques públicos das cidades de almeirim e do cartaxo. Acta Parasitol. Port. 2000, 7, 43–47. [Google Scholar]
- Cruz, A.; Santos, A.; Mateus, T.; Ramalho, F.; Sousa, S.; Madeira de Carvalho, L.M. Avaliação do grau de contaminação ambiental com formas parasitárias zoonóticas com origem em dejectos caninos em áreas públicas da cidade de coimbra. Acta Parasitol. Port. 2012, 19, 35–38. [Google Scholar]
- Neves, D.; Lobo, L.; Simoes, P.B.; Cardoso, L. Frequency of intestinal parasites in pet dogs from an urban area (Greater Oporto, Northern Portugal). Vet. Parasitol. 2014, 200, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.L.; Francisco, F.; Lima, N.R. Environmental contamination by dog faeces with parasitic forms in Ponte De Lima, Portugal. In International Congress on Environmental Health; Almeida, J., Ferreira, A., Paixão, S., Pinto, M.V., Sá, N.L., Santos, C., Simões, H., Eds.; Department of Environmental Health—College of Health Technology of Coimbra: Coimbra, Portugal, 2011; pp. 331–334. [Google Scholar]
- Crespo, M.V.; Rosa, F.; Almeida, J.P. Eliminação parasitária em fezes de canídeos no concelho de óbidos—Estudo geral. Acta Parasitol. Port. 2010, 17, 111. [Google Scholar]
- Ferreira, F.S.; Pereira-Baltasar, P.; Parreira, R.; Padre, L.; Vilhena, M.; Tavora Tavira, L.; Atouguia, J.; Centeno-Lima, S. Intestinal parasites in dogs and cats from the district of Evora, Portugal. Vet. Parasitol. 2011, 179, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, B.; Iorio, R.; Capelli, G.; Sparagano, O.A.E.; Giangaspero, A. Epidemiological scenario of giardiosis in dogs from central Italy. Anim. Biodivers. Emerg. Dis. 2008, 1149, 371–374. [Google Scholar]
- Oliveira-Sequeira, T.C.G.; Amarante, A.F.T.; Ferrari, T.B.; Nunes, L.C. Prevalence of intestinal parasites in dogs from São Paulo State, Brazil. Vet. Parasitol. 2002, 103, 19–27. [Google Scholar]
- Wolfe, A.; Hogan, S.; Maguire, D.; Fitzpatrick, C.; Vaughan, L.; Wall, D.; Hayden, T.J.; Mulcahy, G. Red foxes (Vulpes vulpes) in ireland as hosts for parasites of potential zoonotic and veterinary significance. Vet. Rec. 2001, 149, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.D.; Lynn, R.C.; Eberhard, M.L. Georgis Parasitologia Para Veterinários, 8th ed.; Elsevier: Madrid, España, 2004; p. 440. [Google Scholar]
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.J.; Schurer, J.M.; Gesy, K.M. Old problems on a new playing field: Helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011, 182, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.L.; Barrocas, C. Wild Carnivores as a Source of Zoonotic Helminths in the Northern Portugal. In Proceedings of the Joint 61st Wildlife Disease Association/10th Biennial European Wildlife Disease Association Conference, Lyon, France, 22–27 July 2012; p. 135.
