Urinary 1-Hydroxypyrene is Associated with Oxidative Stress and Inflammatory Biomarkers in Acute Myocardial Infarction
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Collection of Biological Samples
2.3. Laboratorial Analyses
2.4. Quantification of Blood Carboxyhaemoglobin Levels
2.5. 1-Hydroxypyrene Quantification
2.6. Lipid Peroxidation
2.7. Enzymatic Antioxidants
2.8. Non-Enzymatic Antioxidants
2.9. Statistical Analysis
3. Results
| Characteristics | Controls | Post-infarction | ||
|---|---|---|---|---|
| Non-Smokers (n = 25) | Smokers (n = 16) | Non-Smokers (n = 27) | Smokers (n = 31) | |
| Age (years) | 55.7 ± 8.1 | 52.2 ± 7.5 | 63.2 ± 9.1 | 60.1 ± 10.2 |
| Gender (male/female) | (18/7) | (11/5) | (22/5) | (21/10) |
| Alcohol consumption (%) | 38.5 | 6.5 | 40 | 45 |
| Hypocholesterolemic drugs (%) | 7.7 | 0 | 20.4 | 28.3 |
| Anti hypertensive drugs (%) | 30.8 | 3.5 | 43.4 | 40.2 |
| Diuretic drugs (%) | 4 | 0 | 18 | 22.4 |
| Sedentary lifestyle (%) | 8 | 58.8 | 55 | 70 |
| Hypertension (%) | 38.5 | 4.7 | 52 | 48 |
| Biomarkers | Controls | Post-Infarction | ||
|---|---|---|---|---|
| Non-Smokers (n = 25) | Smokers (n = 16) | Non-Smokers (n = 27) | Smokers (n = 31) | |
| Platelets (103/µL) | 251 ± 10.7 | 243 ± 12.6 | 239 ± 8.4 | 259 ± 14.3 |
| Fibrinogen (mg/dL) | 270 ± 9.1 | 286 ± 20.9 | 250 ± 15.1 | 256 ± 13.8 |
| Total cholesterol (mg/dL) | 203 ± 8.8 | 238 ± 13.9 c | 207 ± 8.8 | 197 ± 7.4b |
| HDL cholesterol (mg/dL) | 44.8 ± 2.8 | 44.6 ± 2.7 | 45.1 ± 2.8 | 41.1 ± 1.8 |
| LDL cholesterol (mg/dL) | 120 ± 14.4 | 154 ± 20.5 | 140 ± 17.3 | 135 ± 15.7 |
| Total cholesterol /HDL | 4.9 ± 0.3 | 5.7 ± 0.5 | 4.7 ± 0.3 | 5.0 ± 0.3 |
| Triglycerides (mg/dL) | 187 ± 27.8 | 197 ± 23.1 | 112 ± 13.5 a | 102 ± 8.4 b |

| Biomarkers | Controls | Post-infarction | ||
|---|---|---|---|---|
| Non-Smokers (n = 25) | Smokers (n = 16) | Non-Smokers (n = 27) | Smokers (n = 31) | |
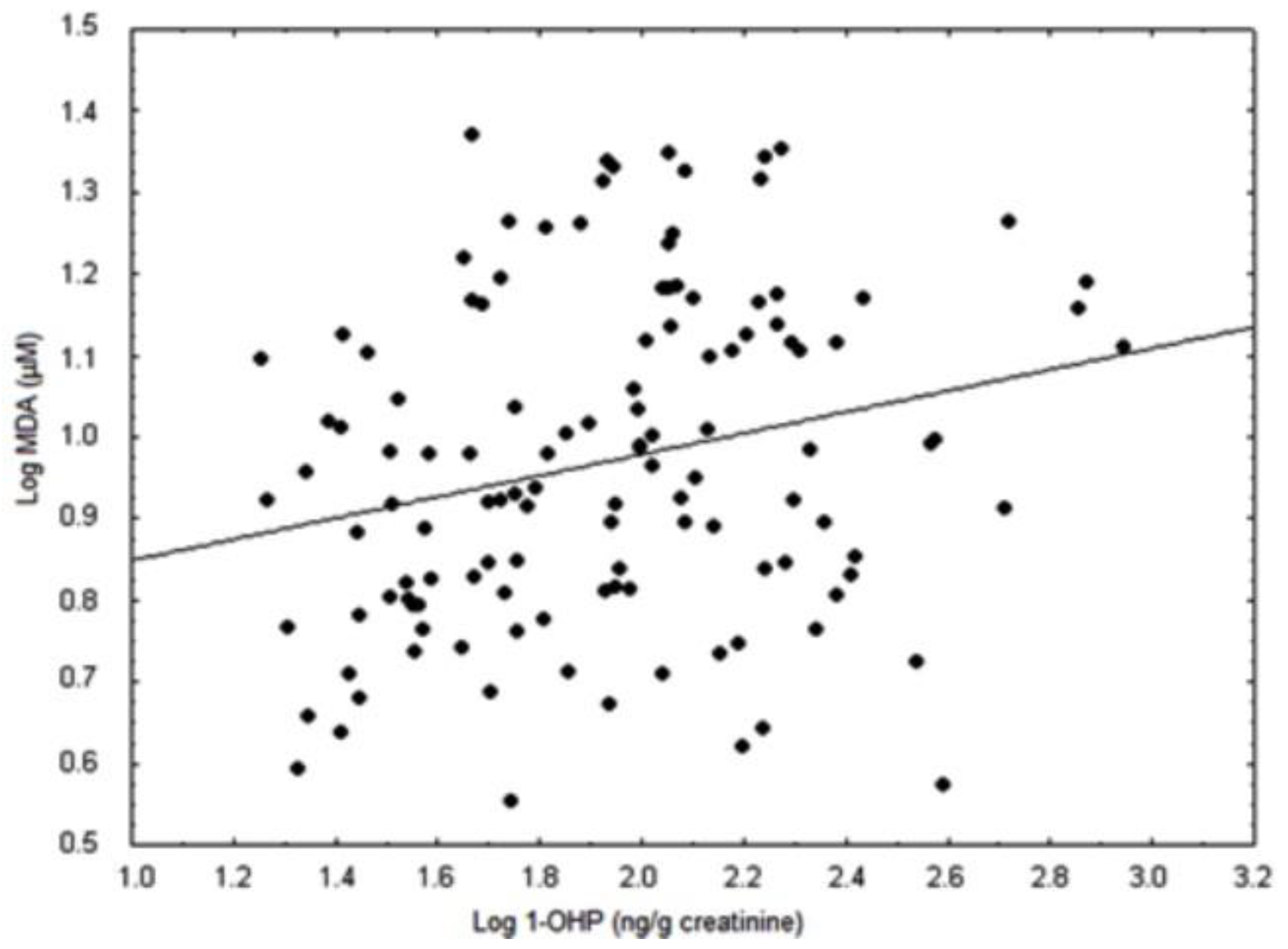
| MDA (µmol/L) | 6.1 ± 0.3 | 7.6 ± 0.7 | 11.7 ± 0.8 a | 11.7 ± 0.9 b |
| SOD (U/mg protein) | 13.8 ± 1.2 | 21.5 ± 1.9 c | 10.3 ± 1.1 a | 12.7 ± 1.2 b |
| CAT (U/mg protein) | 12.1 ± 1.0 | 10.5 ± 1.0 | 30.2 ± 1.8 a | 30.2 ± 2.1 b |
| GPx (µmol NADPH/min/mg protein) | 7.5 ± 0.5 | 8.4 ± 0.8 | 11.3 ± 0.7 a | 12.0 ± 0.7 b |
| Vitamin E (µmol/L) | 29.6 ± 2.0 | 34.0 ± 2.2 | 31.4 ± 2.7 | 30.1 ± 2.4 |
| Retinol (µmol/L) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.7 ± 0.1 | 2.4 ± 0.1 |
| β-Carotene (µmol/L) | 0.8 ± 0.08 | 0.74 ± 0.07 | 0.4 ± 0.1 a | 0.4 ± 0.1 b |
| Lycopene (µmol/L) | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franchini, M.; Mannucci, P.M. Air pollution and cardiovascular disease. Thromb. Res. 2012, 129, 230–234. [Google Scholar] [CrossRef]
- Ilar, A.; Lewné, M.; Plato, N.; Hallqvist, J.; Alderling, M.; Bigert, C.; Hogstedt, C.; Gustavsson, P. Myocardial infarction and occupational exposure to motor exhaust: A population-based case—Control study in Sweden. Eur. J. Epidemiol. 2014, 29, 517–525. [Google Scholar] [CrossRef]
- Associations between Recent Exposure to Ambient Fine Particulate Matter and Blood Pressure in the Multi-Ethnic Study of Atherosclerosis (MESA). Available online: http://deepblue.lib.umich.edu/bitstream/handle/2027.42/58000/Associations%20between%20recent%20exposure%20to%20ambient%20fine%20particulate%20matter%20and%20blood%20pressure%20in%20the%20Multi%20Ethnic%20Study%20of%20Atherosclerosis.pdf?sequence=1 (accessed on 25 August 2014).