- Magalhães, R.; Mateus, T. Desparasitação interna de cães—O que sabem os proprietários de ponte de lima? Acta Parasitol. Port. 2012, 19, 253. [Google Scholar]
- Ramirez-Barrios, R.A.; Barboza-Mena, G.; Munoz, J.; Angulo-Cubillan, F.; Hernandez, E.; Gonzalez, F.; Escalona, F. Prevalence of intestinal parasites in dogs under veterinary care in Maracaibo, Venezuela. Vet. Parasitol. 2004, 121, 11–20. [Google Scholar]
- Xhaxhiu, D.; Kusi, I.; Rapti, D.; Kondi, E.; Postoli, R.; Rinaldi, L.; Dimitrova, Z.M.; Visser, M.; Knaus, M.; Rehbein, S. Principal intestinal parasites of dogs in Tirana, Albania. Parasitol. Res. 2011, 108, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Bwalya, E.C.; Nalubamba, K.S.; Hankanga, C.; Namangala, B. Prevalence of canine gastrointestinal helminths in urban Lusaka and rural Katete Districts of Zambia. Prev. Vet. Med. 2011, 100, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Riggio, F.; Mannella, R.; Ariti, G.; Perrucci, S. Intestinal and lung parasites in owned dogs and cats from central Italy. Vet. Parasitol. 2013, 193, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, D.J.; Tzannes, S.; Graham, P.A.; Wastling, J.M.; Pinchbeck, G.L.; German, A.J. Detection of endoparasites with zoonotic potential in dogs with gastrointestinal disease in the UK. Transbound. Emerg. Dis. 2008, 55, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.; Zunino, G.; Santillan, G.; Wisnivesky, C. Epidemiology of toxocara canis in the dog population from two areas of different socioeconomic status, Greater Buenos Aires, Argentina. Vet. Parasitol. 2003, 115, 275–286. [Google Scholar] [CrossRef]
- Bowman, D.D.; Montgomery, S.P.; Zajac, A.M.; Eberhard, M.L.; Kazacos, K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010, 26, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D. Are we paying too much attention to cardiopulmonary nematodes and neglecting old-fashioned worms like trichuris vulpis? Parasites Vectors 2011, 4. [Google Scholar] [CrossRef]
- Dunn, J.J.; Columbus, S.T.; Aldeen, W.E.; Davis, M.; Carroll, K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002, 40, 2703–2704. [Google Scholar] [CrossRef] [PubMed]
- Keegan, J.D.; Holland, C.V. A comparison of toxocara canis embryonation under controlled conditions in soil and hair. J. Helminthol. 2013, 87, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.; Borecka, A.; Zarnowska, H.; Marczynska, M.; Dobosz, S. Environmental and personal risk factors for toxocariasis in children with diagnosed disease in urban and rural areas of central Poland. Vet. Parasitol. 2008, 155, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Auer, H. Parasitoses of the human central nervous system. J. Helminthol. 2013, 87, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Ulukanligil, M.; Seyrek, A.; Aslan, G.; Ozbilge, H.; Atay, S. Environmental pollution with soil-transmitted helminths in Sanliurfa, Turkey. Mem. Inst. Oswaldo Cruz 2001, 96, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Deutz, A.; Fuchs, K.; Auer, H.; Kerbl, U.; Aspock, H.; Kofer, J. Toxocara-infestations in Austria: A study on the risk of infection of farmers, slaughterhouse staff, hunters and veterinarians. Parasitol. Res. 2005, 97, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.P.; Ogburn, J.; Adegboyega, P. Infection by dipylidium caninum in an infant. Arch. Pathol. Lab. Med. 2003, 127, e157–e159. [Google Scholar] [PubMed]
- Robertson, I.D.; Thompson, R.C. Enteric parasitic zoonoses of domesticated dogs and cats. Microbes Infect. 2002, 4, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Pantchev, N.; Gawlowska, S.; Vrhovec, M.G.; Bauer, C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet. Parasitol. 2008, 157, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Ziadinov, I.; Schweiger, A.; Schnyder, M.; Deplazes, P. Hair coat contamination with zoonotic helminth eggs of farm and pet dogs and foxes. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 503–511. [Google Scholar] [PubMed]
- Dakkak, A. Echinococcosis/hydatidosis: A severe threat in mediterranean countries. Vet. Parasitol. 2010, 174, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.-A.M.; Gray, D.J.; Clements, A.C.A.; Barnes, T.S.; McManus, D.P.; Yang, Y.R. Environmental changes impacting echinococcus transmission: Research to support predictive surveillance and control. Glob. Chang. Biol. 2013, 19, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.M. Intestinal parasites of dogs in Western Australia: Progress in control and new concerns. Vet. J. 1999, 157, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Samantaray, J.C.; Singh, N.; Das, G.B.; Verma, I.C. Trichuris-vulpis infection in an Indian tribal population. J. Parasitol. 1993, 79, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.W.; Peregrine, A.S.; Sargeant, J.M.; Weese, J.S. Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada. BMC Public Health 2013, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Wilkins, P.P. Toxocariasis: America’s most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef]
- Kersting, A.L.; Medeiros, L.C.; LeJeune, J.T. Zoonoses and the physicians’ role in educating farming patients. J. Agromed. 2009, 14, 306–311. [Google Scholar] [CrossRef]
- Grant, S.; Olsen, C.W. Preventing zoonotic diseases in immunocompromised persons: The role of physicians and veterinarians. Emerg. Infect. Dis. 1999, 5, 159–163. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.; Moore, T.A. Emerging helminth zoonoses. Int. J. Parasitol. 2000, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).