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of ambient temperature on the incidence of myocardial infarction. Heart 2009, 95, 1760–1769. [Google Scholar] [CrossRef]
- De Paula Santos, U.; Braga, A.L.F.; Giorgi, D.M.A.; Pereira, L.A.A.; Grupi, C.J.; Lin, C.A.; Bussacos, M.A.; Zanetta, D.M.T.; do Nascimento Saldiva, P.H.; Terra Filho, M. Effects of air pollution on blood pressure and heart rate variability: A panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur. Heart. J. 2005, 26, 193–200. [Google Scholar]
- Miller, K.P.; Ramos, K.S. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 2001, 33, 1–35. [Google Scholar] [CrossRef]
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama 2002, 287, 1132–1141. [Google Scholar]
- Delfino, R. J.; Staimer, N.; Tjoa, T.; Gillen, D. L.; Polidori, A.; Arhami, M.; Kleinman, M. T.; Vaziri, N. D.; Longhurst, J.; Sioutas, C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ. Health Persp. 2009, 117, 1232–1238. [Google Scholar] [CrossRef]
- Yang, Y.; Griffiths, W.J.; Nordling, M.; Nygren, J.; Möller, L.; Bergman, J.; Liepinsh, E.; Otting, G.; Gustafsson, J.-Å.; Rafter, J. Ring opening of benz.a]pyrene in the germ-free rat is a novel pathway for formation of potentially genotoxic metabolites. Biochemestry 2000, 39, 15585–15591. [Google Scholar] [CrossRef]
- Fan, R.; Wang, D.; Mao, C.; Ou, S.; Lian, Z.; Huang, S.; Lin, Q.; Ding, R.; She, J. Preliminary study of children’s exposure to PAHs and its association with 8-hydroxy-2′-deoxyguanosine in Guangzhou, China. Environ. Int. 2012, 42, 53–58. [Google Scholar] [CrossRef]
- Burgaz, S.; Cakmak Demircigil, G.; Karahalil, B.; Karakaya, A.E. Chromosomal damage in peripheral blood lymphocytes of traffic policemen and taxi drivers exposed to urban air pollution. Chemosphere 2002, 47, 57–64. [Google Scholar]
- Demetriou, C.A.; Raaschou-Nielsen, O.; Loft, S.; Møller, P.; Vermeulen, R.; Palli, D.; Chadeau-Hyam, M.; Xun, W.W.; Vineis, P. Biomarkers of ambient air pollution and lung cancer: A systematic review. Occup. Environ. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Hansen, Å.M.; Mathiesen, L.; Pedersen, M.; Knudsen, L.E. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies—A review. Int. J. Hyg. Envir. Heal. 2008, 211, 471–503. [Google Scholar]
- Curfs, D.M.; Lutgens, E.; Gijbels, M.J.; Kockx, M.M.; Daemen, M.J.; van Schooten, F.J. Chronic exposure to the carcinogenic compound benzo[a]pyrene induces larger and phenotypically different atherosclerotic plaques in ApoE-knockout mice. Am. J. Pathol. 2004, 164, 101–108. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Curfs, D.M.; Pachen, D.M.; Gottschalk, R.W.; de Winther, M.P.; Daemen, M.J.; van Schooten, F.J. The environmental carcinogen benzo[a]pyrene induces expression of monocyte-chemoattractant protein-1 in vascular tissue: a possible role in atherogenesis. Mutat. Res-Fund. Mol. M. 2007, 621, 31–41. [Google Scholar] [CrossRef]
- Enomoto, M.; Tierney, W. J.; Nozaki, K. Risk of human health by particulate matter as a source of air pollution. J. Toxicol. Sci. 2008, 33, 251–267. [Google Scholar]
- Vogel, C.F.A.; Sciullo, E.; Wong, P.; Kuzmicky, P.; Kado, N.; Matsumura, F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect. 2005, 113, 1536–1541. [Google Scholar] [CrossRef]
- Podechard, N.; Lecureur, V.; le Ferrec, E.; Guenon, I.; Sparfel, L.; Gilot, D.; Gordon, J.R.; Lagente, V.; Fardel, O. Interleukin-8 induction by the environmental contaminant benzo(a) pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol. Lett. 2008, 177, 130–137. [Google Scholar] [CrossRef]
- Friedewald, W. T.; Levy, R. I.; Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Beutler, E.; West, C. Simplified determination of carboxyhemoglobin. Clin. Chem. 1984, 30, 871–874. [Google Scholar]
- Grotto, D.; Santa Maria, L.; Boeira, S.; Valentini, J.; Charão, M.; Moro, A.; Nascimento, P.; Pomblum, V.; Garcia, S. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography—Visible detection. J. Pharmaceutic. Biomed. 2007, 43, 619–624. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Charão, M.F.; Moro, A.M.; Brucker, N.; Bulcão, R.P.; Baierle, M.; Freitas, F.; Durgante, J.; Nascimento, S.; Bubols, G.B.; Saldiva, P.H. Simultaneous quantification of lycopene, β-carotene, retinol and α-tocopherol in plasma after a simple extraction procedure: stability study and application to human volunteers. J. Brazil Chem. Soc. 2012, 23, 1441–1449. [Google Scholar] [CrossRef]
- Rückerl, R.; Schneider, A.; Breitner, S.; Cyrys, J.; Peters, A. Health effects of particulate air pollution: A review of epidemiological evidence. Inhal. Toxicol. 2011, 23, 555–592. [Google Scholar] [CrossRef]
- Liu, H.-H.; Lin, M.-H.; Chan, C.-I.; Chen, H.-L. Oxidative damage in foundry workers occupationally co-exposed to PAHs and metals. Int. J. Hyg. Envir. Heal. 2010, 213, 93–98. [Google Scholar] [CrossRef]
- Jongeneelen, F.; Anzion, R.; Scheepers, P.; Bos, R.; Henderson, P.T.; Nijenhuis, E.; Veenstra, S.; Brouns, R.; Winkes, A. 1-Hydroxypyrene in urine as a biological indicator of exposure to polycyclic aromatic hydrocarbons in several work environments. Ann. Occup. Hyg. 1988, 32, 35–43. [Google Scholar] [CrossRef]
- Ciarrocca, M.; Rosati, M. V.; Tomei, F.; Capozzella, A.; Andreozzi, G.; Tomei, G.; Bacaloni, A.; Casale, T.; Andrè, J. C.; Fioravanti, M. Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. J. Expo. Anal. Env. Epid. 2013, 24, 17–26. [Google Scholar]
- Wang, C.; Dai, Y.; Feng, G.; He, R.; Yang, W.; Li, D.; Zhou, X.; Zhu, L.; Tan, L. Addition of porphyrins to cigarette filters to reduce the levels of benzo[a]pyrene (B[a]P) and tobacco-specific N-nitrosamines (TSNAs) in mainstream cigarette smoke. J. Agr. Food Chem. 2011, 59, 7172–7177. [Google Scholar]
- Bono, R.; Piccioni, P.; Traversi, D.; Degan, R.; Grosa, M.; Bosello, G.; Gilli, G.; Arossa, W.; Bugiani, M. Urban air quality and carboxyhemoglobin levels in a group of traffic policemen. SCI Total. Environ. 2007, 376, 109–115. [Google Scholar] [CrossRef]
- Bagatini, M.D.; Martins, C.C.; Battisti, V.; Gasparetto, D.; da Rosa, C.S.; Spanevello, R.M.; Ahmed, M.; Schmatz, R.; Schetinger, M.R.C.; Morsch, V.M. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. 2011, 26, 55–63. [Google Scholar]
- Moro, A.M.; Charão, M.; Brucker, N.; Bulcão, R.; Freitas, F.; Guerreiro, G.; Baierle, M.; Nascimento, S.; Waechter, F.; Hirakata, V. Effects of low-level exposure to xenobiotics present in paints on oxidative stress in workers. Sci. Total Environ. 2010, 408, 4461–4467. [Google Scholar] [CrossRef]
- Kiruthiga, P.; Shafreen, R.B.; Pandian, S.K.; Arun, S.; Govindu, S.; Devi, K.P. Protective effect of silymarin on erythrocyte haemolysate against benzo(a)pyrene and exogenous reactive oxygen species (H2O2) induced oxidative stress. Chemosphere 2007, 68, 1511–1518. [Google Scholar] [CrossRef]
- Fridovich, I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986, 247, 1–11. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. The role of O2·− in the production of HO·: In vitro and in vivo. Free Radical Bio. Med. 1994, 16, 29–33. [Google Scholar] [CrossRef]
- Woodall, A.A.; Lee, S. W.-M.; Weesie, R.J.; Jackson, M.J.; Britton, G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta 1997, 1336, 33–42. [Google Scholar]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Freitas, F.; Brucker, N.; Durgante, J.; Bubols, G.; Bulcão, R.; Moro, A.; Charão, M.; Baierle, M.; Nascimento, S.; Gauer, B.; et al. Urinary 1-Hydroxypyrene is Associated with Oxidative Stress and Inflammatory Biomarkers in Acute Myocardial Infarction. Int. J. Environ. Res. Public Health 2014, 11, 9024-9037. https://doi.org/10.3390/ijerph110909024
Freitas F, Brucker N, Durgante J, Bubols G, Bulcão R, Moro A, Charão M, Baierle M, Nascimento S, Gauer B, et al. Urinary 1-Hydroxypyrene is Associated with Oxidative Stress and Inflammatory Biomarkers in Acute Myocardial Infarction. International Journal of Environmental Research and Public Health. 2014; 11(9):9024-9037. https://doi.org/10.3390/ijerph110909024
Chicago/Turabian StyleFreitas, Fernando, Natália Brucker, Juliano Durgante, Guilherme Bubols, Rachel Bulcão, Angela Moro, Mariele Charão, Marília Baierle, Sabrina Nascimento, Bruna Gauer, and et al. 2014. "Urinary 1-Hydroxypyrene is Associated with Oxidative Stress and Inflammatory Biomarkers in Acute Myocardial Infarction" International Journal of Environmental Research and Public Health 11, no. 9: 9024-9037. https://doi.org/10.3390/ijerph110909024
APA StyleFreitas, F., Brucker, N., Durgante, J., Bubols, G., Bulcão, R., Moro, A., Charão, M., Baierle, M., Nascimento, S., Gauer, B., Sauer, E., Zimmer, M., Thiesen, F., Castro, I., Saldiva, P., & Garcia, S. C. (2014). Urinary 1-Hydroxypyrene is Associated with Oxidative Stress and Inflammatory Biomarkers in Acute Myocardial Infarction. International Journal of Environmental Research and Public Health, 11(9), 9024-9037. https://doi.org/10.3390/ijerph110909024





